Key Points
The MUC1-C oncoprotein is aberrantly expressed in AML cells and contributes to activation of the mutant FLT3 receptor.
Targeting MUC1-C thus inhibits FLT3 signaling and represents a potential approach for AML cells resistant to FLT3 inhibitors.
Abstract
Blasts from approximately one-third of patients with acute myeloid leukemia (AML) harbor activating mutations in the FMS-like tyrosine kinase 3 (FLT3) receptor tyrosine kinase that confer a poor prognosis. The Mucin 1-C-terminal subunit (MUC1-C) oncoprotein is aberrantly expressed in AML blasts and stem cells; however, there is no known interaction between MUC1-C and FLT3. The present studies demonstrate that MUC1-C associates with wild-type and mutant FLT3 in AML cells. Targeting MUC1-C with the cell-penetrating peptide inhibitor GO-203 disrupts MUC1-C/FLT3 complexes and downregulates FLT3 activation. GO-203 treatment of AML cells was also associated with inhibition of the FLT3 downstream effectors AKT, extracellular signal-regulated kinase, and STAT5. The results further show that AML cells with FLT3-activating mutations and resistant to the FLT3 inhibitor midostaurin/PKC412 are sensitive to GO-203–induced growth arrest and death. Moreover, GO-203 increases sensitivity of mutant FLT3 AML cells to FLT3 inhibitor treatment. These results indicate that MUC1-C contributes to FLT3 activation in AML cells and that targeting MUC1-C inhibits the FLT3 signaling pathway. Our findings support the development of MUC1-C inhibitors alone and in combination with agents that target FLT3 for the treatment of wild-type and mutant FLT3 AML.
Introduction
The FMS-like tyrosine kinase 3 (FLT3) receptor is a member of the class III subfamily that includes the FMS, KIT, and PDGF receptors. FLT3 is expressed by hematopoietic stem/progenitor cells and functions in the regulation of their proliferation and differentiation.1 The FLT3 receptor is also expressed in more than 90% of acute myeloid leukemia (AML) blasts.2 FLT3 is activated by FLT3 ligand, a transmembrane protein that is widely expressed by cells in the bone marrow, spleen, and epithelial tissues.1,3 Activation of FLT3 by its ligand is associated with autophosphorylation of tyrosine residues in the FLT3 cytoplasmic domain and thereby the generation of docking sites for mitogenic downstream effectors. Specifically, the phosphoinositide 3-kinase (PI3K) p85 subunit interacts with the autophosphorylated FLT3 cytoplasmic domain and, in turn, confers activation of AKT.4,5 FLT3 also interacts with RAS and thereby activates the RAS→RAF→mitogen-activated protein kinase (MEK)→extracellular signal-regulated kinase (ERK) pathway.4,5 Importantly, somatic mutations in the FLT3 gene have been identified in about 30% of patients with AML.1 Among these mutations, the most common type is the internal tandem duplication (ITD).6 The FLT3-ITD mutation results in loss of the FLT3 autoinhibitory function and constitutive activation of the kinase.1 In this way, the FLT3-ITD receptor confers activation of the PI3K→AKT and RAS→RAF→MEK→ERK pathways.7 Of importance clinically, patients with AML blasts harboring FLT3-ITD mutations have an increased risk of relapse and decreased survival.8 Thus, FLT3-ITD has emerged as an attractive target for drug development. Accordingly, the FLT3 inhibitor, PKC412 (midostaurin),9 has been used to treat patients with FLT3 mutant AML with responses that have been typically partial and transient.10,11 Moreover, treatment of patients with FLT3-ITD AML with the FLT3 inhibitor AC220 demonstrated a composite complete response rate of approximately 50%12,13 and that relapses were mediated by reactivation of FLT3 kinase activity.14
Mucin 1 (MUC1) is a heterodimeric protein that is normally expressed at the apical borders of epithelial cells.15,16 Intriguingly, MUC1 is aberrantly expressed in AML blasts17,18 and in AML stem cells19 ; however, the functional role of MUC1 in AML is unknown. Of importance to understanding its function, MUC1 consists of 2 subunits that form a stable complex at the cell surface.15,16 The extracellular N terminal subunit (MUC1-N) contains a glycosylated tandem repeat structure that is characteristic of the mucin family.15,16 The transmembrane C terminal subunit (MUC1-C) contains a 58-amino acid (aa) domain that extends outside the cell, a 28-aa transmembrane region, and a 72-aa cytoplasmic domain.15,16 In epithelial cells, the MUC1-C subunit associates with receptor tyrosine kinases (RTKs), such as epidermal growth factor receptor (EGFR) and ErbB2-4, at the cell membrane and contributes to their downstream signaling.15,16 Phosphorylation of the MUC1-C cytoplasmic domain on tyrosines by RTKs and SRC results in binding sites for PI3K and GRB2/SOS, linking MUC1-C to the AKT and RAS pathways, respectively.15,16 MUC1-C has also been linked to activation of signal transducer and activator of transcription 1/3 (STAT1/3) signaling.20,21 In this capacity to interact with mitogenic pathways, expression of MUC1-C is sufficient to induce anchorage-independent growth and tumorigenicity.22,23 The oncogenic function of the MUC1-C subunit is dependent on the formation of homodimers through a CQC motif in the MUC1-C cytoplasmic domain.15,24 Based on these observations, cell-penetrating peptides have been developed that bind to the MUC1-C CQC motif and block MUC1-C homodimerization and function.25,26 Significantly, treatment of AML cell lines and blasts with MUC1-C inhibitors is associated with growth inhibition and death induction.19,27 These findings provided support for the dependence of AML cells on MUC1-C for their survival.
The present studies demonstrate that MUC1-C associates with FLT3 in AML cells. The results show that targeting MUC1-C (1) disrupts MUC1-C/FLT3 complexes, (2) downregulates FLT3 activation, and (3) suppresses downstream AKT, ERK, and STAT5 signaling. Targeting MUC1-C is also effective in inhibiting growth and inducing death of AML cells resistant to FLT3 inhibitors.
Methods
Cell culture and reagents
Human MOLM-14, MOLM-13, MV4-11, HL-60, mouse BaF3/FLT3 (wild-type [WT]), BaF3/FLT3-ITD, and BaF3/FLT3-D835Y cell lines were maintained in RPMI1640 medium containing 10% heat-inactivated fetal bovine serum, 100 units/mL penicillin, 100 μg/mL streptomycin, and 2 mM L-glutamine. BaF3 cells were grown in the presence of 2 ng/mL interleukin-3 (R&D Systems). MOLM-13 cells were selected for resistance to FLT3 inhibitors, midostaurin/PKC412, and HG-7-85-01 as described.28 Cells were treated with the cell-penetrating GO-203 or control CP2 peptide (Genus Oncology),27 PKC412 (Novartis), HG-7-85-01,29 and AC220 (Selleck). FLT3-ligand was purchased from PeproTech.
Stable silencing of MUC1 expression
MOLM-14 cells were infected with lentiviruses expressing a MUC1 short hairpin RNA (shRNA; Sigma catalog #NM_002456.4-474s1c1) or a control scrambled shRNA (CshRNA; Sigma-Aldrich catalog #SH016) in viral medium containing 8 μg/mL polybrene for 2 hours at 37°C. The cells were pelleted, resuspended in complete RPMI1640 medium, and incubated for 48 hours at 37°C. Selection in the presence of puromycin was then initiated at a concentration of 0.5 μg/mL that was progressively increased to 2 μg/mL with generation of stable cell lines.
Immunoprecipitation, GST-protein precipitation, and immunoblot analysis
Cells were harvested and rinsed with ice-cold phosphate buffered saline (PBS). Ice-cold lysis buffer (0.5 mL; 20 mM Tris [pH 7.5], 150 mM NaCl, 1 mM EDTA, 1% Triton X-100, 2.5 mM sodium pyrophosphate, 1 mM β-glycerophosphate, 1 mM Na3VO4, 1 mg/mL leupeptin, 1 mM phenylmethylsulfonyl fluoride) was added to 1 × 107 cells and sonicated on ice 4 times for 5 seconds each followed by microcentrifugation for 10 minutes at 4°C. Soluble proteins were immunoprecipitated with anti-MUC1-C (Ab5; Neomarkers). The precipitates and lysates not subjected to immunoprecipitation were immunoblotted with anti-FLT3 (Santa Cruz Biotechnology), anti-MUC1-C,30 anti-β-actin (Cell Signaling Technology), anti-p-FLT3, anti-p-AKT S473, anti-AKT, anti-p-ERK, anti-ERK (Cell Signaling Technology), anti-P-Tyr (4G10; Millipore), and anti-p-STAT5 (Cell Signaling Technology) antibodies. Antigen–antibody complexes were visualized by enhanced chemiluminescence (GE Healthcare). Glutathione S-transferase (GST) and GST-MUC1-cytoplasmic domain fusion proteins were prepared as described.31 Total cell lysates were incubated overnight with GST-MUC1-cytoplasmic domain at 4°C. Adsorbates to glutathione-conjugated beads were then analyzed by immunoblotting with anti-FLT3.
Cell viability and death assays
Cell viability was determined by trypan blue exclusion. For analysis of cell death by apoptosis and necrosis, cells were stained with propidium iodide (PI) alone or PI/Annexin V and then analyzed by flow cytometry as described.27
Colony formation assays
CD34+ cells obtained from bone marrow aspirates from AML patients or from mobilized peripheral blood mononuclear cells were isolated by magnetic bead separation as described.19 The CD34+ cells (100 000/plate) were seeded in methylcellulose (MethoCult; StemCell Technologies) in the presence of 5 μM GO-203 or CP2 for 14 days. The number of colonies was scored by visualization under an inverted microscope.
Isolation of primary AML cells
Cells were obtained before therapy from 6 AML patients under an approved protocol (Dana-Farber Cancer Institute 01-206). Patients 1 and 2 had FLT3-WT, patients 3-5 had FLT3-ITD, and patient #6 had FLT3-WT AML. Approval was obtained from the Dana-Farber Cancer Institute institutional review board for these studies. Informed consent was provided according to the Declaration of Helsinki.
Determination of p-FLT3 and p-ERK levels in primary cells
Primary AML cells obtained from patients were washed in PBS/0.1% bovine serum albumin/2 mM EDTA and then incubated with anti-human PE-Cy7 CD13 and CD33 antibodies (used at 1/50 dilution) for 45 minutes. For total FLT3 staining, cells were incubated with an anti-human antigen-presenting cell (APC)-conjugated FLT3 (used at 1/10 dilution) or APC isotype control antibody. After washing, cells were fixed for 20 minutes with BD cytofix/cytoPerm fixation and permeabilization solution (BD Biosciences), and then incubated for 45 minutes with the combination of anti-human APC-conjugated p-ERK1/2 (T202/Y204, used at 1/50 dilution, Cell Signaling Technology), nonconjugated p-FLT3(Y591) (used at 1/50 dilution), or the corresponding combination of isotype control antibodies. Finally, nonconjugated p-FLT3 (Y591) condition was incubated for 35 minutes with an Alexa Fluor 488 goat anti-rabbit immunoglobulin G antibody used at 1/500 dilution (Invitrogen). Samples were analyzed using a BD FACSCanto II analyzer.
AML xenograft model
Four- to 6-week-old BALB/c ν/ν mice (Charles River Laboratories) were injected subcutaneously in the flank with 107 MOLM-14 cells. When tumors were ∼100 mm3, the mice were pair-matched into control and treated groups of 7 mice each, excluding those with tumors not within 15% of the mean volume. Initial dosing was given at the time of pair matching (day 1). PBS (control vehicle) and 7.5 mg/kg GO-203 was administered by intravenous tail vein injection on days 1-5 and 8-12. Mice were weighed twice weekly. Tumor volume (V) was calculated using the formula V = (L × W2)/2, wherein L and W are the larger and smaller diameters, respectively. Survival was also determined by Kaplan-Meier analysis.
Results
MUC1-C associates with FLT3
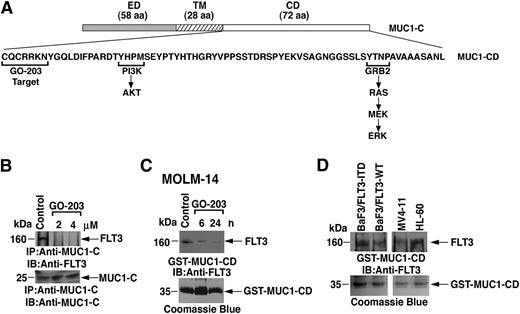
The MUC1-C subunit consists of a 58-aa extracellular domain, a 28-aa transmembrane domain, and 72-aa cytoplasmic domain that interacts with PI3K and GRB2, linking MUC1-C to the AKT and MEK→ERK pathways, respectively (Figure 1A). The MUC1-C cytoplasmic domain also contains a CQC motif that is necessary for its homodimerization and interaction with certain other proteins (Figure 1A).24 GO-203, which contains the D-amino acid sequence CQCRRKN linked at the N terminus to 9 Arg residues ([R]9-CQCRRKN) is a cell-penetrating peptide that binds to the CQC motif and thereby blocks MUC1-C dimerization (Figure 1A; supplemental Figure 1).26,27 To assess the potential interaction between the MUC1-C subunit and FLT3, we performed coimmunoprecipitation studies using MOLM-14 cell lysates. MOLM-14 cells have 1 WT FLT3 allele and 1 FLT3-ITD allele and express MUC1-C.27 Analysis of anti-MUC1-C precipitates demonstrated that MUC1-C associates with FLT3 (Figure 1B). Treatment of MOLM-14 cells with 2 and 4 μM GO-203 for 6 hours was associated with disruption of the MUC1-C/FLT3 complexes (Figure 1B). To determine whether MUC1-C/cytoplasmic domain contributes to the interaction with FLT3, lysates from untreated MOLM-14 cells were incubated with a GST fusion protein containing MUC1-C/cytoplasmic domain (GST-MUC1-C/CD). In pull-down experiments, GST-MUC1-C/cytoplasmic domain formed a complex with FLT3 (Figure 1C). By contrast, treatment of the MOLM-14 cells with GO-203 for 6 and 24 hours attenuated the association between MUC1-C/cytoplasmic domain and FLT3 (Figure 1C). These findings indicate that MUC1-C forms a complex with FLT3 and that this association is mediated, at least in part, by the MUC1-C cytoplasmic domain. As noted previously, MOLM-14 cells express both FLT3-WT and FLT3-ITD. To determine whether MUC1-C interacts with WT and/or mutant FLT3, lysates from BaF3/FLT3-WT, BaF3/FLT3-ITD, MV4-11 (homozygous for FLT3-ITD), and HL-60 (FLT3-WT) cells were incubated with GST-MUC1-C/cytoplasmic domain. Analysis of the adsorbates demonstrated that MUC1-C/cytoplasmic domain interacts with FLT3 from each of these different cell types (Figure 1D), indicating that MUC1-C binds to both FLT3-WT and FLT3-ITD.
Targeting the MUC1-C cytoplasmic domain with GO-203 blocks the interaction with FLT3. (A) Schema of the MUC1-C subunit extracellular domain (ED), transmembrane domain (TM), and the cytoplasmic domain (CD). The amino acid sequence of the MUC1-C cytoplasmic domain (MUC1-CD) is shown with highlighting of the GO-203 target sequence (CQCRRKN) and localization of the PI3K and GRB2 binding sites. (B) Lysates from MOLM-14 cells left untreated (control) or treated with 2 or 4 μM GO-203 for 6 hours were immunoprecipitated with anti-MUC1-C. The precipitates were immunoblotted with the anti-FLT3 antibody (upper panel). As a control, anti-MUC1-C immunoprecipitates were also analyzed by immunoblotting with anti-MUC1-C (lower panel). (C) Lysates from MOLM-14 cells left untreated (control) or treated with 2 μM GO-203 for 6 and 24 hours were incubated with a GST-MUC1-CD fusion protein. The adsorbates were immunoblotted with anti-FLT3 (upper panel). Input of the GST-MUC1-CD proteins was assessed by Coomassie blue staining (lower panel). (D) Lysates from the indicated cells were incubated with the GST-MUC1-CD fusion protein. The adsorbates were immunoblotted with anti-FLT3 (upper panel). Input of the GST-MUC1-CD proteins was assessed by Coomassie blue staining (lower panel).
Targeting the MUC1-C cytoplasmic domain with GO-203 blocks the interaction with FLT3. (A) Schema of the MUC1-C subunit extracellular domain (ED), transmembrane domain (TM), and the cytoplasmic domain (CD). The amino acid sequence of the MUC1-C cytoplasmic domain (MUC1-CD) is shown with highlighting of the GO-203 target sequence (CQCRRKN) and localization of the PI3K and GRB2 binding sites. (B) Lysates from MOLM-14 cells left untreated (control) or treated with 2 or 4 μM GO-203 for 6 hours were immunoprecipitated with anti-MUC1-C. The precipitates were immunoblotted with the anti-FLT3 antibody (upper panel). As a control, anti-MUC1-C immunoprecipitates were also analyzed by immunoblotting with anti-MUC1-C (lower panel). (C) Lysates from MOLM-14 cells left untreated (control) or treated with 2 μM GO-203 for 6 and 24 hours were incubated with a GST-MUC1-CD fusion protein. The adsorbates were immunoblotted with anti-FLT3 (upper panel). Input of the GST-MUC1-CD proteins was assessed by Coomassie blue staining (lower panel). (D) Lysates from the indicated cells were incubated with the GST-MUC1-CD fusion protein. The adsorbates were immunoblotted with anti-FLT3 (upper panel). Input of the GST-MUC1-CD proteins was assessed by Coomassie blue staining (lower panel).
Targeting MUC1-C with GO-203 downregulates FLT3 phosphorylation
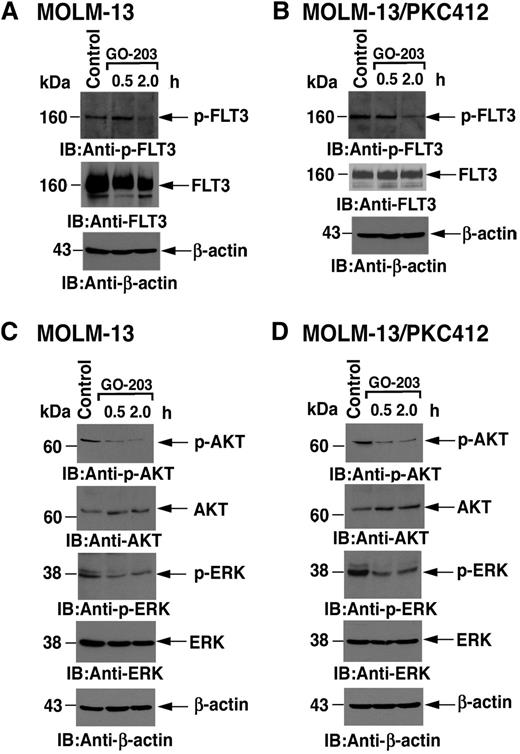
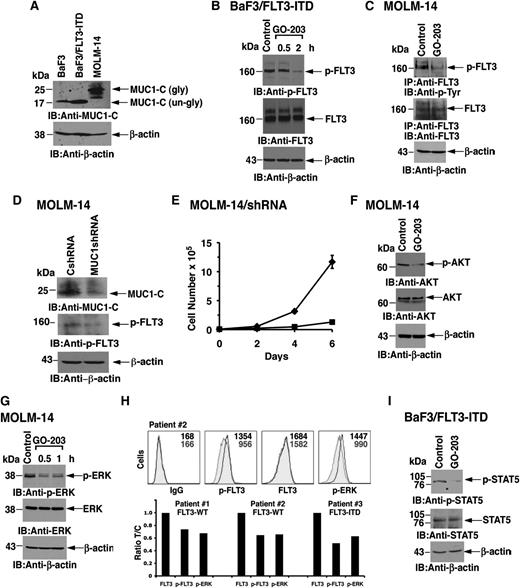
Mouse BaF3 cells are transformed by FLT3-ITD.32 In contrast to MOLM-14 cells, which express the glycosylated 20- to 25-kDa form of MUC1-C, BaF3, and BaF3/FLT3-ITD cells express MUC1-C as the unglycosylated 17-kDa protein (Figure 2A). As shown previously,33 FLT3-ITD expressed in BaF3 cells is constitutively phosphorylated on tyrosine (Figure 2B). Significantly, treatment with GO-203 was associated with decreases in FLT3-ITD phosphorylation, indicating that MUC1-C contributes to FLT3 activation (Figure 2B). To further assess the effects of MUC1-C on FLT3 activation, MOLM-14 cells were treated with GO-203 to disrupt the MUC1-C/FLT3 complex. Analysis of lysates from control and GO-203–treated MOLM-14 cells for p-FLT3 demonstrated that GO-203 treatment is associated with downregulation of constitutive FLT3 activation (Figure 2C). To extend this line of investigation, we stably silenced MUC1-C in MOLM-14 cells. Importantly, MUC1-C silencing was associated with downregulation of p-FLT3 levels, consistent with a decrease in FLT3 activation (Figure 2D) and inhibition of growth (Figure 2E).
Treatment with GO-203 inhibits FLT3 activation and signaling. (A) Lysates from BaF3 cells grown in the presence of interleukin-3, BaF3/FLT3-ITD cells, and MOLM-14 cells were immunoblotted with the indicated antibodies. BaF3 and BaF3/FLT3-ITD express MUC1-C as the 17-kDa unglycosylated (un-gly) form and MOLM-14 cells express the 20- to 25-kDa glycosylated (gly) form. (B) BaF3/FLT3-ITD cells were left untreated (control) or treated with 5 μM GO-203 for 0.5 and 2 hours. Lysates were subjected to immunoblotting with anti-p-FLT3 (upper panel), anti-FLT3 (middle panel), and anti-β-actin (lower panel). (C) MOLM-14 cells were left untreated (control) or treated with 5 μM GO-203 for 30 minutes. Total cell lysates were then subjected to immunoprecipitation with anti-FLT3 antibody. The immunoprecipitates were then analyzed by immunoblotting with anti-P-Tyr (upper panel) or anti-FLT3 (middle panel). As control, total cell lysates were also analyzed by immunoblotting with anti-β-actin (lower panel). (D) Total cell lysates from MOLM-14 cells stably expressing a CshRNA or a MUC1shRNA were analyzed by immunoblotting with the indicated antibodies. (E) MOLM-14/CshRNA (diamonds) and MOLM-14/MUC1shRNA (squares) were seeded at 0.5 × 105 cells/mL. Cell number (mean ± SD of 3 replicates) was determined on the indicated days. (F) MOLM-14 cells were left untreated (control) or treated with 5 μM GO-203 for 60 minutes. Lysates were subjected to immunoblotting with anti-p-AKT (upper panel), anti-AKT (middle panel), and anti-β-actin (lower panel). (G) MOLM-14 cells were left untreated (control) or treated with 5 μM GO-203 for 30 and 60 minutes. Lysates were subjected to immunoblotting with anti-p-ERK antibody (upper panel), anti-ERK antibody (middle panel), and anti-β-actin antibody (lower panel). (H) Primary AML cells were treated with 4 μM GO-203 for 4 hours and then incubated with a combination of anti-human PE-Cy7 CD13 and CD33. For staining, cells were incubated with an anti-human APC-conjugated FLT3 (total FLT3) or APC isotype control antibody. After washing and fixing, the cells were incubated with the combination of anti-human APC-conjugated p-ERK1/2, nonconjugated p-FLT3 (Y591), or the corresponding combination of isotype control antibodies. Following incubation with an Alexa Fluor 488 goat anti-rabbit antibody, samples were analyzed using a FACSCanto II analyzer. A representative flow profile of AML cells from patient 2 is shown in the upper panels. The results are also expressed as the ratio (treated/control; T/C) of fluorescence found for blasts from patients 1-3 (lower panels). (I) BaF3/FLT3-ITD cells were left untreated (control) or treated with 2 μM GO-203 for 2 hours. Total cell lysates were subjected to immunoblotting with anti-p-STAT5 (upper panel), anti-STAT5 (middle panel), and anti-β-actin (lower panel).
Treatment with GO-203 inhibits FLT3 activation and signaling. (A) Lysates from BaF3 cells grown in the presence of interleukin-3, BaF3/FLT3-ITD cells, and MOLM-14 cells were immunoblotted with the indicated antibodies. BaF3 and BaF3/FLT3-ITD express MUC1-C as the 17-kDa unglycosylated (un-gly) form and MOLM-14 cells express the 20- to 25-kDa glycosylated (gly) form. (B) BaF3/FLT3-ITD cells were left untreated (control) or treated with 5 μM GO-203 for 0.5 and 2 hours. Lysates were subjected to immunoblotting with anti-p-FLT3 (upper panel), anti-FLT3 (middle panel), and anti-β-actin (lower panel). (C) MOLM-14 cells were left untreated (control) or treated with 5 μM GO-203 for 30 minutes. Total cell lysates were then subjected to immunoprecipitation with anti-FLT3 antibody. The immunoprecipitates were then analyzed by immunoblotting with anti-P-Tyr (upper panel) or anti-FLT3 (middle panel). As control, total cell lysates were also analyzed by immunoblotting with anti-β-actin (lower panel). (D) Total cell lysates from MOLM-14 cells stably expressing a CshRNA or a MUC1shRNA were analyzed by immunoblotting with the indicated antibodies. (E) MOLM-14/CshRNA (diamonds) and MOLM-14/MUC1shRNA (squares) were seeded at 0.5 × 105 cells/mL. Cell number (mean ± SD of 3 replicates) was determined on the indicated days. (F) MOLM-14 cells were left untreated (control) or treated with 5 μM GO-203 for 60 minutes. Lysates were subjected to immunoblotting with anti-p-AKT (upper panel), anti-AKT (middle panel), and anti-β-actin (lower panel). (G) MOLM-14 cells were left untreated (control) or treated with 5 μM GO-203 for 30 and 60 minutes. Lysates were subjected to immunoblotting with anti-p-ERK antibody (upper panel), anti-ERK antibody (middle panel), and anti-β-actin antibody (lower panel). (H) Primary AML cells were treated with 4 μM GO-203 for 4 hours and then incubated with a combination of anti-human PE-Cy7 CD13 and CD33. For staining, cells were incubated with an anti-human APC-conjugated FLT3 (total FLT3) or APC isotype control antibody. After washing and fixing, the cells were incubated with the combination of anti-human APC-conjugated p-ERK1/2, nonconjugated p-FLT3 (Y591), or the corresponding combination of isotype control antibodies. Following incubation with an Alexa Fluor 488 goat anti-rabbit antibody, samples were analyzed using a FACSCanto II analyzer. A representative flow profile of AML cells from patient 2 is shown in the upper panels. The results are also expressed as the ratio (treated/control; T/C) of fluorescence found for blasts from patients 1-3 (lower panels). (I) BaF3/FLT3-ITD cells were left untreated (control) or treated with 2 μM GO-203 for 2 hours. Total cell lysates were subjected to immunoblotting with anti-p-STAT5 (upper panel), anti-STAT5 (middle panel), and anti-β-actin (lower panel).
FLT3 activates the PI3K→AKT and MEK→ERK pathways.34 In concert with the demonstration that inhibiting MUC1-C downregulates FLT3 activation, treatment of MOLM-14 cells with GO-203 was also associated with decreases in p-AKT (Figure 2F). Moreover, we found that inhibiting MUC1-C results in attenuation of ERK activation (Figure 2G). To further determine whether p-FLT3 and its downstream target ERK are suppressed by GO-203 in primary AML cells, blasts from 3 patients with AML were grown in short-term culture and then treated with GO-203 for 4 hours. As determined by flow cytometry, activation of p-FLT3 and p-ERK in these blasts was suppressed by GO-203 treatment (Figure 2H). Other studies have shown that FLT3 receptor mutants confer activation of STAT5 in AML cells.35-37 Consistent with those observations, we found that treatment of BaF3/FLT3-ITD cells with GO-203 is associated with downregulation of p-STAT5 levels (Figure 2I). These results indicate that targeting MUC1-C results in suppression of FLT3 activation and multiple downstream effectors of the FLT3 pathway.
GO-203 inhibits survival of FLT3-ITD cell lines growing in vitro and as tumor xenografts
In concert with expression of MUC1-C in BaF3 cells, GO-203 was effective in inhibiting their growth in the presence of interleukin-3 (Figure 3A). The GO-203–induced downregulation of FLT3-ITD signaling in BaF3/FLT3-ITD cells was also associated with inhibition of growth (Figure 3B) and induction of cell death (Figure 3C). These findings demonstrate that GO-203–mediated inhibition of BaF3 cell growth is not dependent on FLT3-ITD. Similar effects growth inhibitory effects were observed in the response of MOLM-14 cells to GO-203 treatment (Figure 3D). To assess activity of GO-203 in vivo, MOLM-14 tumor xenografts were established in the flanks of nude mice. Mice bearing tumors of ∼100 mm3 were treated with GO-203 at a dose of 7.5 mg/kg per day administered IV for 5 days on/2 days off × 2 weeks. As compared with the vehicle (PBS) control, GO-203 treatment substantially slowed growth of the MOLM-14 tumors (Figure 3E). In addition, GO-203 had no effect on body weight (supplemental Figure 2). Administration of GO-203 was also associated with a significant increase in survival as determined by Kaplan-Meier analysis (Figure 3F).
GO-203 inhibits cell growth and induces death of BaF3, BaF3/FLT3-ITD, and MOLM-14 cells. BaF3 cells grown in the presence of interleukin-3 (IL-3) were treated with GO-203 at the indicated concentrations for 72 hours (A). BaF3/FLT3-ITD cells were left untreated (open bars) or treated with 2 μM GO-203 (solid bars) for the indicated days (B). Cell number (mean ± standard deviation [SD] of 3 determinations) was determined by trypan blue staining. (C) BaF3/FLT3-ITD cells were treated with the indicated concentrations of GO-203 for 3 days. Percentage cell death (mean ± SD of 3 determinations) was determined by PI staining and flow cytometry. (D) MOLM-14 cells were treated with the indicated concentrations of GO-203 for 3 days. Percentage cell death (mean ± SD of 3 determinations) was determined by PI staining and flow cytometry. (E,F) BALB/c ν/ν mice were injected subcutaneously in the flank with 107 MOLM-14 cells. The mice were pair-matched when the tumors were ∼100 mm3. Treatment groups consisted of 7 mice injected intravenously with PBS (vehicle control; open squares) or 7.5 mg/kg GO-203 (closed squares) each day on days 1-5 and 8-12. Tumor measurements were performed on the indicated days. The results are expressed as tumor volumes (mean ± SE) (D). Percentage survival as determined by Kaplan-Meier analysis (E).
GO-203 inhibits cell growth and induces death of BaF3, BaF3/FLT3-ITD, and MOLM-14 cells. BaF3 cells grown in the presence of interleukin-3 (IL-3) were treated with GO-203 at the indicated concentrations for 72 hours (A). BaF3/FLT3-ITD cells were left untreated (open bars) or treated with 2 μM GO-203 (solid bars) for the indicated days (B). Cell number (mean ± standard deviation [SD] of 3 determinations) was determined by trypan blue staining. (C) BaF3/FLT3-ITD cells were treated with the indicated concentrations of GO-203 for 3 days. Percentage cell death (mean ± SD of 3 determinations) was determined by PI staining and flow cytometry. (D) MOLM-14 cells were treated with the indicated concentrations of GO-203 for 3 days. Percentage cell death (mean ± SD of 3 determinations) was determined by PI staining and flow cytometry. (E,F) BALB/c ν/ν mice were injected subcutaneously in the flank with 107 MOLM-14 cells. The mice were pair-matched when the tumors were ∼100 mm3. Treatment groups consisted of 7 mice injected intravenously with PBS (vehicle control; open squares) or 7.5 mg/kg GO-203 (closed squares) each day on days 1-5 and 8-12. Tumor measurements were performed on the indicated days. The results are expressed as tumor volumes (mean ± SE) (D). Percentage survival as determined by Kaplan-Meier analysis (E).
Targeting MUC1-C suppresses growth of primary AML cells
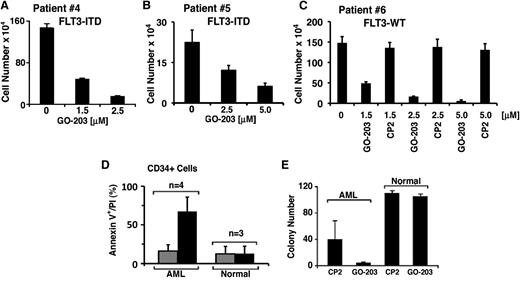
Growth of primary blasts from 2 patients with FLT3-ITD AML was inhibited by GO-203 in a concentration-dependent manner (Figure 4A-B). Similar results were obtained with primary blasts from a patient with FLT3-WT AML (Figure 4C). By contrast, an inactive CP2 peptide in which the critical Cys residues in GO-203 were mutated to Ala ([R]9-CQCRRKN→[R]9-AQARRKN)25,27 had no detectable effect on growth (Figure 4C). These results indicate that sensitivity to GO-203–induced killing is similar in the absence and presence of FLT3-ITD, indicating that this response is independent of the ITD mutation. MUC1 is expressed in most if not all primary AML cells and cell lines.19 Indeed, recent work has shown that AML stem/progenitor cells from 26 patients all express MUC1.19 By contrast, normal hematopoietic stem cells express low to undetectable MUC1 levels.19 Treatment of AML CD34+ cells with GO-203, but not CP2, was associated with the induction of late apoptosis/necrosis, whereas targeting MUC1-C had little effect on death of normal peripheral blood CD34+ stem cells (Figure 4D). In addition, the capacity of AML, but not normal, CD34+ cells to form colonies in methylcellulose was inhibited by GO-203 (Figure 4E), indicating that AML cells are selectively sensitive to targeting MUC1-C.
Targeting MUC1-C selectively inhibits growth and survival of primary AML cells. (A) Primary FLT3-ITD AML blasts from patient 4 were treated with the indicated concentrations of GO-203 for 88 hours. Cell number (mean ± SD of 3 determinations) was determined by trypan blue staining. (B) Primary FLT3-ITD blasts from patient 5 were treated with the indicated concentrations of GO-203 for 72 hours. Cell number (mean ± SD of 3 determinations) was determined by trypan blue staining. (C) Primary FLT3-WT blasts from patient 6 were treated with the indicated concentrations of GO-203 or with the control peptide, CP2, for 72 hours. Cell number (mean ± SD of 3 determinations) was determined by trypan blue staining. (D) CD34+ cells isolated from AML blasts (n = 4) or mobilized normal peripheral blood mononuclear cells (n = 3) were treated with 5 μM GO-203 (solid bars) or CP2 (shaded bars) each day for 3 days. The cells were then analyzed for Annexin V/PI-positive cells by flow cytometry. The results (mean ± SD for 3 independent samples) are expressed as percentage of late apoptotic/necrotic cells. (E) CD34+ cells isolated from AML blasts (n = 3) or mobilized normal peripheral blood mononuclear cells (n = 2) were plated in duplicate in methylcellulose in the presence of 5 μM GO-203 or CP2 for 14 days. Colony number (mean ± SD) is expressed for the indicated samples.
Targeting MUC1-C selectively inhibits growth and survival of primary AML cells. (A) Primary FLT3-ITD AML blasts from patient 4 were treated with the indicated concentrations of GO-203 for 88 hours. Cell number (mean ± SD of 3 determinations) was determined by trypan blue staining. (B) Primary FLT3-ITD blasts from patient 5 were treated with the indicated concentrations of GO-203 for 72 hours. Cell number (mean ± SD of 3 determinations) was determined by trypan blue staining. (C) Primary FLT3-WT blasts from patient 6 were treated with the indicated concentrations of GO-203 or with the control peptide, CP2, for 72 hours. Cell number (mean ± SD of 3 determinations) was determined by trypan blue staining. (D) CD34+ cells isolated from AML blasts (n = 4) or mobilized normal peripheral blood mononuclear cells (n = 3) were treated with 5 μM GO-203 (solid bars) or CP2 (shaded bars) each day for 3 days. The cells were then analyzed for Annexin V/PI-positive cells by flow cytometry. The results (mean ± SD for 3 independent samples) are expressed as percentage of late apoptotic/necrotic cells. (E) CD34+ cells isolated from AML blasts (n = 3) or mobilized normal peripheral blood mononuclear cells (n = 2) were plated in duplicate in methylcellulose in the presence of 5 μM GO-203 or CP2 for 14 days. Colony number (mean ± SD) is expressed for the indicated samples.
GO-203 is effective against AML cells resistant to FLT3 inhibition
As with MOLM-14 cells, MOLM-13 AML cells have 1 WT FLT3 and 1 FLT3-ITD allele and also express MUC1-C. To assess the effects of GO-203 in a setting of resistance to FLT3 inhibitors, we studied MOLM-13 cells that were selected for growth and survival in the presence of midostaurin (MOLM-13/PKC412).28 Treatment of the PKC412-sensitive MOLM-13 cells to GO-203 was associated with downregulation of both p-FLT3 and FLT3 levels (Figure 5A). Decreases in p-FLT3 abundance in response to GO-203 were also observed in PKC412-resistant MOLM-13/PKC412 cells, indicating a lack of cross-resistance between PKC412 and MUC1-C inhibition (Figure 5B). In concert with the downregulation of p-FLT3, GO-203 treatment of MOLM-13 cells was also associated with inhibition of AKT and ERK activation (Figure 5C). Moreover, the resistant MOLM-13/PKC412 cells responded to MUC1-C inhibition with decreases in p-AKT and p-ERK levels (Figure 5D). These results demonstrate that MOLM-13 cells resistant to FLT3 inhibition are sensitive to targeting of MUC1-C and thereby downregulation of FLT3 signaling.
GO-203 suppresses FLT3, AKT, and ERK activation in MOLM-13 cells sensitive and resistant to midostaurin/PKC412. MOLM-13 (A) and MOLM-13/PKC412 (B) cells were left untreated (control) or treated with 4 μM GO-203 for 0.5 and 2 hours. Total cell lysates were analyzed by immunoblotting with the indicated antibodies.MOLM-13 (C) and MOLM-13/PKC412 (D) cells were left untreated (control) or treated with 4 μM GO-203 for 0.5 and 2 hours. Total cell lysates were analyzed by immunoblotting with the indicated antibodies.
GO-203 suppresses FLT3, AKT, and ERK activation in MOLM-13 cells sensitive and resistant to midostaurin/PKC412. MOLM-13 (A) and MOLM-13/PKC412 (B) cells were left untreated (control) or treated with 4 μM GO-203 for 0.5 and 2 hours. Total cell lysates were analyzed by immunoblotting with the indicated antibodies.MOLM-13 (C) and MOLM-13/PKC412 (D) cells were left untreated (control) or treated with 4 μM GO-203 for 0.5 and 2 hours. Total cell lysates were analyzed by immunoblotting with the indicated antibodies.
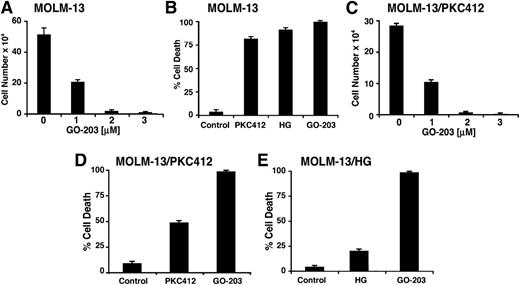
In concert with these findings, treatment of MOLM-13 cells with GO-203 resulted in inhibition of growth that was concentration-dependent (Figure 6A). As shown previously,28 loss of MOLM-13 cell survival was observed in response to treatment with PKC412 or the type II ATP competitive inhibitor HG-7-85-01 (HG) (Figure 6B). However, in studies of the resistant MOLM-13/PKC412 cells, GO-203 was effective in inhibiting growth at the same concentrations used for the treatment of MOLM-13 cells (Figure 6B-C). Moreover, treatment of the MOLM-13/PKC412 cells with GO-203 was associated with induction of cell death (Figure 6D). MOLM-13 cells selected for resistance to HG-7-85-01 (MOLM-13/HG)28 were also sensitive to GO-203 treatment (Figure 6E), indicating that targeting MUC1-C is effective against MOLM-13 cells resistant to diverse FLT3 inhibitors.
GO-203 induces death of MOLM-13 cells resistant to FLT3 inhibitors. (A) MOLM-13 cells were treated with the indicated concentrations of GO-203 for 72 hours. Cell number (mean ± SD of 3 determinations) was determined by trypan blue staining. (B) MOLM-13 cells were left untreated (control) or treated with (1) 50 nM PKC412, (2) 10 nM HG-7-85-01 (HG), and (3) 2 μM GO-203 for 72 hours. Percentage cell death (mean ± SD of 3 determinations) was determined by PI staining and flow cytometry. (C) MOLM-13/PKC412 cells were treated with the indicated concentrations of GO-203 for 72 hours. Cell number (mean ± SD of 3 determinations) was determined by trypan blue staining. (D) MOLM-13/PKC412 cells were left untreated (control) or treated with (1) 5 nM PKC412 and (2) 3 μM GO-203 for 72 hours. Percentage cell death (mean ± SD of 3 determinations) was determined by PI staining and flow cytometry. (E) MOLM-13/HG cells were left untreated (control) or treated with (1) 10 nM HG-7-85-01 (HG) and (2) 3 μM GO-203 for 72 hours. Percentage cell death (mean ± SD of 3 determinations) was determined by PI staining flow cytometry.
GO-203 induces death of MOLM-13 cells resistant to FLT3 inhibitors. (A) MOLM-13 cells were treated with the indicated concentrations of GO-203 for 72 hours. Cell number (mean ± SD of 3 determinations) was determined by trypan blue staining. (B) MOLM-13 cells were left untreated (control) or treated with (1) 50 nM PKC412, (2) 10 nM HG-7-85-01 (HG), and (3) 2 μM GO-203 for 72 hours. Percentage cell death (mean ± SD of 3 determinations) was determined by PI staining and flow cytometry. (C) MOLM-13/PKC412 cells were treated with the indicated concentrations of GO-203 for 72 hours. Cell number (mean ± SD of 3 determinations) was determined by trypan blue staining. (D) MOLM-13/PKC412 cells were left untreated (control) or treated with (1) 5 nM PKC412 and (2) 3 μM GO-203 for 72 hours. Percentage cell death (mean ± SD of 3 determinations) was determined by PI staining and flow cytometry. (E) MOLM-13/HG cells were left untreated (control) or treated with (1) 10 nM HG-7-85-01 (HG) and (2) 3 μM GO-203 for 72 hours. Percentage cell death (mean ± SD of 3 determinations) was determined by PI staining flow cytometry.
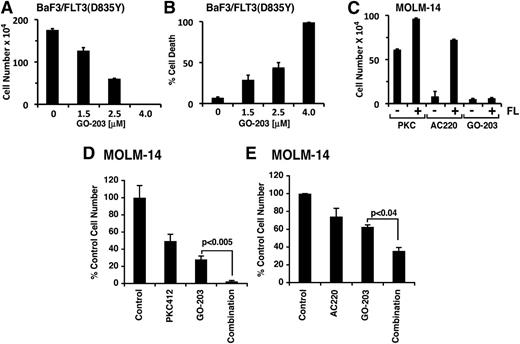
Of note, the mechanistic basis for resistance of MOLM-13 cells to PKC412 and HG has not been defined.28 Therefore, it is possible that a FLT3 point mutation or a non-FLT3–dependent mechanism may be involved in generating these resistant cells. Indeed, other studies have shown that certain point mutations in the FLT3 kinase domain are responsible for conferring resistance to FLT3 inhibitors.38 In this context, the activation loop FLT3(D835Y) mutation confers resistance to AC220.14,39,40 To assess the effects of GO-203 in this setting of FLT3 inhibitor resistance, we generated a BaF3 line that stably expresses FLT3(D835Y). GO-203 was effective in inhibiting growth of BaF3/FLT3(D835Y) cells at concentrations similar to those used for the treatment of BaF3/FLT3 and BaF3/FLT3-ITD cells (Figure 7A). Treatment of BaF3/FLT3(D835Y) cells with GO-203 was also associated with induction of cell death (Figure 7B), indicating that targeting MUC1-C is effective against cells that harbor the FLT3(D835Y) mutation. FLT3 ligand (FL) has been previously shown to influence the in vitro effects of FLT3 inhibitors.41 Moreover, recent studies have shown that exogenous FL at concentrations similar to those observed in patients significantly blocks the activity of PKC412 and AC220.41 Thus, we treated MOLM-14 cells with GO-203 in the absence and presence of FL. The results demonstrate that MOLM-14 cell growth in response to PKC412 or AC220 is increased in the presence of FL (Figure 7C). However, FL had little if any effect on the growth inhibitory response to GO-203 (Figure 7C). Taken together, these findings indicate that targeting MUC1-C with GO-203 is effective against AML cells that are resistant to PKC412 and AC220. This lack of cross-resistance between GO-203 and FLT3 inhibitors invoked the possibility that these agents might be used in combination. To address this possibility, we treated MOLM-14 cells with PKC412 or GO-203 alone and in combination. Exposure of MOLM-14 cells to different concentration of PKC412 demonstrated a 50% inhibition/inhibitory concentration of 25 nM. Consequently, we used a lower dose of PKC412 (10 nM) in combination with 2.5 μM GO-203 (Figure 7D). Treatment with GO-203 in combination with PKC412 was associated with a significant (P < .005) inhibition of growth as compared with either agent alone (Figure 7D). Similar studies were performed with AC220, which was effective against MOLM-14 growth at a 50% inhibition/inhibitory concentration of 2.5 nM. Here the combination of GO-203 and 2 nM AC220 was more effective in inhibiting MOLM-14 cell growth as compared with either agent alone (P < .04) (Figure 7E). These findings indicate that inhibition of MUC1-C is synergistic in combination with FLT3 inhibitors in targeting AML cells.
GO-203 is effective in increasing sensitivity to FLT3 inhibitors. BaF3/FLT3(D835Y) cells were left untreated or treated with the indicated concentrations of GO-203 for 72 hours. Cell number (mean ± SD of 3 determinations) was determined by trypan blue staining (A). Percentage cell death (mean ± SD of 3 determinations) was determined by PI staining and flow cytometry (B). (C) MOLM-14 cells were treated with 25 nM PKC412, 2 nM AC220, or 2 μM GO-203 in the absence (−) and presence (+) of 50 nM FLT3 ligand (FL) for 72 hours. Cell number (mean ± SD of 3 determinations) was determined by trypan blue staining. MOLM-14 cells were treated with 10 nM PKC412 or 2.5 μM GO-203 alone and in combination for 96 hours (D). MOLM-14 cells were treated with 2.5 nM AC220 or 2.5 μM GO-203 alone and in combination for 72 hours (E). Cell number was determined by trypan blue exclusion. The results are expressed as the relative cell number (mean ± SD of 3 determinations) compared with that obtained for the untreated control. P values were determined by the Student’s t test.
GO-203 is effective in increasing sensitivity to FLT3 inhibitors. BaF3/FLT3(D835Y) cells were left untreated or treated with the indicated concentrations of GO-203 for 72 hours. Cell number (mean ± SD of 3 determinations) was determined by trypan blue staining (A). Percentage cell death (mean ± SD of 3 determinations) was determined by PI staining and flow cytometry (B). (C) MOLM-14 cells were treated with 25 nM PKC412, 2 nM AC220, or 2 μM GO-203 in the absence (−) and presence (+) of 50 nM FLT3 ligand (FL) for 72 hours. Cell number (mean ± SD of 3 determinations) was determined by trypan blue staining. MOLM-14 cells were treated with 10 nM PKC412 or 2.5 μM GO-203 alone and in combination for 96 hours (D). MOLM-14 cells were treated with 2.5 nM AC220 or 2.5 μM GO-203 alone and in combination for 72 hours (E). Cell number was determined by trypan blue exclusion. The results are expressed as the relative cell number (mean ± SD of 3 determinations) compared with that obtained for the untreated control. P values were determined by the Student’s t test.
Discussion
MUC1-C interacts with FLT3 in AML cells
FLT3 is expressed in blasts from about 90% of individuals with AML and is mutated in approximately one-third of these patients.1,11 Somewhat paradoxically for an epithelial protein, MUC1 is also expressed at high frequency in blasts and stem cells from AML patients.17-19 However, there has been no previous evidence to support an interaction between MUC1 and FLT3. The present results demonstrate that the oncogenic MUC1-C subunit associates with FLT3 in MOLM-14 AML cells. The MOLM-14 cells have a WT FLT3 allele and a mutant FLT3-ITD allele; thus, MUC1-C may form complexes with either or both FLT3 forms. Studies in HL-60 AML cells, which express only WT FLT3, and in MV4-11 cells, which express only mutant FLT3-ITD, indicate that MUC1-C can associate with both WT and mutant FLT3. The MUC1-C subunit contains a transmembrane domain that positions it in the cell membrane, where it interacts with RTKs.15 Indeed, in carcinoma cells, MUC1-C forms complexes with EGFR that are mediated, at least in part, by galectin-3 bridges.42 MUC1-C also forms complexes with the ErbB2/HER2, ErbB3, MET, and PDGFR RTKs in carcinoma cells.16 The interaction between MUC1-C and RTKs results in phosphorylation of the MUC1-C cytoplasmic domain on tyrosine and the activation of effectors that are downstream to RTK signaling.16 To our knowledge, the present studies provide the first evidence that MUC1-C associates with an RTK, in this case FLT3, in AML cells. Further studies will be needed to determine whether, like EGFR,43 FLT3 phosphorylates the MUC1-C cytoplasmic domain.
Inhibition of MUC1-C downregulates FLT3 downstream signaling
The MUC1-C cytoplasmic domain contains a CQC motif that is necessary and sufficient for the formation of MUC1-C homodimers.24,26 Cell-penetrating peptides that contain the CQC motif, such as GO-203, have been developed to block MUC1-C homodimerization and thereby its oncogenic function.25-27 In the present studies, treatment of MOLM-14 cells with GO-203 disrupted MUC1-C/FLT3 complexes and also resulted in downregulation of FLT3 activation. How MUC1-C homodimerization would contribute to constitutive activation of FLT3 is unclear. However, the formation of MUC1-C homodimers may be of importance at the cell membrane in terms of facilitating the proximity of RTKs, such that transphosphorylation can occur in the absence of ligand. In this context, recent studies have shown that targeting MUC1-C with silencing or GO-203 treatment is associated with downregulation of HER2 activation.44 Mechanistic studies will be needed to address how MUC1-C might contribute to RTK activation. The present results further demonstrate that targeting MUC1-C is associated with downregulation of the PI3K→AKT, MEK→ERK, and STAT5 pathways. This response to GO-203 treatment is likely a consequence of inhibiting the upstream activation of FLT3 signaling. Notably, however, the MUC1-C cytoplasmic domain contains a YHPM motif that, when phosphorylated on tyrosine, functions as a binding site for PI3K and thereby activation of the PI3K→AKT pathway.31,45 Blocking MUC1-C homodimerization with GO-203 is associated with disruption of MUC1-C/PI3K complexes and inactivation of PI3K→AKT signaling.31 Thus, the inhibition of AKT activation observed in the present work may be due in part to loss of MUC1-C–mediated activation of PI3K. The MUC1-C cytoplasmic domain also contains a YTNP motif that, when phosphorylated on tyrosine, functions as a binding site for GRB2/SOS, linking MUC1-C to the RAS→RAF→MEK→ERK pathway.16,46 In the present studies, GO-203 treatment was associated with downregulation of ERK signaling; however, the precise mechanism responsible for this response is presently unclear and could involve inhibition of both FLT3 and MUC1-C.
Targeting MUC1-C in the treatment of AML
Previous studies have demonstrated that targeting MUC1-C in AML cells is associated with disruption of redox balance, induction of differentiation, and loss of survival.27 In support of such findings, MUC1-C attenuates increases in reactive oxygen species (ROS) and thereby protects cells from oxidative stress-induced death.47,48 Other studies have linked FLT3 and FLT3-ITD signaling to the regulation of ROS balance.49,50 Thus, GO-203–induced (1) disruption of the interaction between MUC1-C and FLT3 and (2) downregulation of FLT3 activation could contribute to increases in ROS and thereby loss of survival. In this context, increases in ROS have been linked to a block in self-renewal of hematopoietic cells.51,52 In the present studies, GO-203 treatment of mouse BaF3/FLT3-ITD cells was associated with loss of survival. BaF3/FLT3-ITD cells express MUC1-C and the mouse Muc1-C cytoplasmic domain contains the consensus CQC motif needed for homodimerization. GO-203 treatment of MOLM-13 cells resistant to FLT3 inhibitors was also associated with cell death, suggesting that targeting MUC1-C could be effective in the treatment of AML that is unresponsive to PKC412 or other FLT3 inhibitors. The demonstration that GO-203 promotes the response to PKC412 further indicates that this combination could be more effective than treatment with PKC412 alone, which has induced partial and transient responses in early clinical trials.10 Thus, GO-203 could be used in combination with PKC412 for the treatment of both FLT3 inhibitor-sensitive or inhibitor-resistant AML. A phase I trial of GO-203 has recently been completed in patients with refractory solid tumors to define the maximum tolerated dose. Based on the present findings and the demonstration that MUC1-C is selectively expressed in myeloid leukemia stem/progenitors,19 it is anticipated that a phase I/II trial of GO-203 will be initiated for the treatment of patients with FLT3 mutant AML.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the National Cancer Institute of the National Institutes of Health (grants CA42802, CA100707, and CA66996) and by the Leukemia Lymphoma Society (grant 6226-12).
Authorship
Contribution: S.L., L.Y., D.S., H.R., A.P., and K.S. performed research and analyzed data; D.A., S.K., and R.S. designed research and analyzed data; and D.K. and R.S. wrote the paper.
Conflict-of-interest disclosure: D.K. holds equity in Genus Oncology and is a consultant to the company; S.K. is an employee of Genus Oncology. The remaining authors declare no competing financial interests.
Correspondence: Richard Stone, Dana-Farber Cancer Institute, 450 Brookline Ave, D2053, Boston, MA 02215; e-mail: rstone@partners.org.

![Figure 3. GO-203 inhibits cell growth and induces death of BaF3, BaF3/FLT3-ITD, and MOLM-14 cells. BaF3 cells grown in the presence of interleukin-3 (IL-3) were treated with GO-203 at the indicated concentrations for 72 hours (A). BaF3/FLT3-ITD cells were left untreated (open bars) or treated with 2 μM GO-203 (solid bars) for the indicated days (B). Cell number (mean ± standard deviation [SD] of 3 determinations) was determined by trypan blue staining. (C) BaF3/FLT3-ITD cells were treated with the indicated concentrations of GO-203 for 3 days. Percentage cell death (mean ± SD of 3 determinations) was determined by PI staining and flow cytometry. (D) MOLM-14 cells were treated with the indicated concentrations of GO-203 for 3 days. Percentage cell death (mean ± SD of 3 determinations) was determined by PI staining and flow cytometry. (E,F) BALB/c ν/ν mice were injected subcutaneously in the flank with 107 MOLM-14 cells. The mice were pair-matched when the tumors were ∼100 mm3. Treatment groups consisted of 7 mice injected intravenously with PBS (vehicle control; open squares) or 7.5 mg/kg GO-203 (closed squares) each day on days 1-5 and 8-12. Tumor measurements were performed on the indicated days. The results are expressed as tumor volumes (mean ± SE) (D). Percentage survival as determined by Kaplan-Meier analysis (E).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/123/5/10.1182_blood-2013-04-493858/4/m_734f3.jpeg?Expires=1769523911&Signature=nRhbHG8YYaZqqeWR3H1PhM9S7lxqKemzkj70nkiONYVVnpSFav9WG172AOmfm~MvOOwnkh7sHZs6hqsEK1KtjAeV2f2Ij4PRodwyp1~yNPprhZMy-tBIupK7NbKRaCh7eDEG2HQ3FX2hQ-RPhjtLy0Mh0kUxdmxBStilLZItF-1sylgVfZf1BR5pspHYzdtnVFrb-Iw88M5iL4OUg7BWD6D-vlcVkBUi2BvQz4zHCTG1ZaR4tni62LaOJoge3bf5UickiP1uvgEzgsp2LIxcZUz-2wIkVWWLHs9m-n9umsgWZn0ZwE7i3ew1UFTjwkZQVeWm50J7Z8C132f0LdquIw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)