In this issue of Blood, Klimenkova et al demonstrate that secretory leukocyte protease inhibitor (SLPI), the natural inhibitor of neutrophil elastase (NE), plays a nonredundant role in human neutrophil differentiation. The authors show that NE itself is both required and sufficient to induce expression of SLPI in myeloid progenitors, which subsequently regulates granulocyte colony-stimulating factor receptor (G-CSFR) signaling and thereby cell proliferation, differentiation, and survival. Patients with severe congenital neutropenia (SCN) were found to have strongly reduced SLPI levels, and this article contributes to unraveling the molecular mechanism(s) underlying the block in neutrophil formation in this disease.1
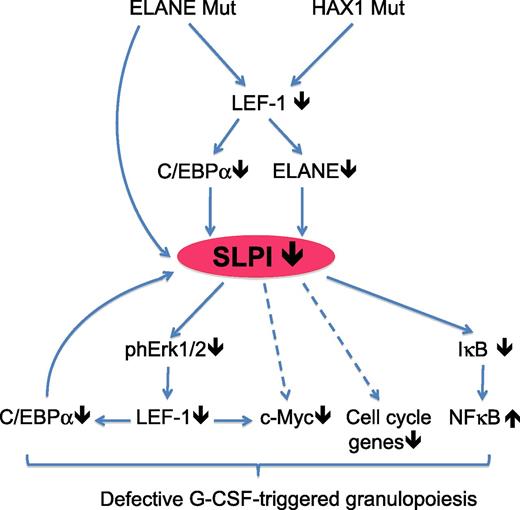
Model for the altered molecular pathways in myeloid progenitor cells of patients suffering from SCN, in which known genetic mutations lead to decreased SLPI expression and subsequently to altered cell signaling and transcription-factor expression, resulting in abrogated G-CSF–induced granulopoiesis. Arrows indicate confirmed (solid) or speculative (dashed) molecular interactions. IκB, inhibitor of NF-κB; Mut, mutated; ph, phosphorylated. See Figure 7 in the article by Klimenkova et al, which begins on page 1239.
Model for the altered molecular pathways in myeloid progenitor cells of patients suffering from SCN, in which known genetic mutations lead to decreased SLPI expression and subsequently to altered cell signaling and transcription-factor expression, resulting in abrogated G-CSF–induced granulopoiesis. Arrows indicate confirmed (solid) or speculative (dashed) molecular interactions. IκB, inhibitor of NF-κB; Mut, mutated; ph, phosphorylated. See Figure 7 in the article by Klimenkova et al, which begins on page 1239.
SCN is a primary immunodeficiency syndrome that occurs in approximately 1 in 200 000 individuals and is characterized by a block in the development of neutrophilic granulocytes. The absence of neutrophils makes these patients much more susceptible to invasive bacterial and fungal infections and increases the risk for the development of myelodysplastic syndrome and acute myelogenous leukemia. Mutations in at least 6 different genes can cause SCN, of which ELANE (or ELA2), the gene encoding NE, is the most frequently affected one.2 Heterozygous mutations in ELANE are sufficient to cause neutropenia, and it has been suggested that the mutated NE protein can accumulate in the cytoplasm and induce apoptosis through activation of the unfolded protein response.3,4 Yet NE expression in myeloid cells and plasma of SCN patients is actually downregulated, independently of the mutation status of ELANE but dependent on the expression of the myeloid transcription factors LEF-1 and c/EBPα.5 Still, the underlying mechanism by which diminished NE expression impairs neutrophil differentiation remains unclear.
NE is a serine proteinase expressed in neutrophils, monocytes, and mast cells that can intracellularly breakdown phagosome content but, upon secretion, NE can also degrade a variety of extracellular proteins, including receptors for chemokines and cytokines, whereas it can also induce the expression of several inflammatory cytokines.6 The natural inhibitor of this potent enzyme is SLPI, which is induced by NE and has strong antiprotease activity to NE, but SLPI can also modulate intracellular signal transduction pathways through ERK and nuclear factor κB (NF-κB).7,8 Therefore, the authors questioned whether reduced levels of NE in SCN could alter intracellular signaling in myeloid progenitors through SLPI and thereby affect the fate of these cells. In their article, Klimenkova et al demonstrate that SLPI expression is strongly reduced in myeloid cells and plasma from SCN patients carrying mutations in ELANE but also in patients with mutations in hematopoietic cell-specific Lyn substrate 1–associated protein X-1 (HAX1), another gene frequently mutated in SCN.1 Importantly, treatment of primary myeloid cells with purified human NE was sufficient to induce SLPI messenger RNA (mRNA) and protein expression, as was transduction with wild-type ELANE, but not with ELANE mutants found in SCN patients. Reciprocally, short hairpin RNA–mediated knockdown experiments revealed that SLPI mRNA and protein expression were dependent on ELANE/NE and HAX1. Further molecular analysis showed that SLPI mRNA expression is regulated by LEF-1–induced activation of c/EBPα and binding of the latter to the SLPI promoter (see figure).
These experiments demonstrate that SLPI expression is positively regulated by an intricate interplay between NE and key myeloid transcription factors, but they do not explain how decreased levels of SLPI can impair neutrophil formation. To address this, the authors inhibited SLPI expression in expanded bone marrow progenitors and could demonstrate that SLPI is in fact required for both G-CSF–driven proliferation and survival and for colony formation of neutrophils, but not of monocytes/macrophages or erythroid cells. These functional experiments were corroborated by the finding that knockdown of SLPI blocked G-CSF–induced phosphorylation of STAT5, ERK1/2, and LEF-1 and led to diminished levels of the LEF-1 target genes c-Myc, survivin, and cyclin D1, which are key drivers of proliferation and survival (see figure). Gene expression analysis by microarrays also showed that c-Myc–triggered signaling and cell-cycle signaling are impaired on SLPI downregulation. Interestingly, the same pathways were also downregulated in myeloid progenitors from SCN patients compared with healthy controls or patients with idiopathic neutropenia. Overall, this article demonstrates that SLPI is an important mediator of neutrophil differentiation, because it positively regulates signal transduction downstream of the G-CSFR in myeloid progenitors and thereby enhances their proliferation and survival. Since both NE and HAX1 control SLPI expression, it becomes more evident why mutations in these genes strongly contribute to the development of SCN. Although the authors did not experimentally address this, it would be of interest to determine whether the block in neutrophil differentiation in SCN patients can be overcome by exogenous treatment with NE or overexpression of SLPI.
The elegant work by Klimenkova et al1 adds another dimension to the anti-inflammatory function that SLPI fulfils in the immune system. In macrophages, SLPI is induced by lipopolysaccharide (LPS) and can suppress LPS-induced activation of NF-κB and thereby the production of nitric oxide and tumor necrosis factor α.7 SLPI is also expressed in dendritic cells, particularly in mucosa-draining lymph nodes, in which it regulates tolerance of T cells to mucosally administered antigens.9 The novel finding that SLPI can also regulate neutrophil differentiation in the bone marrow raises the question of whether this mode of regulation is also important during inflammation. Bone marrow output changes upon infection, and we have shown that interferon γ (IFN-γ) produced on viral infection enhances monocyte formation but strongly inhibits neutrophil formation through the induction of suppressor of cytokine signaling 3 in myeloid progenitors, thereby inhibiting G-CSF–induced neutrophil development.10 Yet since IFN-γ can suppress SLPI expression,7 it would be of interest to determine whether suppression of the newly identified SLPI/G-CSFR/LEF-1/c-Myc pathway1 (see figure) also plays a role in the inflammatory feedback to the bone marrow on viral infection. Conversely, given the important protective function of neutrophils in the defense against bacterial or fungal pathogens, it can be envisaged that this pathway is instead turned on during these infections, but this awaits further investigation.
In conclusion, SLPI is a versatile molecule, not only inside and outside the cell, but also in both developing and fully mature myeloid cells. The discovery that SLPI is an essential regulator in neutrophil differentiation that is downregulated in SCN will open new avenues for further research into this disease, hopefully leading to novel treatment options.
Conflict-of-interest disclosure: The author declares no competing financial interests.