In this issue of Blood, Woyach et al provide compelling evidence that Bruton’s tyrosine kinase (BTK) is a critical target of ibrutinib in chronic lymphocytic leukemia (CLL), and Rushworth et al report data suggesting that BTK may also be a viable therapeutic target in acute myeloid leukemia (AML). These studies exemplify the concept that selectively targeting overactive kinases may be therapeutically useful in neoplasia even in the absence of recurrent genetic mutations in those kinases.1,2
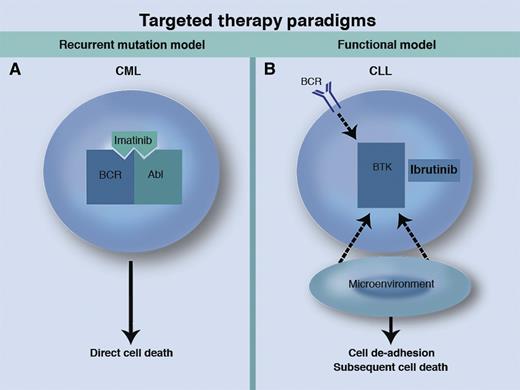
Two paradigms for small molecule–targeted therapies in cancer. (A) In the recurrent mutation model exemplified by CML, selective inhibition of a kinase with recurrent mutation or translocation leads to direct cell death. (B) In the functional model as seen in CLL, kinase inhibition disrupts the prosurvival signals from the BCR and the microenvironment, leading to de-adhesion of malignant cells from the microenvironment and subsequent cell death in the peripheral blood. Professional illustration by Marie Dauenheimer.
Two paradigms for small molecule–targeted therapies in cancer. (A) In the recurrent mutation model exemplified by CML, selective inhibition of a kinase with recurrent mutation or translocation leads to direct cell death. (B) In the functional model as seen in CLL, kinase inhibition disrupts the prosurvival signals from the BCR and the microenvironment, leading to de-adhesion of malignant cells from the microenvironment and subsequent cell death in the peripheral blood. Professional illustration by Marie Dauenheimer.
The foundation of targeted cancer therapy has been selective inhibition of tyrosine kinases that are constitutively activated by DNA mutations or translocations that are recurrent and fundamental to disease pathophysiology. The story of the tremendous success of imatinib and other ABL kinase inhibitors in chronic myeloid leukemia (CML) is the best known example among hematologic malignancies,3 and targeted therapy has also shown promise in several solid tumors such as melanoma4 and lung cancer.5 Targetable kinase mutations with direct relevance to disease pathogenesis have been identified less frequently in lymphoid neoplasms, making the extension of this paradigm to CLL and related diseases challenging.
Despite this limitation, arguably there is no cancer for which more new promising therapeutic agents are available than for CLL, with the rise of novel agents against a variety of targets such as the B-cell receptor (BCR) pathway6 and the intrinsic mitochondrial pathway of apoptosis,7 along with immune-based therapies such as chimeric antigen receptor T cells.8 None of these therapies depends on the presence of a genetically mutated target. This success in CLL unfortunately has not yet been seen in AML, for which several targeted agents are in development, but drugs such as FLT3 inhibitors have thus far shown only limited efficacy in the clinic.9 Can something be learned from the recent experience in CLL that may eventually help patients with AML and other malignancies?
A key target in CLL is BTK, which is known to be a critical mediator of BCR signaling, a fundamental prosurvival pathway for CLL cells nurtured by the stromal microenvironment of the lymph nodes and bone marrow.10 Ibrutinib, the first-in-class oral BTK inhibitor, was recently approved by the US Food and Drug Administration for mantle cell lymphoma on the basis of its high level of activity in that disease.11 In patients with relapsed CLL, the drug has an overall response rate (including patients with residual lymphocytosis) of 91%, with an estimated progression-free survival at 26 months of 75%,12 and it is likely that ibrutinib will soon be approved for CLL as well. Despite the striking activity of ibrutinib in CLL, activating mutations in its primary target BTK have not been described, raising the question of whether off-target effects of ibrutinib on other kinases such as BLK, TEC, and ITK may be more important in determining response to the drug. Until now, little evidence has been published to address this fundamental question.
In this issue, Woyach et al1 report using both CLL patient samples and the Eu-TCL-1 (TCL-1) mouse model to explore the importance of BTK to the pathophysiology of CLL. The investigators found that short interfering RNA–mediated inhibition of BTK in primary human CLL cells promotes apoptosis. When the TCL-1 mouse is crossed with the XID mouse (which has a point mutation in BTK preventing kinase activity), the survival of the XID/TCL-1 crossed mice is longer than that of wild-type TCL-1 mice. Importantly, when TCL-1 mice are treated with ibrutinib starting at 1 month of age, it takes longer for leukemia to develop and the ibrutinib-treated mice live longer than controls.
One limitation of the TCL-1 mouse model used in this paper is the lack of lymphocyte redistribution observed after ibrutinib treatment. This phenomenon is thought to be fundamental to the efficacy of ibrutinib in humans, since it results in CLL cells being mobilized out of protective stromal niches and into peripheral blood, where they eventually die.12 A further limitation of the study is that the contribution of other kinases related to the activity of ibrutinib in CLL is not definitively excluded, and this should be explored further in future work. Despite these caveats, the experiments in the article by Woyach et al1 present us with compelling evidence that BTK is an important target of ibrutinib. These results are consistent with the recent report that C481S BTK mutations in the binding site of ibrutinib confer resistance to the drug in CLL patients.13
Given that the profound activity of ibrutinib in CLL is due primarily to its ability to inhibit BTK, one could imagine that any malignancy that relies on survival signals transmitted by BTK would be sensitive to the drug. As in CLL, AML blasts do not carry mutations in BTK, yet the kinase is highly expressed and phosphorylated, raising the question of whether a BTK inhibitor like ibrutinib might also induce death signaling in AML. Rushworth et al2 now present evidence that BTK is constitutively expressed in blasts from AML patient samples and that the level of BTK phosphorylation is associated with ibrutinib sensitivity. RNA interference–mediated knockdown of BTK reduced the growth potential of both AML cell lines and primary AML blasts. Ibrutinib reduced AML blast adherence to stroma in coculture experiments and was able to induce apoptosis in these blasts even in the presence of stroma. Ibrutinib also enhanced the ability of chemotherapy to kill primary AML blasts in vitro. These findings have clear translational implications and provide a strong rationale for exploring the activity of ibrutinib in a clinical trial for patients with AML. However, many agents have shown a potent ability to kill AML cells in the test tube and in mice only to fail as therapy for patients in the clinic, and therefore our enthusiasm for these exciting results must be tempered until it is demonstrated that ibrutinib has activity in patients with AML.
These two studies should remind us of the importance of ongoing laboratory investigation even for drugs that have already demonstrated convincing clinical activity. Through the further study of ibrutinib in CLL, we now have a clearer understanding of how the drug’s inhibition of BTK contributes to its efficacy. This appreciation validates an emerging paradigm of boldly targeting kinases critical for the survival of malignant cells, even in the absence of recurrent genetic mutations in the target (see figure). The lessons learned about BTK inhibition in CLL may open doorways to new therapeutic approaches for AML, which are urgently needed. Studies such as these are needed for all of the novel agents in development, because the more we understand about the mechanisms underlying the efficacy of these new drugs, the better we will be able to use them to improve the treatment of patients with hematologic malignancies.
Conflict-of-interest disclosure: The author has participated on an advisory board for Infinity Pharmaceuticals.