Key Points
Deubiquitylating enzymes USP14 and UCHL5 are involved in the tumorigenesis of MM.
b-AP15 is a specific USP14 and UCHL5 inhibitor, which blocks growth and induces apoptosis in MM cells.
Abstract
Proteasome inhibitors have demonstrated that targeting protein degradation is effective therapy in multiple myeloma (MM). Here we show that deubiquitylating enzymes (DUBs) USP14 and UCHL5 are more highly expressed in MM cells than in normal plasma cells. USP14 and UCHL5 short interfering RNA knockdown decreases MM cell viability. A novel 19S regulatory particle inhibitor b-AP15 selectively blocks deubiquitylating activity of USP14 and UCHL5 without inhibiting proteasome activity. b-AP15 decreases viability in MM cell lines and patient MM cells, inhibits proliferation of MM cells even in the presence of bone marrow stroma cells, and overcomes bortezomib resistance. Anti-MM activity of b-AP15 is associated with growth arrest via downregulation of CDC25C, CDC2, and cyclin B1 as well as induction of caspase-dependent apoptosis and activation of unfolded protein response. In vivo studies using distinct human MM xenograft models show that b-AP15 is well tolerated, inhibits tumor growth, and prolongs survival. Combining b-AP15 with suberoylanilide hydroxamic acid, lenalidomide, or dexamethasone induces synergistic anti-MM activity. Our preclinical data showing efficacy of b-AP15 in MM disease models validates targeting DUBs in the ubiquitin proteasomal cascade to overcome proteasome inhibitor resistance and provides the framework for clinical evaluation of USP14/UCHL5 inhibitors to improve patient outcome in MM.
Introduction
The ubiquitin proteasome pathway is a validated therapeutic target in multiple myeloma (MM), evidenced by the US Food and Drug Administration approval of bortezomib and carfilzomib.1,2 Recent studies have focused on targeting enzymes that modulate protein ubiquitin conjugation/deconjugation upstream of the proteasome rather than the proteasome itself, with the goal of producing more specific, potent, and less toxic therapies targeting the ubiquitin proteasome pathway. Importantly, many human diseases are linked to dysfunction of ubiquitin ligases and/or deubiquitylating enzymes (DUBs), suggesting that inhibitors of ubiquitylating or DUB enzymes represent a potential therapeutic strategy.3,4
The human genome encodes approximately 100 putative DUBs, which are classified into five families: USP (ubiquitin-specific processing protease), UCH (ubiquitin C-terminal hydrolase), OTU (ovarian tumor ubiquitin), MJD (Josephin domain), and JAMM (Jab1/Mov34 metalloenzyme).5,6 Note that the first 4 families are cysteine proteases, whereas the fifth family consists of metalloproteases; to date, USP and UCH are the best characterized families.7 DUBs play a central role in regulating cellular processes, such as cell growth, proliferation, apoptosis, DNA repair, kinase activation, and transcription.8,9 In mammalian cells, three DUBs are associated with the proteasome: USP14, UCHL5/Uch37, and Rpn11.6,10-12 Both USP14 and UCHL5 reversibly associate with the 19S regulatory particle, whereas Rpn11 is an intrinsic subunit of the proteasome lid subcomplex of the 19S regulatory particle; therefore, modulating their functions may affect the proteasomal uptake of protein substrate for degradation. These DUBs have also been implicated in cancer. Screening for genetic abnormalities by using retroviral expression libraries and the 3T3 focus formation assay shows involvement of USP14 in ovarian carcinogenesis.13 A recent study shows that USP14 is highly expressed in colorectal cancer and correlates with pathologic stage as well as liver and lymph node metastases.14 Deubiquitylation of CXCR4 by USP14 is also crucial for both CXCR4 degradation and chemotaxis.15 UCLH5, like USP14, plays an important role in regulating oncogenic signaling.16 For example, transforming growth factor-β (TGF-β) is a critical regulator of cell proliferation, differentiation, and tumor pathogenesis; UCHL5 regulates TGF-β/Smad signaling by binding to intracellular Smad transcription factors, thereby allowing for deubiquitylation and stabilization of the TGF receptor.17 UCHL5/Rpn13 complex also regulates nuclear factor κB signaling pathway via interaction with inhibitor of κB.18 Additionally, knockdown of UCHL5 induces apoptosis, whereas overexpression of UCHL5 promotes cell proliferation in A549 cells. Finally, the clinical significance of UCHL5 was demonstrated in a recent analysis of tumor specimens from 111 patients with esophageal squamous cell carcinoma, showing a direct correlation between the elevated expression of UCHL5 and lymph node metastasis.19
A recent study described a small molecule inhibitor of USP14 and UCHL5.20 In contrast to the proteasome inhibitors, b-AP15 blocks the deubiquitylating activity of 19S regulatory particle–associated USP14 and UCHL5 without affecting proteolytic activities of the 20S core particle.20 Interestingly, b-AP15 triggers cancer cell apoptosis, regardless of TP53 status and BCL2 expression level, and inhibits tumor growth and metastasis in vivo.21 Given the therapeutic success of proteasome inhibition in MM, we attempted to examine whether b-AP15 blockade of USP14 and UCHL5 had anti-MM activity. Our in vitro and in vivo studies suggest that targeting DUBs can overcome proteasome inhibitor resistance, providing the basis for their clinical evaluation.
Materials and methods
Cell culture and reagents
Human MM cell lines MM.1S, MM.1R, KMS-11, ARP-1, RPMI 8226, DOX40, LR5, ANBL-6 (ANBL6.WT), and ANBL-6-bortezomib-resistant (ANBL-6.BR), solid tumor cell lines COLO320, LS174, and SHSY5Y, and peripheral blood mononuclear cells (PBMCs) from normal healthy donors were cultured in RPMI-1640 medium supplemented with complete medium (10% fetal bovine serum, 100 units/mL penicillin, 100 µg/mL streptomycin, and 2 mM l-glutamine) at 37°C and 5% CO2. Solid tumor cell lines DLD-1, 7860, and U2OS cells were cultured in Dulbecco’s modified Eagle medium supplemented with complete medium. Patient MM cells and bone marrow stromal cells (BMSCs) were prepared as described previously.22 Informed consent was obtained from all patients, in accordance with the Helsinki Protocol. b-AP15 was provided by Vivolux AB (Uppsala Science Park, Uppsala, Sweden); lenalidomide, bortezomib, and suberoylanilide hydroxamic acid (SAHA) were purchased from Selleck Chemicals LLC (Houston, TX); and dexamethasone was obtained from Calbiochem (San Diego, CA).
Western blotting
Cell lysis was prepared followed by sodium dodecyl sulfate polyacrylamide gel electrophoresis transferring and blocking as described previously.22 Immunoblotting was performed by using antibodies against poly ADP ribose polymerase (PARP) (BD Bioscience Pharmingen, San Diego, CA), caspase 8, caspase 9, caspase 3, cyclin B1, cyclin E1, cyclin D1, CDC25C, CDC2, p21, cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) (D46F1 rabbit monoclonal antibody; Cell Signaling, Beverly, MA), USP14 (Bethyl Laboratories), UCHL5, p-eIF2α (Abcam), XBP-1 (Santa Cruz Biotechnology), and p-IREα (Thermo Scientific). Blots were then developed by enhanced chemiluminescence (Amersham, Arlington Heights, IL).
Transient transfection
MM.1S cells were transiently transfected with genome control short interfering RNA (siRNA), USP14 siRNA, UCHL5 siRNA, or USP14 plus UCHL5 ON-TARGETplus SMARTpool siRNA (Dharmacon, Inc., Lafayette, CO) using the cell line Nucleofector Kit V (Amaxa Biosystems, Cologne, Germany). Cells were harvested 24 hours posttransfection, followed by analysis using both immunoblotting and cell viability assay.
Ub-VS labeling
MM.1S cells were treated with dimethylsulfoxide or b-AP15 (100 nM) for 3 hours, harvested, and lysed in lysis buffer (50 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid; pH 7.4], 250 mM sucrose, 10 mM MgCl2, 2 mM adenosine triphosphate, 1 mM dithiothreitol), followed by removal of debris by centrifugation. Total protein (25 μg) was labeled with 1 μM hemagglutinin [HA] -tagged ubiquitin-vinyl sulfone (HA-Ub-VS) probe for 30 minutes at 37°C and then subjected to western blotting.21
Analysis of proteasome and HtrA2/Omi activities
Proteasome activity assay was performed by using 20S Proteasome Assay Kit, SDS-Activated (Calbiochem), as previously described.1 High temperature requirement protein A2 (HtrA2)/Omi serine protease activity was determined by measuring cleavage of HtrA2/Omi substrate β-casein using an in vitro enzyme-based assay.22
Cytotoxicity and cell migration assays
Cell viability in MM cell lines, patient MM cells, and normal PBMCs was assessed as described previously.23 Migration was performed by using 24-well transwell plates (Millipore, Billerica, MA) in the presence of 10% fetal bovine serum or stromal-derived factor-1 (SDF-1), and migrating cells were quantified by measuring the intensity of fluorescence.22
Apoptosis and cell cycle analysis
MM.1S cells were treated with vehicle or b-AP15 (100 nM) for 24 hours and then stained with Annexin V-fluorescein isothiocyanate/PI (BD Biosciences, San Jose; Clontech, Mountain View, CA), followed by quantification of apoptotic cells using FACSCalibur (BD Biosciences). Caspase 8 and Caspase-9 Fluorometric Assay Kit (Enzo Life Sciences, Farmingdale, NY) was used to measure caspase activity. Cell cycle analysis was performed as described previously.21
Human plasmacytoma xenograft models
All experiments involving animals were approved by an institutional Animal Care and Use Committee. The subcutaneous MM.1S mouse xenograft model and the disseminated KMS-11 xenograft model were performed as previously described.23,24 b-AP15 (4 mg/kg) was injected for 14 consecutive days in the subcutaneous model. For the disseminated xenograft model, mice were treated 7 days postinjection with either vehicle or b-AP15 (4 mg/kg) for 10 consecutive days. Imaging was done on days 16, 24, and 32 using a Xenogen Imaging System.
Immunostaining
Statistical analysis
A Student t test was used to determine a significant difference, and P < .05 was considered statistically significant. Mouse survival was determined by using GraphPad Prism software (GraphPad Software, La Jolla, CA). Isobologram analysis was performed by using the CalcuSyn software program (Biosoft, Ferguson, MO, and Cambridge, United Kingdom). A combination index (CI) <1.0 indicates synergism, and CI = 1 indicates additive activity.
Results
USP14 and UCHL5 are highly expressed in patient MM cells and MM cell lines
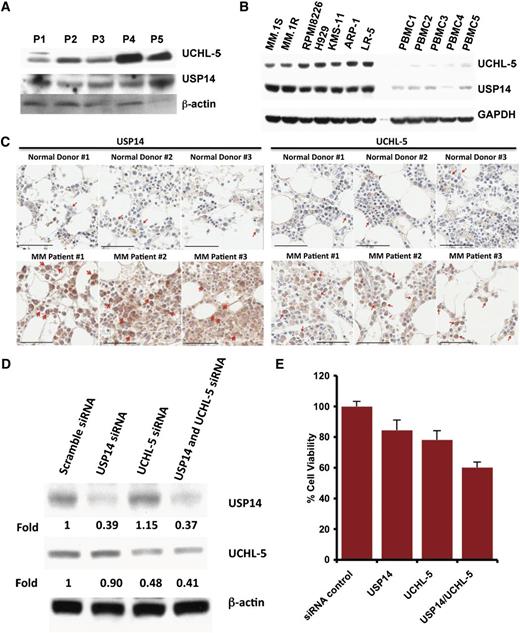
Examination of the expression of USP14 and UCHL5 in MM cells showed that both MM cell lines and patient cells expressed USP14 and UCHL5 (Figure 1A-B); notably, USP14 and UCHL5 expression is higher in MM cell lines than in normal PBMCs (Figure 1B). Similarly, immunostaining of bone marrow biopsies from MM patients and normal healthy donors showed that USP14 and UCHL5 are highly expressed in MM cells; in contrast, normal plasma cells lack detectable USP14 and UCHL5 expression (Figure 1C and supplemental Figure 1, available on the Blood Web site).
Characterization of 19S-associated deubiquitylating enzyme USP14 and UCHL5 in MM. (A) Purified CD138+ patient MM cells were lysed in protein lysis buffer and then subjected to immunoblotting with USP14 and UCHL5 antibodies. (B) MM cell lines and PBMCs from healthy donors were lysed in lysis buffer; lysates were then subjected to immunoblotting with USP14 and UCHL5 antibodies. Blots shown are representative of 3 independent experiments. (C) IHC analysis of bone marrow biopsies from normal donors and MM patients showing USP14 and UCHL5 expression. Scale bar = 60 µM. Red arrowheads indicate USP14 or UCHL5-positive cells (brown). (D) MM.1S cells were transfected with scramble siRNA, USP14 siRNA, UCHL5 siRNA, or USP14 plus UCHL5 siRNA by using the cell line Nucleofector Kit V. The cells were harvested 24 hours posttransfection and subjected to immunoblotting with USP14 and UCHL5 antibodies. (E) MM.1S cells were transfected with scramble siRNA, USP14 siRNA, UCHL5 siRNA, or both USP14 and UCHL5 siRNA, followed by viability analysis using a CellTiter-Glo assay. Percentage of cell viability was normalized to scramble siRNA control.
Characterization of 19S-associated deubiquitylating enzyme USP14 and UCHL5 in MM. (A) Purified CD138+ patient MM cells were lysed in protein lysis buffer and then subjected to immunoblotting with USP14 and UCHL5 antibodies. (B) MM cell lines and PBMCs from healthy donors were lysed in lysis buffer; lysates were then subjected to immunoblotting with USP14 and UCHL5 antibodies. Blots shown are representative of 3 independent experiments. (C) IHC analysis of bone marrow biopsies from normal donors and MM patients showing USP14 and UCHL5 expression. Scale bar = 60 µM. Red arrowheads indicate USP14 or UCHL5-positive cells (brown). (D) MM.1S cells were transfected with scramble siRNA, USP14 siRNA, UCHL5 siRNA, or USP14 plus UCHL5 siRNA by using the cell line Nucleofector Kit V. The cells were harvested 24 hours posttransfection and subjected to immunoblotting with USP14 and UCHL5 antibodies. (E) MM.1S cells were transfected with scramble siRNA, USP14 siRNA, UCHL5 siRNA, or both USP14 and UCHL5 siRNA, followed by viability analysis using a CellTiter-Glo assay. Percentage of cell viability was normalized to scramble siRNA control.
We next assessed the functional significance of USP14 and UCHL5 in MM cells by using siRNA strategy. USP14 and UCHL5 protein expression was markedly knocked down by USP14 and UCHL5 smart pool siRNAs compared with scrambled siRNA, as quantified by densitometric analysis (Figure 1D). Transfection of MM.1S cells with either USP14 siRNA or UCHL5 siRNA alone triggered cell death compared with scrambled siRNA transfected cells (Figure 1E). Importantly, simultaneous knockdown of USP14 and UCHL5 induced significantly greater cell death vs cells transfected with siRNA against either USP14 or UCHL5 (Figure 1E). Together, these data suggest a growth-promoting role for USP14 and UCHL5 in MM cells and provide a rationale for potential therapeutic targeting of USP14 and UCHL5 in MM.
b-AP15 inhibits USP14 and UCHL5 activity in MM cells
It is well established that inhibition of DUBs or proteasome causes accumulation of ubiquitinated proteins.25 Our result showed that treatment of MM.1S cells with b-AP15 (100 nM), like bortezomib, induced time-dependent accumulation of ubiquitinated proteins (Figure 2A). The disappearance of b-AP15 activity on poly-ubiquitination at later time points might be due to the onset of cell-cycle arrest and apoptosis. Additionally, b-AP15 inhibited the activity of both USP14 and UCHL5 as evident by decreased Ub-VS labeling when compared with mock vehicle–treated MM.1S cells (Figure 2B). While b-AP15 blocks the deubiquitylating activity of USP14 and UCHL5, it does not inhibit proteasome activity. Specifically, treatment of MM.1S cells with b-AP15 for 3 hours did not affect chymotrypsin-like, trypsin-like, or caspase-like proteasome activities (Figure 2C). The proteasome inhibitor bortezomib served as a control for chymotrypsin-like activity inhibitor (Figure 2C). To further confirm b-AP15–specific activity against USP14 and UCHL5, we examined the effect of b-AP15 on other DUBs such as USP7 or UCHL3 by using additional MM cell lines RPMI-8226 and KMS-11. No inhibition of total DUBs or proteasome proteolytic activity in b-AP15–treated MM cells was observed (supplemental Figure 2A-B). In addition, b-AP15 significantly inhibited both USP14 and UCHL5, whereas no inhibition of USP7 or UCHL3 was noted (supplemental Figure 2C-D). Furthermore, there was no inhibition in HA-Ub-VS binding in protein lysates from b-AP15–treated MM cells (supplemental Figure 2E). Together, our results show that b-AP15 potently inhibits USP14 and UCHL5 without affecting enzymatic activity of other DUBs.
b-AP15 targets USP14 and UCHL5 in MM cells. (A) MM.1S cells were treated with dimethylsulfoxide (DMSO) or b-AP15 (100 nM) for indicated times or treated with bortezomib for 3 hours; protein lysates were subjected to immunoblotting with anti-polyubiquitin or anti-actin antibodies. (B) MM.1S cells were treated with DMSO or b-AP15 for 3 hours; cells were lysed in lysis buffer and total protein was labeled with HA-Ub-VS probe and then subjected to immunoblotting with USP14 and UCHL5 antibodies. (C) MM.1S cells were treated with DMSO, b-AP15, or bortezomib for 3 hours; protein lysates were analyzed for proteasome activity. The percentage of proteasome activity was normalized to DMSO control (mean ± standard deviation [SD], n = 3). (D) Recombinant human HtrA2 enzyme was incubated with its substrate β-casein in assay buffer, followed by sodium dodecyl sulfate polyacrylamide gel electrophoresis, silver staining, and quantification of cleaved β-casein. The bar graph represents the percentage of HtrA2-induced β-casein cleavage in the presence of bortezomib (3 nmol/L) or b-AP15 (100 nmol/L) vs DMSO control. Data represent means ± SD (n = 2; P < .05 for bortezomib). CT-L, chymotrypsin-like; T-L, trypsin-like; C-L, caspase-like.
b-AP15 targets USP14 and UCHL5 in MM cells. (A) MM.1S cells were treated with dimethylsulfoxide (DMSO) or b-AP15 (100 nM) for indicated times or treated with bortezomib for 3 hours; protein lysates were subjected to immunoblotting with anti-polyubiquitin or anti-actin antibodies. (B) MM.1S cells were treated with DMSO or b-AP15 for 3 hours; cells were lysed in lysis buffer and total protein was labeled with HA-Ub-VS probe and then subjected to immunoblotting with USP14 and UCHL5 antibodies. (C) MM.1S cells were treated with DMSO, b-AP15, or bortezomib for 3 hours; protein lysates were analyzed for proteasome activity. The percentage of proteasome activity was normalized to DMSO control (mean ± standard deviation [SD], n = 3). (D) Recombinant human HtrA2 enzyme was incubated with its substrate β-casein in assay buffer, followed by sodium dodecyl sulfate polyacrylamide gel electrophoresis, silver staining, and quantification of cleaved β-casein. The bar graph represents the percentage of HtrA2-induced β-casein cleavage in the presence of bortezomib (3 nmol/L) or b-AP15 (100 nmol/L) vs DMSO control. Data represent means ± SD (n = 2; P < .05 for bortezomib). CT-L, chymotrypsin-like; T-L, trypsin-like; C-L, caspase-like.
Anti-MM activity of b-AP15 in vitro
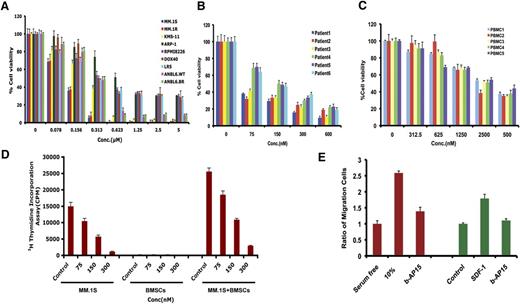
Various human MM cell lines, including drug-resistant cell lines, were treated with b-AP15 for 48 hours, followed by assessment for cell viability. A significant concentration-dependent decrease in viability of all MM cell lines was observed in response to treatment with b-AP15 (Figure 3A). b-AP15 showed 50% inhibitory concentration (IC50) in drug-resistant–derived cell lines similar to that in parental isogenic cell lines. Interestingly, a potent antitumor activity of b-AP15 was also observed in solid tumor cell lines (supplemental Figure 3). b-AP15 also significantly decreased the cell viability of all primary MM cells from patients, including those who had relapsed after multiple prior therapies with lenalidomide, dexamethasone, or bortezomib (Figure 3B). It is worth noting that b-AP15 at IC50 for MM cells does not significantly affect the viability of normal PBMCs (Figure 3C).
Anti-MM activity of b-AP15. (A) MM cell lines were treated with DMSO or b-AP15 at different concentrations for 48 hours, followed by measurement of cell viability with 3-(4,5-dimethylthiazol-2-yl)-2,5 diphenyl tetrazolium bromide (MTT) assay. (B) Purified CD138+ patient MM cells were treated with DMSO or b-AP15 for 24 hours, followed by assessment of viability using CellTiter-Glo assay. (C) PBMCs from healthy donors were treated with DMSO or b-AP15 for 48 hours, and cell viability was measured by CellTiter-Glo assay. (D) MM.1S cells were cultured alone or with BMSCs for 48 hours in the presence or absence of b-AP15, and DNA synthesis was measured by 3H-TdR (tritiated thymidine) uptake. (E) MM.1S cells were pretreated with DMSO or b-AP15 overnight and then allowed to migrate for 4 hours in a transwell plate in the presence or absence of 10% serum or SDF-1. Migrating cells were detached and quantified by fluorescence (mean ± SD; n = 3; P < .001).
Anti-MM activity of b-AP15. (A) MM cell lines were treated with DMSO or b-AP15 at different concentrations for 48 hours, followed by measurement of cell viability with 3-(4,5-dimethylthiazol-2-yl)-2,5 diphenyl tetrazolium bromide (MTT) assay. (B) Purified CD138+ patient MM cells were treated with DMSO or b-AP15 for 24 hours, followed by assessment of viability using CellTiter-Glo assay. (C) PBMCs from healthy donors were treated with DMSO or b-AP15 for 48 hours, and cell viability was measured by CellTiter-Glo assay. (D) MM.1S cells were cultured alone or with BMSCs for 48 hours in the presence or absence of b-AP15, and DNA synthesis was measured by 3H-TdR (tritiated thymidine) uptake. (E) MM.1S cells were pretreated with DMSO or b-AP15 overnight and then allowed to migrate for 4 hours in a transwell plate in the presence or absence of 10% serum or SDF-1. Migrating cells were detached and quantified by fluorescence (mean ± SD; n = 3; P < .001).
BMSCs confer a cytoprotective effect to MM cells. In our results, significant inhibition of BMSC-induced proliferation of MM.1S cells was noted in response to b-AP15 treatment (Figure 3D).
SDF-1 binds to G-protein–coupled CXCR4, the known substrate of USP14,15 and most important, CXCR4 is implicated in MM metastasis.26 We therefore examined serum or SDF-1–induced MM cell migration. Serum or SDF-1 alone increased MM.1S cell migration; conversely, b-AP15 inhibits serum or SDF-1–dependent migration (Figure 3E). These cells were >95% viable prior to and after performing the migration assay.
b-AP15 induces apoptosis and cell cycle arrest in MM cells
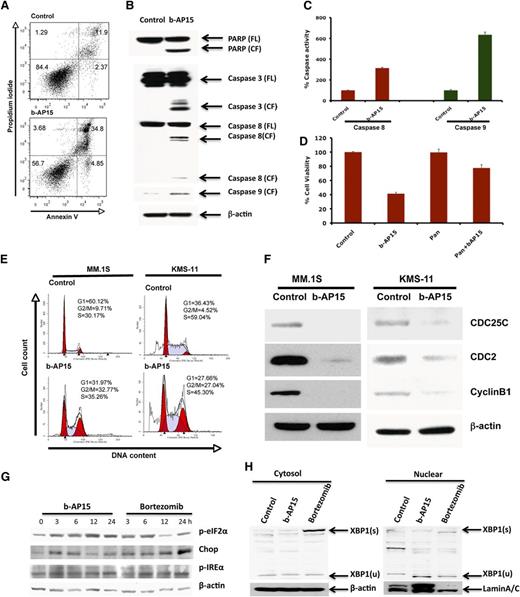
To further understand the mechanism of action underlying b-AP15–induced MM cell death, MM.1S cells were treated with b-AP15 (100 nM) for 12 hours, and then stained with Annexin V/PI. b-AP15 triggered a significant increase in both early (Annexin V+/PI−) and late (Annexin V+/PI+) apoptotic cell populations (Figure 4A). Moreover, b-AP15 markedly increased the cleavage of PARP, a hallmark of apoptosis. We next examined whether b-AP15 affects the intrinsic (via caspase 9) and extrinsic (via caspase 8) apoptotic signaling pathways. Our data show that b-AP15 activates caspase 8 and caspase 9 followed by activation of caspase 3 (Figure 4B). Additionally, b-AP15 induced caspase 8 and caspase 9 enzymatic activity, as determined by using enzyme-linked immunosorbent assay (Figure 4C). Importantly, blockade of caspases by pan-caspase inhibitor significantly attenuated b-AP15–triggered MM cell death (Figure 4D).
Mechanisms of b-AP15–induced MM cell death. (A) MM.1S cells were treated with DMSO or b-AP15 (100 nM) for 24 hours, followed by analysis for apoptosis with Annexin V/PI double staining (mean ± SD; n = 3; P < .001). (B) MM.1S cells were treated with DMSO or b-AP15 for 12 hours; protein lysates were subjected to immunoblotting with anti-PARP, caspase 3, caspase 8, or caspase 9 antibodies. (C) MM.1S cells were treated with b-AP15 (100 nM) for 12 hours, followed by measurement of caspase 8 and caspase 9 enzymatic activity (mean ± SD; n = 3; P < .001). (D) MM.1S cells were incubated with or without pan-caspase inhibitor for 1 hour and then treated with DMSO or b-AP15 (150 nM) for an additional 24 hours, followed by assessment of cell viability using MTT assay (mean ± SD; n = 3; P < .005 for b-AP15 alone vs b-AP15 plus pan-caspase inhibitor). (E) MM.1S and KMS-11 cells were treated with DMSO or b-AP15 (100 nM) for 48 hours and fixed in 70% ethanol. After washing with phosphate-buffered saline, cells were stained with PI, and DNA content of cells was analyzed using fluorescence-activated cell sorter. (F) MM.1S and KMS-11 cells were treated with DMSO or b-AP15 (100 nM) for 24 hours; protein lysates were subjected to immunoblotting with anti-CDC25C, CDC2, cyclin B1, or β-actin antibodies. (G) MM.1S MM cells were treated with DMSO or b-AP15 (100 nM) for the indicated times; protein lysates were subjected to immunoblotting with anti–p-eIF2α, p-IREα, CHOP, or β-actin antibodies. (H) MM.1S MM cells were treated with DMSO or b-AP15 (100 nM) for 24 hours; cytosolic and nuclear proteins were prepared with NE-PERNuclear and Cytoplasmic Extraction Reagents (Thermo Scientific, Rockford, IL) and subjected to immunoblotting with anti–XBP-1 and Lamin A/C antibodies. FL, full length of the protein; CF, cleaved form of the protein; s, splicing form; u, unsplicing form.
Mechanisms of b-AP15–induced MM cell death. (A) MM.1S cells were treated with DMSO or b-AP15 (100 nM) for 24 hours, followed by analysis for apoptosis with Annexin V/PI double staining (mean ± SD; n = 3; P < .001). (B) MM.1S cells were treated with DMSO or b-AP15 for 12 hours; protein lysates were subjected to immunoblotting with anti-PARP, caspase 3, caspase 8, or caspase 9 antibodies. (C) MM.1S cells were treated with b-AP15 (100 nM) for 12 hours, followed by measurement of caspase 8 and caspase 9 enzymatic activity (mean ± SD; n = 3; P < .001). (D) MM.1S cells were incubated with or without pan-caspase inhibitor for 1 hour and then treated with DMSO or b-AP15 (150 nM) for an additional 24 hours, followed by assessment of cell viability using MTT assay (mean ± SD; n = 3; P < .005 for b-AP15 alone vs b-AP15 plus pan-caspase inhibitor). (E) MM.1S and KMS-11 cells were treated with DMSO or b-AP15 (100 nM) for 48 hours and fixed in 70% ethanol. After washing with phosphate-buffered saline, cells were stained with PI, and DNA content of cells was analyzed using fluorescence-activated cell sorter. (F) MM.1S and KMS-11 cells were treated with DMSO or b-AP15 (100 nM) for 24 hours; protein lysates were subjected to immunoblotting with anti-CDC25C, CDC2, cyclin B1, or β-actin antibodies. (G) MM.1S MM cells were treated with DMSO or b-AP15 (100 nM) for the indicated times; protein lysates were subjected to immunoblotting with anti–p-eIF2α, p-IREα, CHOP, or β-actin antibodies. (H) MM.1S MM cells were treated with DMSO or b-AP15 (100 nM) for 24 hours; cytosolic and nuclear proteins were prepared with NE-PERNuclear and Cytoplasmic Extraction Reagents (Thermo Scientific, Rockford, IL) and subjected to immunoblotting with anti–XBP-1 and Lamin A/C antibodies. FL, full length of the protein; CF, cleaved form of the protein; s, splicing form; u, unsplicing form.
Recent studies indicate that DUBs play a pivotal role in the regulation of the cell cycle.7 We therefore examined the effect of b-AP15 on cell cycle profile by using 2 distinct MM cell lines—MM.1S and KMS-11. b-AP15 induced significant G2/M phase growth arrest in both cell lines (Figure 4E). In accordance with these findings, b-AP15 treatment decreased G2/M phase cell cycle regulatory proteins cdc25c and its downstream protein cdc2, as well as cyclin B1 (Figure 4F). We also examined the effect of b-AP15 on G1/S phase marker proteins including cyclin E1, cyclin D1, and p21. Results showed that b-AP15 induced p21 without any significant change in cyclin E1 or cyclin D1 (supplemental Figure 4A).
b-AP15 triggers endoplasmic reticulum stress response signaling in MM cells
Accumulation of ubiquitylated proteins (misfolded/unfolded) induces unfolded protein response (UPR) and apoptosis.25 A head-to-head experiment comparing the effect of b-AP15 and bortezomib on endoplasmic reticulum (ER) stress response showed that treatment of MM.1S with b-AP15 or bortezomib activates PERK and IRE1-mediated ER stress signaling, evidenced by significant induction of p-IREα and p-eIF2α (Figure 4G). XBP-1 plays an important role in MM pathogenesis and drug resistance.27 Although we observed the induction of the splicing form of XBP-1, XBP-1(s) with bortezomib treatment, no induction of XBP-1(s) was detected with treatment with b-AP15 (Figure 4H). We did not detect the active form of ATF6 in cells treated with either b-AP15 or bortezomib (data not shown). Additionally, b-AP15 and bortezomib triggered induction of CHOP (Figure 4E). We next compared the effect of b-AP15 vs bortezomib on induced ER stress response signaling by using ANBL6.WT and ANBL6.BR cell lines. b-AP15 upregulated p-eIF2α and CHOP in both ANBL6.WT and ANBL6.BR cells (supplemental Figure 4B). Bortezomib-induced cytotoxicity in ANBL6.WT correlated with induction of p-eIF2α and CHOP. In contrast, treatment of ANBL6.BR with IC50 for ANBL6.WT triggered no significant decrease in viability, but it upregulated p-eIF2α without altering CHOP expression (supplemental Figure 4B). No basal-level expression change in p-eIF2α and CHOP expression was noted in ANBL6.WT vs the ANBL6.BR cell line.
Anti-MM activity of b-AP15 in MM xenograft mouse models
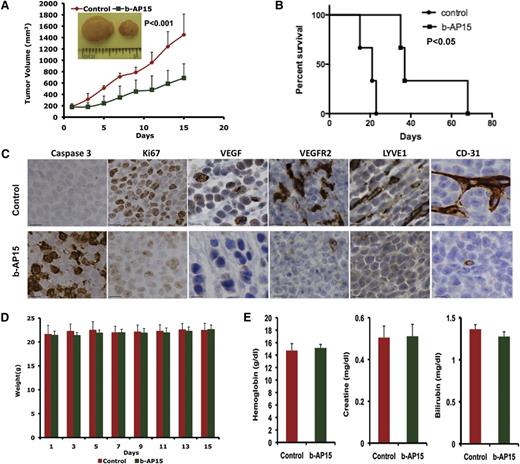
Having shown that b-AP15 induced apoptosis in MM cells in vitro, we next examined the in vivo efficacy of b-AP15 by using two distinct MM xenograft mouse models. A marked reduction in tumor growth as well as prolongation of survival was noted in b-AP15–treated mice vs mice receiving vehicle alone (Figure 5A-B). Mechanistic study using immunohistochemical (IHC) staining showed a decrease in proliferation marker Ki67 and an increase in the number of caspase 3 cleavage–positive cells in tumors excised from mice receiving b-AP15 (Figure 5C). Vascular endothelial growth factor (VEGF) and VEGF receptor 2 (VEGFR2) play a central role in tumor angiogenesis, and we therefore examined the expression of VEGF and VEGFR2 as well as other tumor growth–associated angiogenesis markers LYVE1 and CD31 in control vs b-AP15–treated mouse tumors. A marked reduction in VEGF, VEGFR2, LYVE1 and CD31 expression was observed in tumors from b-AP15–treated mice vs mice receiving vehicle alone (Figure 5C). Moreover, the doses of b-AP15 administered were well tolerated by mice, since no significant weight loss was noted in the study (Figure 5D). As seen in Figure 5E, b-AP15–treated mice retained normal creatinine, hemoglobin, and bilirubin levels.
b-AP15 inhibits tumor growth and prolongs survival in xenograft mouse model. MM.1S cells (5 × 106) were subcutaneously inoculated into mouse hind flank region. On days 21 to 23, when the tumors reached 150 to 200 mm3, mice were randomized to control or b-AP15 treatment groups (7 mice in each group) and treated with vehicle control or b-AP15 (4 mg/kg) for 14 consecutive days. (A) Tumor volume was measured every other day. Data shown are mean ± SD (n = 7; P < .001). (B) Mice were euthanized when tumor volume reached 2000 mm3, and survival is shown in a Kaplan-Meier plot (P < .05). (C) Tumor sections from control and b-AP15–treated mice were immunostained with anti–caspase 3, Ki67, VEGF, VEGFR2, LYVE1, or CD31 antibodies. Scale bar = 10 μM. (D) Mouse body weight was measured every other day. Data shown are mean ± SD. (E) Hemoglobin, creatinine, and bilirubin from mouse serum were measured at the end of treatment. Data shown are mean ± SD.
b-AP15 inhibits tumor growth and prolongs survival in xenograft mouse model. MM.1S cells (5 × 106) were subcutaneously inoculated into mouse hind flank region. On days 21 to 23, when the tumors reached 150 to 200 mm3, mice were randomized to control or b-AP15 treatment groups (7 mice in each group) and treated with vehicle control or b-AP15 (4 mg/kg) for 14 consecutive days. (A) Tumor volume was measured every other day. Data shown are mean ± SD (n = 7; P < .001). (B) Mice were euthanized when tumor volume reached 2000 mm3, and survival is shown in a Kaplan-Meier plot (P < .05). (C) Tumor sections from control and b-AP15–treated mice were immunostained with anti–caspase 3, Ki67, VEGF, VEGFR2, LYVE1, or CD31 antibodies. Scale bar = 10 μM. (D) Mouse body weight was measured every other day. Data shown are mean ± SD. (E) Hemoglobin, creatinine, and bilirubin from mouse serum were measured at the end of treatment. Data shown are mean ± SD.
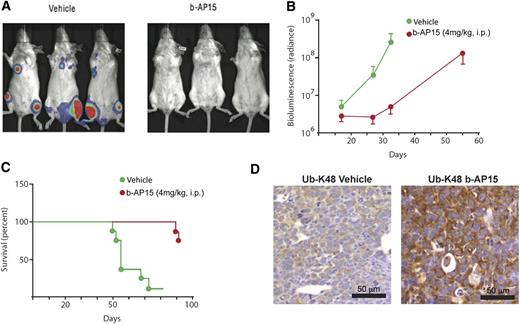
To further validate the in vivo anti-MM activity of b-AP15, we used a KMS-11 disseminated MM xenograft mouse model. Treatment of KMS-11–bearing mice with b-AP15 significantly decreased the tumor burden of the mice (Figure 6A); of note, only 2 of 8 mice receiving b-AP15 treatment showed signs of disease. As shown in Figure 6B, a significant decrease in bioluminescence intensity was observed in b-AP15– vs vehicle-treated mice at day 32. Furthermore, b-AP15 treatment dramatically prolonged survival, with the median survival extending from 55 to >93 days (Figure 6C). IHC analysis in tumor tissues showed that b-AP15 enhanced ubiquitination in tumor cells in vivo. Specifically, the K48-linked ubiquitination was clearly evident in tumor sections from b-AP15–treated mice vs the vehicle control group (Figure 6D). Taken together, our findings using two distinct murine xenograft models of human MM show potent in vivo antitumor activity of b-AP15 at doses that are well tolerated.
b-AP15 reduces tumor burden and prolongs survival in disseminated KMS-11 MM xenograft model. b-AP15 was dissolved in Cremophor EL/polyethylene glycol 400 (1:1) to a stock concentration of 8 mg/mL, which was diluted in saline immediately before injection. KMS-11-LUC2 cells (5 × 106) were injected intravenously in female SCID mice. After being injected for 7 days, mice were randomized into control and b-AP15 treatment groups (8 mice per group). Mice were treated with vehicle or b-AP15 (4 mg/kg) for 10 consecutive days. (A) The representative imaging was taken at day 32 by using a charge-coupled device (CCD camera, Xenogen IVIS Lumina System). (B) The bioluminescence in the animal was measured at days 16, 24, and 32 of tumor cell injection. Signal intensity was quantified by using IGOR Pro version 4.09A Software (WaveMetrics, Inc., Lake Oswego, OR). The effect of the treatments was expressed as percentage of radiance inhibition with respect to control group values (P < .001). (C) Mice were sacrificed when paralysis was observed, and the number of days of survival is shown in a Kaplan-Meier plot (P < .001). (D) Tumor sections from control and b-AP15–treated mice were immunostained with anti–Ub-K48 antibody. Scale bar = 50 μM.
b-AP15 reduces tumor burden and prolongs survival in disseminated KMS-11 MM xenograft model. b-AP15 was dissolved in Cremophor EL/polyethylene glycol 400 (1:1) to a stock concentration of 8 mg/mL, which was diluted in saline immediately before injection. KMS-11-LUC2 cells (5 × 106) were injected intravenously in female SCID mice. After being injected for 7 days, mice were randomized into control and b-AP15 treatment groups (8 mice per group). Mice were treated with vehicle or b-AP15 (4 mg/kg) for 10 consecutive days. (A) The representative imaging was taken at day 32 by using a charge-coupled device (CCD camera, Xenogen IVIS Lumina System). (B) The bioluminescence in the animal was measured at days 16, 24, and 32 of tumor cell injection. Signal intensity was quantified by using IGOR Pro version 4.09A Software (WaveMetrics, Inc., Lake Oswego, OR). The effect of the treatments was expressed as percentage of radiance inhibition with respect to control group values (P < .001). (C) Mice were sacrificed when paralysis was observed, and the number of days of survival is shown in a Kaplan-Meier plot (P < .001). (D) Tumor sections from control and b-AP15–treated mice were immunostained with anti–Ub-K48 antibody. Scale bar = 50 μM.
Combining b-AP15 with SAHA, lenalidomide, or deexamethasone triggers synergistic anti-MM activity
Preclinical and clinical studies showed that combining anti-MM agents can enhance cytotoxicity, reduce toxicity, and overcome drug resistance. Therefore, we next examined whether b-AP15 can be combined with other drugs to enhance cytotoxicity. MM.1S cells were treated with b-AP15 and SAHA, lenalidomide, or dexamethasone across a range of concentrations for 24 hours and then analyzed for viability. An analysis of synergistic anti-MM activity demonstrated that the combination of low concentrations of b-AP15 and lenalidomide, histone deacetylase (HDAC) inhibitor SAHA, or dexamethasone triggered synergistic anti-MM activity, with a CI <1.0 (Figure 7A-C).
Combination of b-AP15 with other anti-MM agents shows additive to synergistic effect in MM. (A) MM.1S cells were treated for 48 hours with b-AP15, SAHA, or b-AP15 plus SAHA and then assessed for viability by using the MTT assay. Isobologram analysis shows the synergistic cytotoxic effect of b-AP15 and SAHA. The graphs (right panel) are derived from the values given in the tables (left panel). CI <1 indicates synergy. (B) MM.1S cells were treated for 48 hours with b-AP15, lenalidomide, or b-AP15 plus lenalidomide and then assessed for viability by using an MTT assay. Synergistic anti-MM activity was analyzed as in panel (A). (C) MM.1S cells were treated for 48 hours with b-AP15, dexamethasone, or b-AP15 plus dexamethasone and then assessed for viability by using an MTT assay. Synergistic anti-MM activity was analyzed as in (A). Fa, fraction of viable cells.
Combination of b-AP15 with other anti-MM agents shows additive to synergistic effect in MM. (A) MM.1S cells were treated for 48 hours with b-AP15, SAHA, or b-AP15 plus SAHA and then assessed for viability by using the MTT assay. Isobologram analysis shows the synergistic cytotoxic effect of b-AP15 and SAHA. The graphs (right panel) are derived from the values given in the tables (left panel). CI <1 indicates synergy. (B) MM.1S cells were treated for 48 hours with b-AP15, lenalidomide, or b-AP15 plus lenalidomide and then assessed for viability by using an MTT assay. Synergistic anti-MM activity was analyzed as in panel (A). (C) MM.1S cells were treated for 48 hours with b-AP15, dexamethasone, or b-AP15 plus dexamethasone and then assessed for viability by using an MTT assay. Synergistic anti-MM activity was analyzed as in (A). Fa, fraction of viable cells.
Discussion
Recent reports have identified several DUBs,7,16,28 and elucidation of their structures and functional roles in tumorigenesis has provided the impetus for validating them as novel therapeutic targets in cancer.29-31 Although USP14 and UCHL5 have been implicated in the tumorigenesis and progression of colorectal cancer and esophageal squamous cell carcinoma,14,19 their role in MM is undefined. Consistent with previous studies,15,16,21 we found that USP14 and UCHL5 are highly expressed in MM cells vs normal plasma cells and PBMCs, suggesting a role of USP14 and UCHL5 in MM pathogenesis. Most notably, knockdown of USP14 and UCHL5 decreased MM cell viability, indicating involvement of USP14 and UCHL5 in MM growth and survival.
A recent study identified b-AP15, a novel small molecule inhibitor of USP14 and UCHL5.21 Here, we examined the anti-MM activity of b-AP15 by using our in vitro and in vivo models of MM. b-AP15 induced a marked increase in ubiquitylated proteins and inhibited the labeling of USP14 and UCHL5 with HA-Ub-VS. No difference in the labeling of other DUBs with Ub-VS probe was observed, confirming that b-AP15 is a selective DUB inhibitor. In addition, a previous study found that treatment with b-AP15 achieved the free ubiquitin pool decreases to an extent similar to that of bortezomib in colon carcinoma and melanoma cells, and overexpression of K48 and WT ubiquitin chains only slightly changed the apoptotic activity vs control.21 These data suggest that although depletion of the free ubiquitin pool is likely to be a factor, overexpression of ubiquitin does not appear to reverse this process.21 Moreover, b-AP15 triggered no alteration in the proteolytic activities of the proteasome and total DUB activity. Together, these data demonstrate that b-AP15 targets the deubiquitylating function of USP14 and UCHL5 in the cellular environment.
Peripheral neuropathy associated with bortezomib therapy is partly the result of blockade of neuronal cell survival protease HtrA2/Omi.2 In this study, we found no significant inhibition of HtrA2/Omi in response to b-AP15 treatment, whereas bortezomib inhibited HtrA2/Omi activity. These data highlight another distinction between bortezomib and b-AP15 and further indicate the selectivity of b-AP15.
We next characterized the mechanism underlying anti-MM activity of b-AP15. We showed that b-AP15 decreased viability of MM cell lines and primary patient tumor cells without markedly affecting the normal viability of PBMCs, suggesting selective anti-MM activity and a favorable therapeutic index for b-AP15. The difference of b-AP15 IC50 in MM cell lines may be due to the genetic heterogeneity characteristics of MM.23,32 Moreover, b-AP15 decreased the viability of both ANBL-6.WT and ANBL-6.BR MM cells, confirming the ability of b-AP15 to overcome bortezomib resistance. We found similar responses to b-AP15 in tumor cells from patients in whom MM was resistant to therapies such as bortezomib, lenalidomide, and dexamethasone.
It is well established that BMSCs confer resistance to anti-MM agents via cell adhesion–mediated drug resistance. In our study, b-AP15 inhibits BMSC-induced proliferation of MM.1S cells in a concentration-dependent fashion, suggesting that b-AP15 has the ability to overcome cell adhesion–mediated drug resistance.
DUBs play a role in diverse signaling pathways including apoptosis and cell cycle progression.5,9,33 Cell cycle is tightly regulated by numerous cyclins, cyclin-dependent kinases, and checkpoint proteins which, in turn, are degraded in a timely manner by the ubiquitin proteasome signaling pathway. For example, CDC25C dephosphorylates cyclin B–bound CDC2 and triggers entry into mitosis.34 Moreover, CDC25C and cyclin B are degraded through a proteasome-mediated degradation pathway.9,35,36 Prior studies have established that p21 mediates growth arrest in different cell-cycle phases. Activation of p21 triggers inhibition of CDK1-cyclin B1 kinase activity which, in turn, blocks the progression of cells through G2 or G2/M.37 Our results show that b-AP15 induces G2/M phase cell cycle arrests, downregulates CDC25C, CDC2, and cyclin B1, and upregulates p21 in MM cells. The mechanism of b-AP15–mediated depletion of cyclin B1, CDC2, and CDC25C is unclear, but it is likely due to enhanced degradation of proteasome-regulated cyclins.
Our mechanistic study shows that b-AP15 triggers apoptosis in MM cells via activation of caspases, and addition of a pan-caspase inhibitor attenuates b-AP15–induced MM cell death. We also found that both b-AP15 and bortezomib triggered UPR, evidenced by induction of p-IREα, p-eIF2α, and CHOP. We did not detect an active form of ATF6 in either b-AP15– or bortezomib-treated cells. However, in contrast to bortezomib, b-AP15 did not affect XBP-1 splicing. It is possible that b-AP15 activates the IRE1/JNK pathway instead of the IRE1/XBP-1 pathway,38 and this remains to be further examined. Nonetheless, these data show that both b-AP15 and bortezomib trigger ER stress signaling in MM.1S cells, albeit with some distinction.
Bortezomib-induced cytotoxicity in ANBL6.WT correlated with induction of p-eIF2a and CHOP. In contrast, treatment of ANBL6.BR with IC50 of bortezomib for ANBL6.WT triggered no significant decrease in viability, but it upregulated p-eIF2α without altering CHOP (supplemental Figure 4). These data suggest that bortezomib triggered a weaker UPR in ANBL6.BR compared with ANBL6.WT cells. Our data are consistent with a previous report showing that a weak UPR induction can be resolved by induction of p-eIF2α via protein synthesis inhibition; this may prevent both CHOP upregulation and apoptosis.39 Together, our data suggest that b-AP15–triggered apoptosis in MM cells occurs in a caspase-dependent manner and involves induction of UPR signaling pathways.
In addition to our in vitro studies, we also examined anti-MM activity of b-AP15 in vivo. A marked growth inhibitory effect of b-AP15 was observed in two distinct human MM xenograft mouse models. Our data show that b-AP15 is well tolerated, inhibits tumor growth, and prolongs survival in mice. These findings are consistent with the reported antitumor activity of b-AP15 in solid tumor xenograft models.21 In addition, b-AP15 treatment was not associated with any significant toxicity, since no differences in body weight and/or alteration in blood chemistry were noted. The remarkable anti-MM activity of b-AP15 in vivo was confirmed by IHC analysis for apoptosis (caspase 3 cleavage), proliferation (Ki67), and associated angiogenesis (VEGF, VEGFR2, LYVE1, and CD31) of tumor sections from tumors harvested from control and b-AP15–treated mice. In agreement with our in vitro data, analysis of tumors excised from mice showed accumulation of K48-linked polyubiquitylated proteins in b-AP15–treated mice but not in mice treated with vehicle, reflecting blockade of proteasome function in tumor cells. Together, these data demonstrated a dual effect of b-AP15: increased MM cell apoptosis and decreased angiogenesis.
Finally, we also examined whether b-AP15 can be combined with other conventional (dexamethasone) and novel (SAHA, lenalidomide) anti-MM agents. Besides proteasomal degradation, intracellular protein homeostasis also regulated via an HDAC-dependent aggressome-autophagic signaling pathway. Blockade of both mechanisms of protein catabolism by combining bortezomib and HDAC inhibitor induces significant cytotoxicity in MM cells. Consistent with these findings, our data show synergistic anti-MM activity of b-AP15 with HDAC inhibitor SAHA. We also found that b-AP15 adds to the anti-MM activity of dexamethasone and lenalidomide. Together, these data confirm the potential clinical benefit of combining DUB inhibitors with HDAC inhibitors, lenalidomide, or dexamethasone.
Collectively, our preclinical studies therefore demonstrate potent in vitro and in vivo anti-MM activity of b-AP15 at doses that are well tolerated in human MM xenograft mouse models. These findings provide the proof of concept for evaluation of USP14/UCHL5 DUB inhibitors, alone and in combination, as potential therapy to improve patient outcome in MM.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
ANBL6.WT and ANBL6.BR cells were kindly provided by Dr Robert Orlowski (MD Anderson Cancer Center, Houston, TX).
This work was supported by National Institutes of Health Specialized Programs of Research Excellence (SPORE) grants P50100707, PO1-CA078378, and RO1 CA050947. K.C.A. is an American Cancer Society Clinical Research Professor (ACS grant CRP-10-198-01-LIB). Funding was also provided by Cancerfonden (S.L.), Vetenskapsrådet (S.L.), Alex and Eva Wallströms Foundation (P.D.), and Åke Olssons Foundation (P.D.).
Authorship
Contribution: Z.T. designed the research, performed the experiments, and wrote the manuscript; P.D. and X.W. helped with some experiments; A.R. helped with cell culture; Y.-T.T. and P.R. provided patient samples; Y.H. helped with animal experiments; R.D.C. performed immunohistochemistry in bone marrow biopsy from patients and healthy donors; S.L. wrote the manuscript; D.C. designed the research and wrote the manuscript; and K.C.A. wrote the manuscript.
Conflict-of-interest disclosure: K.C.A. is on the advisory boards of Celgene, Onyx Pharmaceuticals, Gilead, and Sanofi-Aventis; he is a scientific founder of Acetylon Pharmaceuticals and Oncoprep. S.L. owns shares in Vivolux, and D.C. is a consultant to Vivolux. The remaining authors declare no competing financial interests.
Correspondence: Dharminder Chauhan, Dana-Farber Cancer Institute, M561, 450 Brookline Ave, Boston, MA; e-mail: dharminder_chauhan@dfci.harvard.edu.
References
Author notes
D.C. and K.C.A. are senior coauthors.

![Figure 2. b-AP15 targets USP14 and UCHL5 in MM cells. (A) MM.1S cells were treated with dimethylsulfoxide (DMSO) or b-AP15 (100 nM) for indicated times or treated with bortezomib for 3 hours; protein lysates were subjected to immunoblotting with anti-polyubiquitin or anti-actin antibodies. (B) MM.1S cells were treated with DMSO or b-AP15 for 3 hours; cells were lysed in lysis buffer and total protein was labeled with HA-Ub-VS probe and then subjected to immunoblotting with USP14 and UCHL5 antibodies. (C) MM.1S cells were treated with DMSO, b-AP15, or bortezomib for 3 hours; protein lysates were analyzed for proteasome activity. The percentage of proteasome activity was normalized to DMSO control (mean ± standard deviation [SD], n = 3). (D) Recombinant human HtrA2 enzyme was incubated with its substrate β-casein in assay buffer, followed by sodium dodecyl sulfate polyacrylamide gel electrophoresis, silver staining, and quantification of cleaved β-casein. The bar graph represents the percentage of HtrA2-induced β-casein cleavage in the presence of bortezomib (3 nmol/L) or b-AP15 (100 nmol/L) vs DMSO control. Data represent means ± SD (n = 2; P < .05 for bortezomib). CT-L, chymotrypsin-like; T-L, trypsin-like; C-L, caspase-like.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/123/5/10.1182_blood-2013-05-500033/4/m_706f2.jpeg?Expires=1765920190&Signature=GJ8OnRjtzvVIqavh9QzHAOKu2aAG4qZwJpsBVl9GkfWz9IPv4c-fhTEXjJnefMzOC9kBe1wap7bxAe0LuvUi3ZbpdE-vBNcXzDP040W5W7iCYuh2gMv-RLKXwv~Q7vtmI7ZFxXUL7kJj~Q6aV6HxDKBjtJoAdSRZBhk~9XIbGkydSBAHERlifGqPt7Tw4tAj4kshI6WFiEXoZwapnToAxModl49X8Wu0kLctC3p-IiYt1rL13bNcvnbWGSUmWy-88KaWrGK~8~02kabtgj1O8rJvY6SgZMWqmZ-os06~a-BnWvvYRFSs0eOssyY4m6-XZYIzsZ5BKmo~AHydaxdZoQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal