Abstract
Acute myeloid leukemia (AML) cells are characterized by unlimited self-renewal and an impaired capacity to undergo terminal differentiation. The MUC1 oncoprotein is aberrantly expressed in AML cells; however, there has been no evidence for involvement of MUC1 in myeloid leukemogenesis. Cell-penetrating peptide inhibitors of the MUC1-C subunit block its oligomerization and thereby oncogenic function. The present results demonstrate that treatment of human MOLM-14 and MV4-11 AML cells with these inhibitors is associated with arrest of growth, induction of late apoptosis/necrosis, and loss of self-renewal capacity. Similar results were obtained with primary blasts from patients with AML. Inhibition of MUC1-C was associated with increases in reactive oxygen species (ROS) and depletion of glutathione. Increases in ROS have been linked to induction of hematopoietic cell differentiation along the myeloid lineage. In this regard, inhibition of MUC1-C was associated with induction of a terminally differentiated myeloid phenotype in AML cell lines and primary blasts by an ROS-dependent mechanism. These findings indicate that MUC1-C function is of importance to AML cell self-renewal and that inhibition of MUC1-C represents a potential therapeutic approach to induce terminal differentiation of AML cells.
Introduction
Acute myelogenous leukemia (AML) is a clonal disorder of hematopoietic progenitor cells that is characterized by accumulation of blasts with an unrestrained proliferative capacity and a block at various stages of myeloid differentiation.1 The development of AML involves a multistep process with the acquisition of alterations in genes that confer a proliferative advantage or affect differentiation.2 Allogeneic bone marrow transplantation and therapy with cytosine arabinoside and daunorubicin have had a significant effect on long-term survival of AML patients younger than age 60. However, patients older than age 60 and those with secondary AML or a previous myelodysplastic syndrome have a poor prognosis. The concept that AML occurs as a result of a block in maturation has led to therapeutic approaches based on the use of agents that induce terminal AML cell differentiation. However, such strategies have been limited, in terms of effectiveness, to the treatment of acute promyelocytic leukemia (APL). For example, all-trans retinoic acid (ATRA) and arsenic trioxide induce differentiation of APL cells and have had a marked effect on the treatment of this disease.3 Other studies have provided support for the involvement of reactive oxygen species (ROS) in the regulation of AML cell survival and induction of myeloid cell differentiation.4 In this context, AML cell self-renewal is decreased by agents that increase ROS levels.5-7 Moreover, differentiation of AML cells has been associated with increases in ROS.8 These findings have suggested that targeting redox balance may overcome the block in terminal AML cell differentiation.
The MUC1 heterodimeric protein is aberrantly expressed in blasts from patients with AML.9,10 MUC1 includes an extracellular N-terminal subunit (MUC1-N) that has the structural characteristics of mucins and is tethered to the cell surface in a complex with a C-terminal transmembrane subunit (MUC1-C).11 MUC1-C consists of a 58–amino acid extracellular domain, a transmembrane domain, and a 72–amino acid cytoplasmic domain.11 In transformed cells with up-regulation of MUC1 expression, the MUC1-C subunit accumulates in the cytoplasm and is targeted to the nucleus and mitochondria.11 The MUC1-C cytoplasmic domain is phosphorylated by c-Src and certain receptor tyrosine kinases, and interacts with effectors, such as β-catenin and NF-κB, that have been linked to transformation.11 Notably in this regard, the MUC1-C cytoplasmic domain is sufficient to induce anchorage-independent growth and tumorigenicity.12 Overexpression of MUC1-C also blocks death induced in the response to DNA damage, ROS, and other forms of stress.11,13,14 Targeting of MUC1-C to the nucleus and mitochondria, and thereby its transforming function, is dependent on the formation of oligomers through a CQC motif in the MUC1-C cytoplasmic domain.11,15 These observations led to the development of cell-penetrating peptides that bind to the CQC motif and block MUC1-C oligomerization and function.16 Moreover, treatment of human carcinoma cells with one of the cell-penetrating MUC1-C peptide inhibitors, designated GO-201, was associated with induction of a MUC1-dependent necrotic cell death response.16,17 These findings provided support for the dependence of carcinoma cells on MUC1-C for their survival.
The present studies demonstrate that human AML cell lines and primary AML blasts respond to targeting of MUC1-C with increases in ROS and late apoptotic/necrotic cell death. The results also show that treatment of AML cells with MUC1-C inhibitors is associated with ROS-dependent induction of terminal myeloid differentiation.
Methods
Cell culture
Human MOLM-14 and MV4-11 AML cells were cultured in RPMI 1640 medium (Cellgro) supplemented with 10% heat-inactivated fetal bovine serum (FBS; Cellgro), 100 units/mL penicillin, 100 μg/mL streptomycin, and 2mM l-glutamine. In certain experiments, the MOLM-14 and MV4-11 cells were grown in complete media containing 20 ng/mL human IL-3 (Gibco). Cells were treated with GO-201, GO-202, GO-203, CP-1, and CP-2 peptides (AnaSpec) and 5mM N-acetylcysteine (NAC; Calbiochem). Viability was determined by trypan blue exclusion.
FACS analysis
Cells were incubated with anti-MUC1-N (MAb DF3)18 and then a secondary PE-labeled goat anti–mouse IgG (Invitrogen). In certain experiments, the MUC1-positive and MUC1-negative cells were collected using the ARIA cell sorter (BD Biosciences). Alternatively, cells were incubated with FITC-conjugated antibodies against CD11b or CD11c (NOVUS Biologicals). Reactivity was analyzed by FACScan (Becton Dickinson).
Immunoblot analysis
Cells were lysed as described.19 Soluble proteins were analyzed by immunoblotting with anti–MUC1-C (Ab5; LabVision), anti–β-actin (Sigma-Aldrich), and anti-C/EBPβ (Santa Cruz Biotechnology). Antigen-antibody complexes were visualized by enhanced chemiluminescence (GE Healthcare).
Isolation of primary AML blasts
Bone marrow aspirates and peripheral blood samples were obtained from patients with AML under approval of the Institutional Review Board. Mononuclear cells were isolated by density gradient centrifugation through Ficoll-Plaque Plus (GE Healthcare Bio-Sciences AB) for 30 minutes, washed twice with PBS, and then cultured in RPMI 1640 medium containing FBS, antibiotics, and l-glutamine.
Measurement of ROS levels
Cells were incubated with 5μM c-H2DCFDA (Molecular Probes) for 20 minutes at 37°C to assess hydrogen peroxide (H2O2)–mediated oxidation to the fluorescent compound DCF. Fluorescence of oxidized DCF was measured at an excitation wavelength of 480 nm and an emission wavelength of 525 nm using a flow cytometer (BD Biosciences). For the assessment of superoxide (O2−) levels, cells were incubated with 2μM hydroethidine (HE; Polyscience Inc) for 20 minutes at room temperature. Superoxide-mediated conversion of HE to ethidium was measured by excitation at 470 nm and emission at 590 nm.
Determination of GSH levels
Intracellular GSH concentrations were measured using the Bioxytech GSH-400 kit (OXIS International).
Analysis of cell death
Cells were incubated with propidium iodide (PI)/annexin V-FITC (BD Biosciences) for 15 minutes at room temperature and then analyzed by flow cytometry.
Clonogenic survival
MOLM-14, MV4-11, and AML patient no. 1 cells were seeded at 1500, 10 000 or 1500 cells per well, respectively, in a 6-well plate containing 0.29%-0.34% select agar (Invitrogen) in RPMI 1640 medium supplemented with 10% FBS. At 2 weeks, colonies were counted under an inverted microscope.
Results
MUC1-C inhibitor GO-201 suppresses growth of AML cells
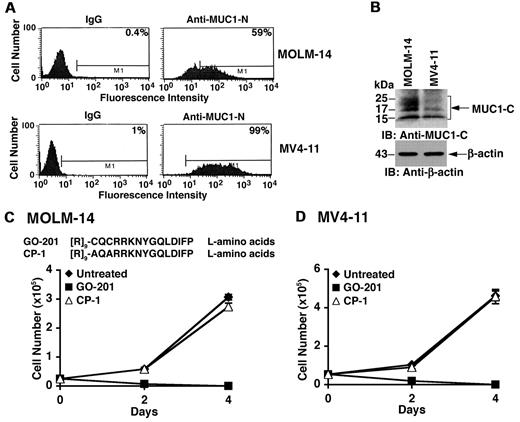
MUC1 is expressed in blasts from about 70% of AML patients.9,10 Analysis of the MOLM-14 AML cell line by flow cytometry demonstrated cell surface expression of the MUC1-N subunit (Figure 1A). MUC1-N expression was also detectable on the surface of MV4-11 AML cells (Figure 1A). Immunoblot analysis of these AML cells further demonstrated expression of the MUC1-C subunit (Figure 1B). As shown in other cell types,20 MUC1-C is expressed in MOLM-14 cells as a broad 25- to 20 kDa N-glycosylated form, a 17-kDa unglycosylated form and a processed 15-kDa form (Figure 1B). In MV4-11 cells, MUC1-C is predominantly expressed as the 17- and 15-kDa species (Figure 1B). The relationship between detection of the MUC1-N and MUC1-C subunits may vary depending on stability of MUC1-N at the cell surface. To evaluate the effects of inhibiting MUC1-C, we treated MOLM-14 cells with GO-201, a peptide inhibitor of MUC1-C oligomerization.16 GO-201 contains the L-amino acid CQCRRKNYGQLDIFP sequence linked at the N-terminus to 9 Arg residues ([R]9-CQCRRKNYGQLDIFP) for cell penetration (Figure 1C). The MOLM-14 cells were also treated with a control L-amino acid peptide (CP-1; [R]9-AQARRKNYGQLDIFP), which is altered at the critical CQC motif to AQA, is devoid of binding to the MUC1-C cytoplasmic domain, and is thereby ineffective in blocking MUC1-C oligomerization16 (Figure 1C). Growth of MOLM-14 cells was inhibited by treatment with 5μM GO-201 (Figure 1C). By contrast, 5μM CP-1 had little if any effect (Figure 1C). Similar results were obtained with MV4-11 cells (Figure 1D), indicating that inhibition of MUC1-C blocks growth of these AML cell lines.
Growth of MOLM-14 and MV4–11 AML cells is inhibited by GO-201. (A) MOLM-14 (top panels) and MV4-11 (bottom panels) were incubated with a control IgG or anti–MUC1-N (MAb DF3) and analyzed by flow cytometry. The percentage of MUC1-positive cells is indicated in the panels. (B) Lysates from MOLM-14 and MV4-11 cells were subjected to immunoblotting with the indicated antibodies. (C) Amino acid sequences of GO-201 and CP-1. MOLM-14 cells were left untreated (♦), and treated with 5μM GO-201 (■) or 5μM CP-1 (▵) each day for the indicated days. Viable cell number as determined by trypan blue exclusion is expressed as the mean ± SD of 3 determinations. (D) MV4-11 cells were left untreated (♦), and treated with 5μM GO-201 (■) or 5μM CP-1 (▵) each day for the indicated days. Viable cell number was determined by trypan blue exclusion.
Growth of MOLM-14 and MV4–11 AML cells is inhibited by GO-201. (A) MOLM-14 (top panels) and MV4-11 (bottom panels) were incubated with a control IgG or anti–MUC1-N (MAb DF3) and analyzed by flow cytometry. The percentage of MUC1-positive cells is indicated in the panels. (B) Lysates from MOLM-14 and MV4-11 cells were subjected to immunoblotting with the indicated antibodies. (C) Amino acid sequences of GO-201 and CP-1. MOLM-14 cells were left untreated (♦), and treated with 5μM GO-201 (■) or 5μM CP-1 (▵) each day for the indicated days. Viable cell number as determined by trypan blue exclusion is expressed as the mean ± SD of 3 determinations. (D) MV4-11 cells were left untreated (♦), and treated with 5μM GO-201 (■) or 5μM CP-1 (▵) each day for the indicated days. Viable cell number was determined by trypan blue exclusion.
AML cell growth is inhibited by the GO-203 derivative
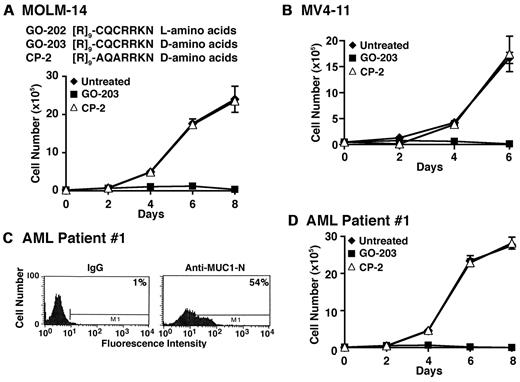
GO-201 derivatives were synthesized to determine whether shorter versions retain activity. Treatment with GO-202 ([R]9-CQCRRKN; L-amino acids) was found to be as active as GO-201 against MOLM-14 and MV4-11 cells (Figure 2A and data not shown). Conversion of GO-202 to the D-amino acid configuration, designated GO-203, was then tested to see whether this modification affects activity (Figure 2A). As observed with GO-201 and GO-202, treatment of MOLM-14 cells with 5μM GO-203 was associated with inhibition of growth (Figure 2A). By contrast, a control D-amino acid peptide, designated CP-2, with alteration of the CQC motif to AQA had no apparent effect (Figure 2A). Similar results were obtained with MV4-11 cells (Figure 2B). GO-203 was also effective in inhibiting growth of MOLM-14 and MV4-11 cells when cultured in the presence of 20 ng/mL IL-3 (supplemental Figure 1A-B, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). To determine whether primary AML cells also respond to GO-203, blasts from patient 1 with AML were grown in short-term culture. As determined by flow cytometry, AML patient 1 blasts expressed MUC1 (Figure 2C). In addition, growth of AML patient 1 blasts was inhibited by GO-203 and not CP-2 (Figure 2D). AML blasts from 2 other patients (2 and 3) were also sensitive to growth inhibition by GO-203 (supplemental Figure 2A-D), indicating that this response is observed in diverse AML cell lines and patient blasts.
GO-203 is effective in inhibiting growth of AML cells. (A) Amino acid sequences of GO-202, GO-203, and CP-3. MOLM-14 cells were left untreated (♦), and treated with 5μM GO-203 (■) or 5μM CP-2 (▵) each day for the indicated days. Viable cell number was determined by trypan blue exclusion. (B) MV4–11 cells were left untreated (♦), and treated with 5μM GO-203 (■) or 5μM CP-2 (▵) each day for the indicated days. Viable cell number was determined by trypan blue exclusion. (C) AML patient 1 cells were incubated with a control IgG or anti–MUC1-N (MAb DF3) and analyzed by flow cytometry. The percentage of MUC1-positive cells is indicated in the panels. (D) AML patient 1 cells were left untreated (♦), and treated with 5μM GO-203 (■) or 5μM CP-2 (▵) each day for the indicated days. Viable cell number was determined by trypan blue exclusion.
GO-203 is effective in inhibiting growth of AML cells. (A) Amino acid sequences of GO-202, GO-203, and CP-3. MOLM-14 cells were left untreated (♦), and treated with 5μM GO-203 (■) or 5μM CP-2 (▵) each day for the indicated days. Viable cell number was determined by trypan blue exclusion. (B) MV4–11 cells were left untreated (♦), and treated with 5μM GO-203 (■) or 5μM CP-2 (▵) each day for the indicated days. Viable cell number was determined by trypan blue exclusion. (C) AML patient 1 cells were incubated with a control IgG or anti–MUC1-N (MAb DF3) and analyzed by flow cytometry. The percentage of MUC1-positive cells is indicated in the panels. (D) AML patient 1 cells were left untreated (♦), and treated with 5μM GO-203 (■) or 5μM CP-2 (▵) each day for the indicated days. Viable cell number was determined by trypan blue exclusion.
GO-203 disrupts redox balance in AML cells
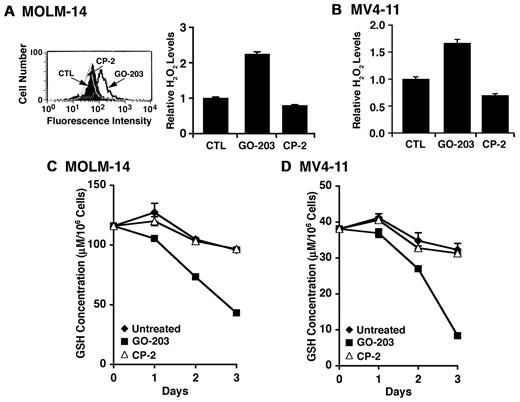
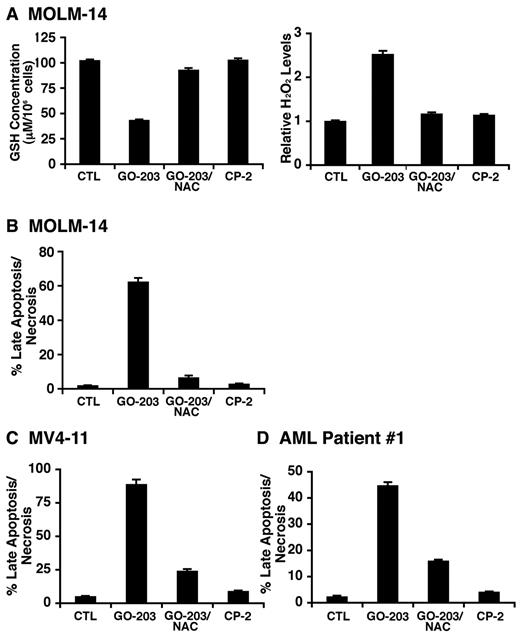
Previous studies have shown that MUC1 regulates intracellular levels of reactive oxygen species (ROS).14,19,21,22 To determine whether targeting MUC1 with GO-203 affects redox balance, we incubated GO-203–treated MOLM-14 cells with c-H2DCFDA and oxidation of the fluorochrome by hydrogen peroxide was assessed by flow cytometry.14 The results show that treatment with 5μM GO-203 and not CP-2 is associated with increases in hydrogen peroxide levels (Figure 3A left). These results were confirmed in repetitive experiments with similar results (Figure 3A right). Increases in hydrogen peroxide levels were also observed in GO-203–treated MV4-11 cells (Figure 3B). Superoxide levels were monitored by oxidation of hydroethidine as another measure of disrupting redox balance.14 As found for hydrogen peroxide, GO-203 treatment of MOLM-14 and MV4-11 cells was associated with increases in superoxide levels that were confirmed in additional experiments (supplemental Figure 3A-B). Oxidative stress is associated with decreases in intracellular GSH levels.23 In this regard, GO-203 treatment of MOLM-14 cells was associated with a marked depletion of GSH that was clearly detectable by day 3 (Figure 3C). Similar results were obtained in the response of MV4-11 cells to GO-203 treatment (Figure 3D). These findings indicate that inhibition of MUC1-C in MOLM-14 and MV4-11 AML cells is associated with disruption of redox balance.
Redox balance is disrupted in AML cells by GO-203 treatment. (A) MOLM-14 cells were left untreated, and treated with 5μM GO-203 or 5μM CP-2 each day for 3 days. The cells were then incubated with c-H2DCFDA for 30 minutes. Fluorescence of oxidized DCF was measured by flow cytometry (left). The results are expressed as the relative H2O2 level (mean ± SD for 3 determinations) compared with that obtained with control cells (right). (B) MV4-11 cells were left untreated, and treated with 5μM GO-203 or 5μM CP-2 each day for 3 days. Oxidation of DCF was measured by flow cytometry. The results are expressed as the relative H2O2 level (mean ± SD for 3 determinations) compared with that obtained with control cells. (C-D) MOLM-14 (C) and MV4-11 (D) cells were left untreated, and treated each day with 5μM GO-203 or 5μM CP-2. The cells were harvested at the indicated times and analyzed for GSH levels. The results are expressed as GSH levels/106 cells.
Redox balance is disrupted in AML cells by GO-203 treatment. (A) MOLM-14 cells were left untreated, and treated with 5μM GO-203 or 5μM CP-2 each day for 3 days. The cells were then incubated with c-H2DCFDA for 30 minutes. Fluorescence of oxidized DCF was measured by flow cytometry (left). The results are expressed as the relative H2O2 level (mean ± SD for 3 determinations) compared with that obtained with control cells (right). (B) MV4-11 cells were left untreated, and treated with 5μM GO-203 or 5μM CP-2 each day for 3 days. Oxidation of DCF was measured by flow cytometry. The results are expressed as the relative H2O2 level (mean ± SD for 3 determinations) compared with that obtained with control cells. (C-D) MOLM-14 (C) and MV4-11 (D) cells were left untreated, and treated each day with 5μM GO-203 or 5μM CP-2. The cells were harvested at the indicated times and analyzed for GSH levels. The results are expressed as GSH levels/106 cells.
GO-203 induces late apoptosis/necrosis of AML cells
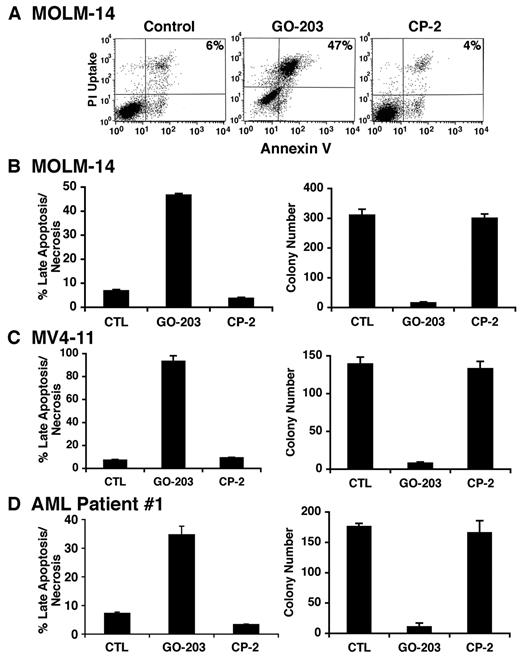
To determine whether the induction of oxidative stress is associated with cell death, cells were treated with GO-203 and then analyzed by staining with propidium iodide (PI) and annexin V. MOLM-14 cells responded to 5μM GO-203 with an increase in both PI and annexin V staining, consistent with the induction of late apoptosis/necrosis (Figure 4A). By contrast, CP-2 had little if any effect (Figure 4A). These results were confirmed in repetitive experiments and demonstrated late apoptosis/necrosis in more than 50% of the MOLM-14 cells on day 3 (Figure 4B left). To extend these observations, MOLM-14 cells were treated with 5μM GO-203 for 4 days, washed, and then seeded in agar in the absence of drug. Analysis of colony number after 14 days demonstrated that GO-203 induces a marked decrease in clonogenic survival compared with that obtained for control and CP-2–treated cells (Figure 4B right). MOLM-14 cells were selected by flow cytometry into MUC1-positive and MUC1-negative populations (supplemental Figure 4A), and then seeded in agar. The results demonstrate that the MOLM-14/MUC1–positive cells represent the predominant clonogenic, self-renewing population compared with that obtained with MOLM-14/MUC1–negative cells (supplemental Figure 4B). Induction of late apoptosis/necrosis (Figure 4C left and supplemental Figure 5A) and a decrease in colony formation (Figure 4C right) were also observed with GO-203–treated MV4-11 cells. Moreover, treatment of AML patient 1 blasts with GO-203 was associated with PI/annexin V staining (Figure 4D left and supplemental Figure 5B) and loss of clonogenic survival (Figure 4D right). These findings indicate that inhibition of MUC1-C in AML blasts is associated with induction of late apoptosis/necrosis and loss of self-renewal capacity.
GO-203 induces late apoptosis/necrosis of AML cells. (A) MOLM-14 cells were left untreated, and treated with 5μM GO-203 or 5μM CP-2 each day for 3 days. The cells were stained with PI and annexin V, and analyzed by flow cytometry. The percentage of MOLM-14 cells staining with both PI and annexin V is indicated in the upper right panels. (B-D) MOLM-14 (B), MV4-11 (C) and AML patient 1 (D) cells were left untreated, and treated each day with 5μM GO-203 or 5μM CP-2. The MOLM-14, MV4-11 and AML patient 1 cells were harvested on days 3, 3, and 4, respectively. The results obtained from staining with PI/annexin V are expressed as the percentage of cells (mean ± SD of 3 determinations) with late apoptosis/necrosis (left). The cells were also seeded in agar in the absence of drug and colonies were counted at 14 days. The results are expressed as the number of colonies (mean ± SD of 3 determinations) (right).
GO-203 induces late apoptosis/necrosis of AML cells. (A) MOLM-14 cells were left untreated, and treated with 5μM GO-203 or 5μM CP-2 each day for 3 days. The cells were stained with PI and annexin V, and analyzed by flow cytometry. The percentage of MOLM-14 cells staining with both PI and annexin V is indicated in the upper right panels. (B-D) MOLM-14 (B), MV4-11 (C) and AML patient 1 (D) cells were left untreated, and treated each day with 5μM GO-203 or 5μM CP-2. The MOLM-14, MV4-11 and AML patient 1 cells were harvested on days 3, 3, and 4, respectively. The results obtained from staining with PI/annexin V are expressed as the percentage of cells (mean ± SD of 3 determinations) with late apoptosis/necrosis (left). The cells were also seeded in agar in the absence of drug and colonies were counted at 14 days. The results are expressed as the number of colonies (mean ± SD of 3 determinations) (right).
GO-203–induced AML cell death is blocked by NAC
N-acetylcysteine (NAC), a precursor for GSH synthesis, was added to GO-203–treated cells to determine whether it attenuates the disruption of redox balance. Indeed, in experiments with MOLM-14 cells, the concurrent addition of NAC with GO-203 restored the depletion of GSH associated with GO-203 treatment alone (Figure 5A left) and blocked GO-203–induced increases in hydrogen peroxide (Figure 5A right) and superoxide (supplemental Figure 6A). Moreover, NAC blocked GO-203–induced MOLM-14 cell late apoptosis/necrosis (Figure 5B and supplemental Figure 6B) and loss of clonogenic survival (data not shown). Treatment of MV4-11 cells with NAC was also associated with attenuation of GO-203–induced (1) decreases in GSH levels (supplemental Figure 6C), (2) increases in hydrogen peroxide (supplemental Figure 6D) and superoxide (supplemental Figure 6E), and (3) late apoptosis/necrosis (Figure 5C). In concert with these effects, NAC also blocked GO-203–induced late apoptosis/necrosis of AML patient 1 blasts (Figure 5D). These findings indicate that inhibition of MUC1-C results in disruption of redox balance and thereby AML cell death.
GO-203–induced disruption of redox balance and death is reversed by NAC. (A) MOLM-14 cells were left untreated, and treated with 5μM GO-203 or 5μM CP-2 each day for 3 days. The GO-203–treated cells were also incubated concurrently with 5mM NAC. The cells were analyzed for GSH and hydrogen peroxide levels (A). The results are expressed as GSH levels/106 cells (mean ± SD of 3 determinations) (A, left) and relative hydrogen peroxide levels (mean ± SD of 3 determinations) compared with that obtained with control cells (A, right). The cells were also stained with PI/annexin V (B). The results are expressed as the percentage of cells (mean ± SD of 3 determinations) with late apoptosis/necrosis (B). (C-D) MV4-11 (C) and AML patient 1 (D) cells were left untreated, and treated with 5μM GO-203 or 5μM CP-2 each day for 3 (C) and 4 (D) days. The GO-203–treated cells were also incubated in the presence of 5mM NAC. The cells were stained with PI/annexin V. The results are expressed as the percentage of cells (mean ± SD of 3 determinations) with late apoptosis/necrosis.
GO-203–induced disruption of redox balance and death is reversed by NAC. (A) MOLM-14 cells were left untreated, and treated with 5μM GO-203 or 5μM CP-2 each day for 3 days. The GO-203–treated cells were also incubated concurrently with 5mM NAC. The cells were analyzed for GSH and hydrogen peroxide levels (A). The results are expressed as GSH levels/106 cells (mean ± SD of 3 determinations) (A, left) and relative hydrogen peroxide levels (mean ± SD of 3 determinations) compared with that obtained with control cells (A, right). The cells were also stained with PI/annexin V (B). The results are expressed as the percentage of cells (mean ± SD of 3 determinations) with late apoptosis/necrosis (B). (C-D) MV4-11 (C) and AML patient 1 (D) cells were left untreated, and treated with 5μM GO-203 or 5μM CP-2 each day for 3 (C) and 4 (D) days. The GO-203–treated cells were also incubated in the presence of 5mM NAC. The cells were stained with PI/annexin V. The results are expressed as the percentage of cells (mean ± SD of 3 determinations) with late apoptosis/necrosis.
AML cells respond to GO-203 with induction of a terminally differentiated phenotype
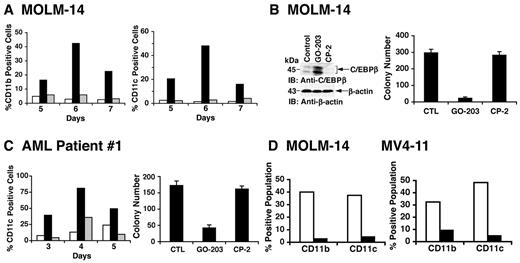
AML blasts are characterized in part by a block in terminal differentiation. To determine whether GO-203 treatment is associated with the induction of a differentiated phenotype, MOLM-14 cells were treated with a lower (2.5μM) concentration of GO-203 that slows growth without the rapid induction of death observed at 5μM GO-203 (supplemental Figure 7). Analysis of the growth-arrested MOLM-14 cells demonstrated increased expression of the transmembrane CD11b glycoprotein myeloid differentiation marker on days 5-7 (Figure 6A left). In concert with the induction of differentiation, GO-203 treatment of the MOLM-14 cells was also associated with increased expression of (1) CD11c, a transmembrane glycoprotein found on the surface of granulocytes and monocytes/macrophages (Figure 6A right) and (2) C/EBPβ, a transcription factor that promotes myeloid differentiation24,25 (Figure 6B left). MV4-11 cells also responded to 2.5μM GO-203 with (1) arrest of growth (supplemental Figure 8A), (2) increased expression of CD11b and CD11c (supplemental Figure 8B), (3) up-regulation of C/EBPβ (supplemental Figure 8C), and (4) loss of clonogenic survival (supplemental Figure 8D). In addition, treatment of AML patient 1 blasts with 2.5μM GO-203 resulted in (1) arrest of growth (supplemental Figure 9), (2) increased expression of CD11b (data not shown) and CD11c (Figure 6C left), and (3) decreases in colony formation (Figure 6C right). In concert with the effects on survival, NAC treatment of MOLM-14 (Figure 6D left) and MV4-11 (Figure 6D right) cells blocked the GO-203–induced increases in CD11b and CD11c expression. These findings demonstrate that treatment of AML cells with GO-203 is associated with ROS-mediated induction of a differentiated phenotype.
AML cells respond to GO-203 with induction of ROS-mediated myeloid differentiation. (A) MOLM-14 cells were left untreated (□), and treated with 2.5μM GO-203 (■) or 2.5μM CP-2 (▩) each day for the indicated days. Cells were analyzed for the percentage with expression of CD11b (left) and CD11c (right). (B) MOLM-14 cells were left untreated, and treated with 2.5μM GO-203 or 2.5μM CP-2 each day for 6 days. Lysates were immunoblotted with the indicated antibodies (left). Cells were seeded in agar in the absence of drug and colonies were counted at 14 days. The results are expressed as the number of colonies (mean ± SD of 3 determinations) (right). (C) AML patient 1 cells were left untreated (□), and treated with 2.5μM GO-203 (■) or 2.5μM CP-2 (▩) each day for the indicated days. Cells were analyzed for the percentage with expression of CD11c (left). On day 6, the cells were seeded in agar in the absence of drug and colonies were counted at 14 days. The results are expressed as the number of colonies (mean ± SD of 3 determinations) (right). (D) MOLM-14 (left) and MV4-11 (right) cells were treated with 2.5μM GO-203 in the absence (□) and presence (■) of 5mM NAC for 6 days. The cells were then analyzed for the percentage with expression of CD11b and CD11c.
AML cells respond to GO-203 with induction of ROS-mediated myeloid differentiation. (A) MOLM-14 cells were left untreated (□), and treated with 2.5μM GO-203 (■) or 2.5μM CP-2 (▩) each day for the indicated days. Cells were analyzed for the percentage with expression of CD11b (left) and CD11c (right). (B) MOLM-14 cells were left untreated, and treated with 2.5μM GO-203 or 2.5μM CP-2 each day for 6 days. Lysates were immunoblotted with the indicated antibodies (left). Cells were seeded in agar in the absence of drug and colonies were counted at 14 days. The results are expressed as the number of colonies (mean ± SD of 3 determinations) (right). (C) AML patient 1 cells were left untreated (□), and treated with 2.5μM GO-203 (■) or 2.5μM CP-2 (▩) each day for the indicated days. Cells were analyzed for the percentage with expression of CD11c (left). On day 6, the cells were seeded in agar in the absence of drug and colonies were counted at 14 days. The results are expressed as the number of colonies (mean ± SD of 3 determinations) (right). (D) MOLM-14 (left) and MV4-11 (right) cells were treated with 2.5μM GO-203 in the absence (□) and presence (■) of 5mM NAC for 6 days. The cells were then analyzed for the percentage with expression of CD11b and CD11c.
Discussion
Targeting MUC1-C in AML cells is associated with increases in ROS levels
MUC1 is functionally involved in suppressing increases in ROS levels in the response to oxidative stress.14 This function is mediated by the MUC1-C cytoplasmic domain and, at least in part, the induction of genes that confer protection against the disruption of redox balance.14,19 In the present studies, human AML cell lines that express MUC1 were treated with peptide inhibitors (GO-201, GO-202, and GO-203) of MUC1-C oligomerization and thereby function.16 Somewhat surprisingly, these MUC1-C inhibitors blocked growth and induced death of the MOLM-14 and MV4-11 AML cells. In this regard, MUC1 has been shown to be expressed in AML blasts,9,10 but had heretofore not been identified as a potential target for inducing AML cell death. Significantly, these findings were not restricted to AML cell lines, but included primary blasts from AML patients, indicating that MUC1-C function is of importance to AML cell survival. In concert with a role for MUC1-C in regulating ROS levels, treatment of the AML cell lines and primary blasts with GO-203 was associated with marked increases in hydrogen peroxide and superoxide. In addition, GSH levels decreased in the response to MUC1-C inhibition, conceivably as a result of depletion from the induction of oxidative stress.23,26 Indeed, restoration of GSH levels with NAC, a precursor of GSH synthesis, attenuated the MUC1-C inhibitor-induced increases in ROS. These findings provided support for the involvement of MUC1-C in the regulation of redox balance in AML cells.
MUC1-C confers AML cell survival by a ROS-dependent mechanism
Studies in Drosophila melanogaster and mammalian cells have demonstrated that ROS are critical in regulating self-renewal of normal hematopoietic cells.4,27,28 Moreover, in this context, self-renewal of leukemic cells appears to be sensitive to increases in ROS levels, indicating that disruption of redox balance in AML could be effective therapeutically.5 For example, agents that increase ROS, such as CDDO and parthenolide, have been shown to induce apoptosis of AML cells.6,7 The present results provide further evidence that inhibition of MUC1-C and the associated increases in ROS induce late apoptosis/necrosis of the MOLM-14 and MV4-11 AML cells. Late apoptosis/necrosis was also induced in primary AML blasts in the response to MUC1-C inhibition. Analysis of clonogenic survival of the AML cell lines and primary blasts further indicated that blocking MUC1-C function is associated with loss of self-renewal capacity. Significantly, the induction of AML cell death by MUC1-C inhibition was reversed in large part by NAC, confirming the involvement of increases in ROS levels. The link between redox balance and loss of hematopoietic cell self-renewal initially came from studies that ataxia telangiectasia mutated (ATM)–deficient mice develop early bone marrow failure as a function of increases in ROS.27 Other studies demonstrated that FoxO family members protect hematopoietic cells from oxidative stress.4 As found in the ATM-deficient mice, the size of the hematopoietic cell compartment was restored in the FoxO-deficient mice by treatment with NAC.4,27 MUC1-C participates in stress-induced activation of ATM29 and FoxO3a19 and, thus, inhibition of MUC1-C in AML cells could also increase ROS and block self-renewal by affecting these pathways.
MUC1-C is a target for inducing terminal differentiation of AML cells
The present results further demonstrate that inhibition of MUC1-C in AML cells is associated with the induction of a differentiated myeloid phenotype and that this response is dependent on increases in ROS. These observations are in concert with the premise that ROS may act as an inducer of normal hematopoietic cell differentiation along the myeloid lineage.4 In addition, studies demonstrating that CDDO, which increases intracellular ROS, also induces maturation of leukemic blasts6,30 have indicated that ROS both decrease AML cell self-renewal in concert with promoting myeloid differentiation. Another recent study has shown that iron chelation treatment of AML cell lines and primary blasts increases ROS and induces myelomonocytic differentiation.8 Thus, the available evidence collectively indicates that AML cells have retained the capacity to overcome the block in differentiation by an ROS-dependent mechanism. Based on this line of reasoning, the targeting of MUC1-C and thereby increasing intracellular ROS could be exploited to induce AML cell differentiation. Of importance to such a therapeutic strategy is that the induction of differentiation is associated with loss of self-renewal. In this regard, inhibition of MUC1-C in AML cell lines and primary blasts resulted in both maturation along the myeloid lineage and loss of self-renewal, consistent with induction of terminal differentiation. Toxicity studies of GO-203 in mice, which share the highly conserved CQCRRKN motif in the MUC1-C cytoplasmic domain that is targeted by this agent, have not been associated with myelosuppression (S.K., D.K., unpublished data, 2010). Moreover, up-regulated expression of MUC1-C in myeloid lineage cells may be restricted to leukemic clones.9,10,31 Indeed, recent work has shown that MUC1 is selectively expressed in myeloid leukemia stem cells and not normal hematopoietic stem cells.32 Moreover, inhibition of MUC1-C suppresses growth of myeloid leukemia progenitors, but has little effect on expansion of normal hematopoietic cells.32 Thus, MUC1-C could represent a novel target for the treatment of AML with peptides or small molecule inhibitors that in turn increase ROS and thereby induce terminal differentiation. These and other findings have supported the approval of an Investigational New Drug application by the Food and Drug Administration to begin a phase 1 trial of GO-203 in patients with refractory solid tumors in early 2011, and subsequently a phase 1/2 trial in patients with AML.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by grants from the National Cancer Institute (CA42802), the Leukemia & Lymphoma Society, and the Multiple Myeloma Research Foundation.
National Institutes of Health
Authorship
Contribution: L.Y. performed research and analyzed data; Z.W. performed research; D.A, J.R., and R.S. contributed clinical samples; S.K. contributed vital new reagents; and D.K. designed the research and wrote the paper.
Conflict-of-interest disclosure: D.K. is an equity holder of and consultant to Genus Oncology. S.K. is an employee of Genus Oncology. The remaining authors declare no competing financial interests.
Correspondence: Donald Kufe, Dana-Farber Cancer Institute, Harvard Medical School, 450 Brookline Ave, Boston, MA 02215; e-mail: donald_kufe@dfci.harvard.edu.