Key Points
We have developed a novel in vitro system to model how shear force and transient interaction with endothelial cells alter chronic lymphocytic leukemia cell phenotype and behavior.
We have used our model to investigate chronic lymphocytic leukemia cell migration and have determined the critical role for integrin α4β1 in this process.
Abstract
There is growing evidence that lymphocyte trafficking contributes to the clinical course of chronic lymphocytic leukemia (CLL), but to date, only static in vitro cultures have been used to study these phenomena. To address this lack of data, we have developed a dynamic in vitro model in which CLL cells experience shear forces equivalent to those in capillary beds and are made to flow through capillary-like hollow fibers lined with endothelial cells. CLL cells treated in this way increased their expression of CD62L and CXCR4 (both P < .0001) and of CD49d and CD5 (both P = .003) directly as a result of the shear force. Furthermore, CLL cells migrated through the endothelium into the “extravascular” space (mean migration, 1.37% ± 2.14%; n = 21). Migrated CLL cells had significantly higher expression of CD49d (P = .02), matrix metallopeptidase-9 (P = .004), CD38 (P = .009), CD80 (P = .04), and CD69 (P = .04) compared with CLL cells that remained in the circulation. The degree of migration observed strongly correlated with CD49d expression (r2, 0.47; P = .01), and treatment with the CD49d-blocking antibody natalizumab resulted in significantly decreased migration (P = .01). Taken together, our data provide evidence for a novel, dynamic, and tractable in vitro model of lymphocyte migration and confirm that CD49d is a critical regulator of this process in CLL.
Introduction
Chronic lymphocytic leukemia (CLL) is characterized by a clonal expansion of CD5+CD19+ B cells found predominantly in the peripheral blood and lymphoid tissues. Although CLL is a proliferative disease,1 the majority of circulating CLL cells are arrested in growth 0 phase/growth 1 phase of the cell cycle. As a consequence, the peripheral vasculature is often viewed as a passive transit zone, with very little known about the dynamics of tissue homing, migration, and recirculation out of and into this microenvironment or the critical molecular interactions that drive these processes. To date, most in vitro studies of migration have been confined to static cultures using transwell plates, which are devoid of vascular shear force and the influence of endothelial cell interactions.2 Shear stress on endothelial cells results in alignment and elongation of the cells, tight junction formation, decreased DNA synthesis, and cell cycle arrest.3 These limitations have become increasingly pertinent, as there is growing evidence that trafficking to the microenvironmental niches of the bone marrow and lymph nodes affords cytoprotection to CLL cells.4 Furthermore, in the lymph nodes, CLL cells interact with accessory cells, including T cells, stromal cells, and endothelial cells, resulting in the activation of nuclear factor κB (NF-κB) in the CLL cells,5,6 which promotes CLL cell proliferation.7-10 Hence, the predisposition of CLL cells to return to prosurvival, pro-proliferative tissues from the peripheral vasculature may be a critical factor in determining the clinical course of the disease. In keeping with this concept, gene expression profiling revealed that increased expression of genes that code for proteins involved in the control of cell movement were correlated with poor prognosis.11,12 One such protein, CD49d, which interacts with vascular cell adhesion molecule 1 (VCAM-1) on endothelial cells during the process of CLL cell migration, has emerged as an important modulator of the pathology of CLL, as evidenced by the fact that it is an independent prognostic marker in this disease.13-15
Here we describe the development and characterization of a novel circulating model system designed to investigate the interactions of CLL cells and endothelial cells under physiologically relevant shear forces. Endothelial cells were seeded into hollow fibers and subjected to shear to encourage the formation of pseudovessels. Subsequently, CLL cells were circulated through the hollow fibers to mimic the transient contact with endothelial cells encountered in the capillary beds. Using this model system, we demonstrated that shear induced a number of significant changes in endothelial cell and CLL cell phenotypes. In addition, we were able to demonstrate a time-dependent increase in CLL cell transendothelial migration, which was associated with the upregulation of CD49d, matrix metallopeptidase (MMP)-9, CD38, and CD80 on CLL cells recovered from the “extravascular” space (EVS). Targeted inhibition of CD49d, using an anti-CD49d blocking antibody, resulted in a significant reduction in the level of migration, providing evidence for the key role this integrin plays in CLL cell migration.
Methods
Cells and patient characteristics
Peripheral blood samples were obtained from patients with CLL attending outpatient clinics in accordance with the Declaration of Helsinki; ethical approval was obtained from the South East Wales Local Research Ethics Committee (LREC #02/4806). CLL peripheral blood mononuclear cells (PBMCs) were isolated by density gradient centrifugation, using Lymphoprep (Axis-Shield). The clinical characteristics of the patients are shown in Table 1.
Patient characteristics
| Factor . | Number . |
|---|---|
| Median age | 67 years |
| Range | 29 - 85 years |
| Median follow-up | 6.2 years |
| Required treatment | |
| Treated | 19 |
| Untreated | 26 |
| Binet stage | |
| A | 39 |
| B/C | 6 |
| LDT, months | |
| >12 | 25 |
| <12 | 6 |
| Not determined | 14 |
| CD38 expression | |
| <20% | 26 |
| ≥20% | 19 |
| Not determined | 0 |
| Genetics | |
| Normal | 5 |
| 13q− | 12 |
| 12+ | 2 |
| 11q−/ 17p− | 2 |
| Not determined | 24 |
| IGHV status | |
| <98% | 15 |
| ≥98% | 9 |
| Not determined | 21 |
| Factor . | Number . |
|---|---|
| Median age | 67 years |
| Range | 29 - 85 years |
| Median follow-up | 6.2 years |
| Required treatment | |
| Treated | 19 |
| Untreated | 26 |
| Binet stage | |
| A | 39 |
| B/C | 6 |
| LDT, months | |
| >12 | 25 |
| <12 | 6 |
| Not determined | 14 |
| CD38 expression | |
| <20% | 26 |
| ≥20% | 19 |
| Not determined | 0 |
| Genetics | |
| Normal | 5 |
| 13q− | 12 |
| 12+ | 2 |
| 11q−/ 17p− | 2 |
| Not determined | 24 |
| IGHV status | |
| <98% | 15 |
| ≥98% | 9 |
| Not determined | 21 |
13q−, abnormalities involving loss of chromosome 13q; 12+, trisomy 12; 11q− and 17p−, any fluorescent in situ hybridization or karyotypic abnormality involving chromosome 11q or 17p; <98%, sequence homology with the closest germline sequence; CD38 expression, <20%/≥20% of the CLL B cells expressing the antigen; IGHV status: ≥98% sequence homology with the closest germline sequence; LDT, lymphocyte doubling time <12 months/>12 months; Normal, no detectable cytogenetic aberration by fluorescent in situ hybridization.
Modifying a hollow fiber bioreactor
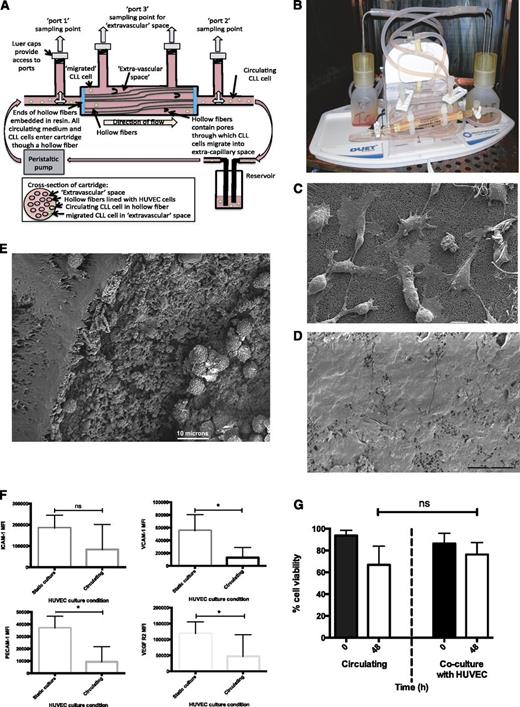
A hollow fiber bioreactor system (FiberCell Systems Inc) was adapted to generate an in vitro model of circulating CLL. The bioreactor uses a peristaltic pump to create a flow of medium at a defined shear force (dynes/cm2) through a closed system consisting of a reservoir of medium, wide tubing, and a cartridge containing porous hollow fibers (Figure 1A). The FiberCell Systems Polysulfone Plus cartridge (C2025) hollow fibers were coated with matrix proteins to allow attachment of endothelial cells to the insides of the hollow fiber. The fibers in the cartridge were activated by 70% ethanol, followed by water, and then gelatin (0.2% solution; Sigma Aldrich) was allowed to adhere to the insides of the hollow fibers for 2 hours. M199 medium (Sigma Aldrich) supplemented with 20% fetal bovine serum (Life Technologies) was used to gently wash unbound matrix protein from the hollow fibers and to allow its removal from the system. In experiments in which the hollow fibers were coated with endothelial cells, 15 × 106 human umbilical vein endothelial cells (HUVEC) or human mammary epithelial cells (HMEC-1 cells; Life Technologies) were introduced into the hollow fibers and allowed to adhere to the gelatin-coated fibers for an additional 2 hours. Nonadhered cells were then collected and removed from the system. The system was filled with 50 mL M199 medium supplemented with 20% fetal bovine serum, and circulation was initiated. Medium was circulated through the system with a shear force of 1.5 dynes/cm2 overnight before the shear force was increased to 10 dynes/cm2 for 6 hours before being decreased to 5 dynes/cm2 for a minimum of 2 hours. Shear force was increased in these increments to encourage the HUVEC cells in the hollow fibers to form firm adhesion and to align along the fibers to form pseudovessels. CLL cells were subsequently introduced into the circulating system through port 2 of the cartridge (Figure 1A). At the point of introduction of CLL to the circulating system, recombinant human interleukin 4 (R&D Systems) was added to the medium at a final concentration of 5 ng/mL.
Development of a novel circulating model of CLL. (A) HUVEC cells were seeded into the hollow fibers of the bioreactor and exposed to physiologically relevant shear forces. Medium containing CLL cells was pumped around the system, permitting transient interaction with the endothelial layer. The hollow fibers in the bioreactor have pores through which CLL cells can migrate into the space outside fibers; the EVS is shown in the cross-sectional image of the cartridge. CLL cells were recovered from the circulating compartment and the EVS of the system via the access ports. (B) Photograph of the adapted bioreactor. (C) Scanning electron micrograph of HUVEC cells on the interior of the hollow fibers after 5 hours of alignment at 5 dynes/cm2. The beginning of flattening and spreading of the cells is visible. (D) Scanning electron micrograph of HUVEC cells inside a hollow fiber after 6 hours of alignment under 10 dynes/cm2, followed by 24 hours at 5 dynes/cm2. The HUVEC cells showed increased spreading and flattening, resulting in coverage of the interior of the hollow fiber. (E) Scanning electron micrograph of a transverse section of a lined hollow fiber showing the wall of the fiber, with CLL cells visible in the inside of the fiber. Scale bars represent 10 microns in all scanning electron micrograph images. (F) Expression of endothelial cell markers was measured by flow cytometry on HUVEC cells grown in both noncirculating static tissue culture flasks and on cells recovered from the hollow fibers after alignment under shear force. Expression of VCAM-1 (P = .01), PECAM-1 (P = .002), and VEGFR2 (P = .05) were reduced in HUVEC cells under shear force. (G) CLL cell viability in circulating and static coculture with HUVEC cells. There was no significant difference between CLL viability in circulating culture and in static coculture with HUVEC cells. All culture conditions were supplemented with interleukin 4 (5 ng/mL).
Development of a novel circulating model of CLL. (A) HUVEC cells were seeded into the hollow fibers of the bioreactor and exposed to physiologically relevant shear forces. Medium containing CLL cells was pumped around the system, permitting transient interaction with the endothelial layer. The hollow fibers in the bioreactor have pores through which CLL cells can migrate into the space outside fibers; the EVS is shown in the cross-sectional image of the cartridge. CLL cells were recovered from the circulating compartment and the EVS of the system via the access ports. (B) Photograph of the adapted bioreactor. (C) Scanning electron micrograph of HUVEC cells on the interior of the hollow fibers after 5 hours of alignment at 5 dynes/cm2. The beginning of flattening and spreading of the cells is visible. (D) Scanning electron micrograph of HUVEC cells inside a hollow fiber after 6 hours of alignment under 10 dynes/cm2, followed by 24 hours at 5 dynes/cm2. The HUVEC cells showed increased spreading and flattening, resulting in coverage of the interior of the hollow fiber. (E) Scanning electron micrograph of a transverse section of a lined hollow fiber showing the wall of the fiber, with CLL cells visible in the inside of the fiber. Scale bars represent 10 microns in all scanning electron micrograph images. (F) Expression of endothelial cell markers was measured by flow cytometry on HUVEC cells grown in both noncirculating static tissue culture flasks and on cells recovered from the hollow fibers after alignment under shear force. Expression of VCAM-1 (P = .01), PECAM-1 (P = .002), and VEGFR2 (P = .05) were reduced in HUVEC cells under shear force. (G) CLL cell viability in circulating and static coculture with HUVEC cells. There was no significant difference between CLL viability in circulating culture and in static coculture with HUVEC cells. All culture conditions were supplemented with interleukin 4 (5 ng/mL).
Scanning electron microscopy
Cartridges were flushed with PBS to remove all medium and were then fixed for 24 hours in 2.5% vol/vol glutaraldehyde in PBS. Fixative was removed by washing twice with double-distilled water for 5 minutes. An adapted version of the Progressive Lowering of Temperature protocol was employed.16,17 Fibers were cut transversely and longitudinally, placed on self-adhesive carbon tape adhered to the surface of an aluminum stub, and sputter-coated with a gold target for 8 minutes in an EMscope sputter coater. Samples were viewed on a JEOL 840A scanning electron microscope operating at 5 kV, and images were recorded using SIS digital software.
Characterization of endothelial cells pre- and postcirculation
HUVEC and HMEC-1 cells were removed from liquid culture or from the hollow fibers of the circulating system after 48 hours, using an enzyme-free cell dissociation buffer (Life Technologies) and gentle agitation. Expression of intercellular adhesion molecule 1 (ICAM-1; BD), platelet endothelial cell adhesion molecule 1 (PECAM-1; R&D Systems), VCAM-1, and vascular endothelial growth factor receptor 2 (VEGFR2; Life Technologies) were determined by flow cytometry.
Antibody labeling of CLL cells
CLL cells were circulated through the system for 1 hour before samples were removed from the circulating compartment (Figure 1A). CLL cells were then circulated for a further 48 hours before additional samples were removed. After the retrieval of CLL cells from the circulating system, the cells were labeled with fluorescence-tagged antibodies: CD19-Brilliant Violet-520, CD49d-PE-Cy7, CD38-Brilliant Violet-605, CD62L-eFluor450, CXCR4-PerCP-Cy5.5, CD80-PE, CD11a-APC, CD44-APC-Cy7, CD69-Alexa Fluor-700 (BioLegend), CD5-ECD (Beckman Coulter), and MMP-9-fluorescein isothiocyanate (R&D Systems). Cells labeled with anti-MMP-9 antibody were fixed and permeabilized before labeling. Circulated CLL cells were compared with CLL cells before circulation and with cells maintained in static culture conditions. Flow cytometry was performed on an Aria III FACS machine (Becton Dickinson), and data were analyzed using FlowJo software (Tree Star, Inc.).
Migration
The FiberCell Systems Polysulfone Plus cartridges (C2025) are packed with hollow fibers with an internal radius of 350 μM. The hollow fibers contain pores of ∼0.2 μM along their length (Figure 1A) to allow the exchange of medium (and potentially cells) with the EVS. The EVS was accessed via port 3 in the cartridge (Figure 1A). Migration of CLL cells from the circulating system to the EVS was measured by collecting 100 μL circulating medium from the circulating compartment and from the EVS at 1 and 48 hours postintroduction of CLL cells. These samples were analyzed by flow cytometry, and the total number of live cells in 100 μL sampled from each port was measured. The percentage of the circulating population that migrated was calculated according to the formula
where NP2 is the number of live cells in 100 μL sample from the circulating compartment and NP3 is the number of live cells in 100 μL sample from the EVS.
Separate samples from the circulating and EVS compartments of the model were labeled with the panel of antibodies detailed earlier, and expression of the markers was compared on CLL cells recovered from the 2 compartments.
Polystyrene beads (unlabeled [10 μm] [Beckman Coulter] or fluorescein isothiocyanate-labeled [2.5 μm; Dako]) were added to the circulating system and circulated for 1 hour before samples were taken from the circulating and EVS compartments. These samples were analyzed by flow cytometry to determine whether the polystyrene beads could passively pass into the EVS.
Blocking CD49d functionality
Freshly isolated CLL cells were pretreated (30 minutes) with 20 μg/106 CLL cells of the anti-CD49d antibody natalizumab (Elan Pharma International Ltd) before their introduction into the circulating system. In addition, untreated CLL cells from the same patient were introduced into a parallel cartridge. Untreated and natalizumab-treated CLL cells were harvested from the circulating and extravascular compartments of each system after 1 and 48 hours. The level of CLL cell migration into the EVS in the presence and absence of natalizumab at both times was then calculated.
Results
Endothelial cells adhered to the inside of hollow fibers and aligned under shear force
The circulating model of CLL that we have developed is composed of an adapted hollow fiber bioreactor with the interior of the hollow fibers lined with primary HUVEC cells (Figure 1A-B). We also evaluated a microvascular endothelial cell line (HMEC-1) to compare and contrast the effects of different endothelium on CLL cell survival, phenotype, and migratory capacity.18,19 Under the peristaltic action of the pump in the bioreactor, the HUVEC (or HMEC-1) cells were subjected to shear force (5 dynes/cm2) equivalent to that present in the capillary beds.20 Scanning electron microscopy of the interior of the lined hollow fibers confirmed that the HUVEC cells subjected to 5 dynes/cm2 for 24 hours showed evidence of flattening and spreading along the inside surface of the hollow fiber (Figure 1C). However, we noted that short-term exposure to higher shear forces (10 dynes/cm2 for 6 hours) encouraged further flattening and elongation and more complete coverage of the hollow fibers (Figure 1D). We therefore employed this strategy in subsequent experiments before reducing the shear forces to 5 dynes/cm2 before the introduction of CLL cells into the model. As the hollow fibers are embedded in resin at the ends of the cartridges, the medium containing CLL cells could only circulate via the inside of the hollow fibers, allowing interactions with endothelial cells under shear stress (Figure 1E).
Shear force induced an altered phenotype on endothelial cells
HUVEC and HMEC-1 cells recovered from the interior of the hollow fibers retained the expression of endothelial cell markers VCAM-1, PECAM-1, ICAM-1, and VEGFR2 but showed shear-induced reductions in all the markers when compared with cells grown in static cultures (Figure 1F and supplemental Figure 1 [available on the Blood Web site], respectively). These data are consistent with previous reports showing that the expression of ICAM-1 and VCAM-1 is markedly decreased on endothelial cells placed under shear.21,22
Transient interaction with endothelial cells maintained CLL cell viability
Although long-lived in vivo, CLL cells rapidly undergo apoptosis when cultured in vitro23,24 unless they are supported by coculture.25-27 We have previously shown that static coculture on endothelial cells provides cytoprotection for CLL cells,6,28 so we set out to establish whether transient interactions with endothelial cells under shear force could also maintain CLL cell viability. Figure 1G shows there was no significant loss in CLL cell viability when the CLL cells were cultured in our HUVEC-lined circulating model system when compared with static HUVEC coculture. Similar results were obtained using HMEC-1-lined cartridges (supplemental Figure 1).
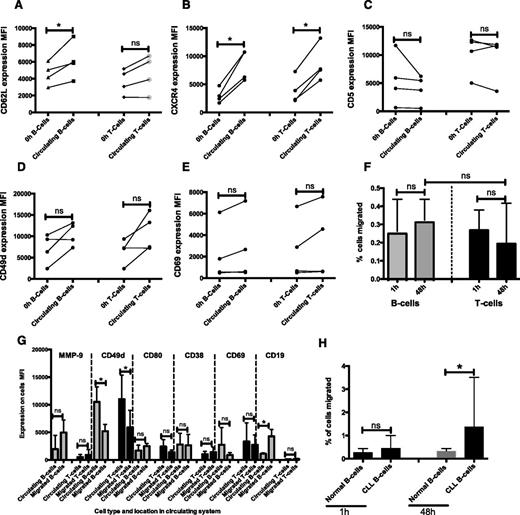
CLL cells increased their expression of CD62L, CXCR4, CD5, and CD49d as a result of circulation
CLL cells recovered from our circulating system showed a number of significant changes in phenotype when compared with baseline expression (time 0). After 48 hours in circulation, CLL cells showed significantly increased expression of the selectin CD62L (Figure 2A; P < .0001); the chemokine receptor CXCR4 (Figure 2B; P < .0001); the negative regulator of BCR signaling, CD5 (Figure 2C; P = .003); the adhesion molecule CD49d (Figure 2D; P = .004); and the early-activation antigen CD69 (Figure 2E; P = .007). In contrast, no significant change in the expression of CD38, CD80, MMP-9, and CD19 was observed as a result of transient interaction with endothelial cells under shear (Figure 2F-I). Furthermore, experiments performed with the HMEC-1 cell line induced similar phenotypic changes (supplemental Figure 2).
CLL cells circulating under physiologically relevant shear force showed increased expression of CD62L, CXCR4, CD5, CD49d, and CD69. CLL cells were circulated through the circulating system with hollow fibers lined with HUVEC cells for 48 hours. Cell surface expression of (A) CD62L, (B) CXCR4, (C) CD5, (D) CD49d, (E) CD69, (F) CD38, (G) CD80, (H) MMP-9, and (I) CD19 was measured by flow cytometry gated on doublet-discriminated lymphocytes represented as mean fluorescence intensity (MFI) values (**P < .0001; *P < .05).
CLL cells circulating under physiologically relevant shear force showed increased expression of CD62L, CXCR4, CD5, CD49d, and CD69. CLL cells were circulated through the circulating system with hollow fibers lined with HUVEC cells for 48 hours. Cell surface expression of (A) CD62L, (B) CXCR4, (C) CD5, (D) CD49d, (E) CD69, (F) CD38, (G) CD80, (H) MMP-9, and (I) CD19 was measured by flow cytometry gated on doublet-discriminated lymphocytes represented as mean fluorescence intensity (MFI) values (**P < .0001; *P < .05).
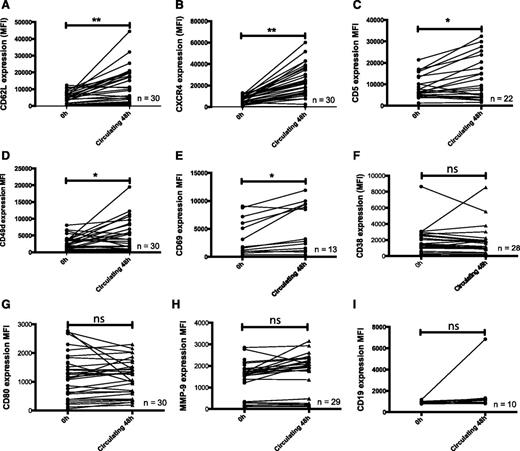
CLL cells actively migrated into the EVS
The ends of the hollow fibers in the circulating system are embedded in resin, ensuring all cells flowing through the system pass through the lumen of the hollow fibers. Therefore, no cells can passively leave the circulation and enter the space surrounding the hollow fibers (described here as the EVS). However, we consistently recovered CLL cells from the EVS even after just 1 hour of circulation (Figure 3A and supplemental Figure 3) and noted that the number of cells recovered was time-dependent (1 hour, 0.44% ± 0.56%; 48 hours, 1.37% ± 2.14%). Figure 3B shows that the percentage of migration was significantly increased after 48 hours (P = .03). The hollow fibers are perforated along their entire length by a network of pores (∼0.2 μm) that could potentially facilitate the escape of CLL cells into the EVS. To establish whether objects approximately the size of CLL cells could be recovered from the EVS, we introduced polystyrene beads (10 μm) into the system for 1 hour and then sampled 100 μL medium from the circulating and extravascular compartments (Figure 3Ci). Beads were readily recovered from the circulating compartment, but no beads were recovered from the EVS. We next introduced smaller (2.5 μm) fluorescent beads into the circulation system (Figure 3Cii). Again, no beads were recovered from the EVS of the circulating system, confirming that the beads were not able to passively move from the circulating compartment into the EVS. Therefore, the time-dependent recovery of CLL cells from the EVS indicated that these cells were actively migrating, rather than passively diffusing, out of the circulating compartment.
CLL cells actively underwent transendothelial cell migration associated with the upregulation of MMP-9, CD49d, CD80, CD38, and CD69. CLL cells were circulated through the hollow fiber model system lined with HUVEC endothelial cells for 48 hours. Paired samples were taken from the circulating compartment of the model and from the EVS. (A) Scanning electron micrograph of the outside of a hollow fiber showing CLL cells that migrated from the inside of the fiber to the outside. Scale bar represents 10 microns. (B) CLL cells showed a significant increase in migration at 48 hours when compared with 1 hour (n = 21; P = .03). (C) Polystyrene beads were introduced into the circulating compartment of the circulating system and circulated for 1 hour. Aliquots of 100 μL were removed from both the circulating and extravascular compartment, and the number of beads in each 100-μL aliquot was measured by flow cytometry. (Ci) Ten-micrometer beads and (Cii) 2.5-μm fluorescent beads in the circulating compartment and below the dashed line in the extravascular compartment after 1 hour. No beads were recovered from the EVS in each of the 3 replicates of the experiment. In contrast, CLL cells were consistently recovered from the extravascular compartment at this time. Compared with CLL cells remaining in the circulating compartment, CLL cells recovered from the EVS had increased expression of MMP-9, CD49d, CD80, CD38, CD69, and CD19 when measured by flow cytometry after (D) 1-hour and (E) 48-hour circulation around the system. Error bars indicate ± 1 SD around the mean fluorescence intensity on a population of doublet-discriminated CD19+ lymphocytes. *P < .05.
CLL cells actively underwent transendothelial cell migration associated with the upregulation of MMP-9, CD49d, CD80, CD38, and CD69. CLL cells were circulated through the hollow fiber model system lined with HUVEC endothelial cells for 48 hours. Paired samples were taken from the circulating compartment of the model and from the EVS. (A) Scanning electron micrograph of the outside of a hollow fiber showing CLL cells that migrated from the inside of the fiber to the outside. Scale bar represents 10 microns. (B) CLL cells showed a significant increase in migration at 48 hours when compared with 1 hour (n = 21; P = .03). (C) Polystyrene beads were introduced into the circulating compartment of the circulating system and circulated for 1 hour. Aliquots of 100 μL were removed from both the circulating and extravascular compartment, and the number of beads in each 100-μL aliquot was measured by flow cytometry. (Ci) Ten-micrometer beads and (Cii) 2.5-μm fluorescent beads in the circulating compartment and below the dashed line in the extravascular compartment after 1 hour. No beads were recovered from the EVS in each of the 3 replicates of the experiment. In contrast, CLL cells were consistently recovered from the extravascular compartment at this time. Compared with CLL cells remaining in the circulating compartment, CLL cells recovered from the EVS had increased expression of MMP-9, CD49d, CD80, CD38, CD69, and CD19 when measured by flow cytometry after (D) 1-hour and (E) 48-hour circulation around the system. Error bars indicate ± 1 SD around the mean fluorescence intensity on a population of doublet-discriminated CD19+ lymphocytes. *P < .05.
Migrated CLL cells had higher expression of MMP-9, CD49d, CD80, CD69, and CD38
CLL cells recovered from the EVS had a distinct phenotype when compared with paired samples recovered from the circulating compartment at the same time. Migrated cells showed increased expression of the B-cell coreceptor CD19 (P = .0008), the gelatinase MMP-9 (P = .004), the adhesion molecule CD49d (P = .02), the costimulatory molecule CD80 (P = .04), and the activation marker CD69 (P = .05) at 1 hour (Figure 3D). After 48 hours, expression of these markers was further increased on the migrated CLL cells (Figure 3E), and this was accompanied by significantly elevated expression of CD38 at this time (P = .009). Consistent with these findings, higher expression of CD3829 and CD695 have been reported on CLL cells derived from tissue microenvironments. It is worthy noting that none of the changes in CLL cell phenotype were specific to HUVEC interactions, as the HMEC-1 cell line also promoted CLL cell migration and induced very similar changes in phenotype (supplemental Figure 4).
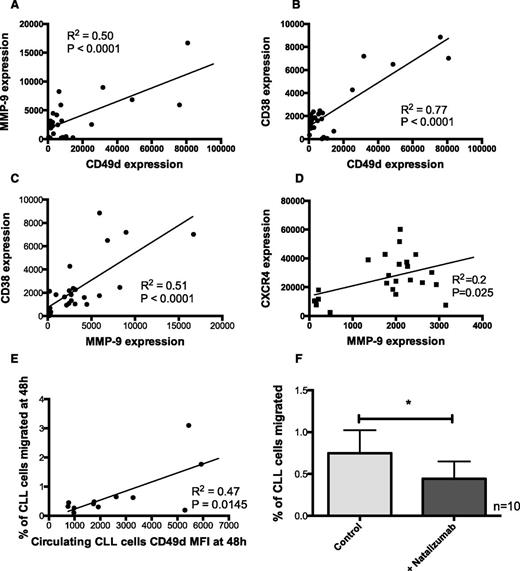
CD49d expression correlated with CLL cell migration
CD49d expression on CLL cells that migrated into the EVS correlated with MMP-9 expression (Figure 4A; r2, 0.5; P < .0001) and CD38 (Figure 4B; r2, 0.8; P < .0001). These findings are in keeping with previous studies that showed that MMP-9 is regulated by CD49d30 and is correlated with CD38.13,14 In addition, CD38 expression correlated with MMP-9 expression (Figure 4C; r2, 0.5; P < .0001), consistent with these molecules functioning in a macro-molecular complex and with all being involved in trafficking of CLL cells into the protective microenvironments found in lymph nodes.31 This was further supported by the correlation found between MMP-9 and CXCR4 (Figure 4D; r2, 0.2; P = .025). CD49d expression on CLL cells has been previously associated with worse clinical prognosis.13,14,32 Here we show for the first time that the expression of CD49d on circulating CLL cells correlates with the level of migration out of the circulation into the EVS (Figure 4E; r2, 0.4658; P = .01). The relationships between all the other markers measured and migration are shown in supplemental Figure 5.
CLL cell migration correlated with CD49d expression and was inhibited by the CD49d antagonist natalizumab. CLL cells that had been circulated in a closed system containing hollow fibers lined with HUVEC cells were recovered from both the circulating compartment and the extravascular compartment. CD49d expression correlated with (A) MMP-9 (r2, 0.5; n = 28) and (B) CD38 (r2, 0.8; n = 27). In addition, (C) MMP-9 and CD38 expression correlated with each other (r2, 0.5; n = 25) and (D) MMP-9 and CXCR4 expression levels correlated with each other (r2, 0.2; n = 24). (E) CD49d expression on circulating CLL cells after 48 hours showed the strongest correlation with the percentage of migration of CLL cells into the EVS (r2, 0.47; n = 12). (F) To establish a functional role for CD49d in CLL cell migration, CLL cells were pretreated with the anti-CD49d monoclonal antibody and then introduced into the circulating system. Natalizumab-treated CLL cells showed a significant reduction in their capacity to migrate into the EVS at 48 hours.
CLL cell migration correlated with CD49d expression and was inhibited by the CD49d antagonist natalizumab. CLL cells that had been circulated in a closed system containing hollow fibers lined with HUVEC cells were recovered from both the circulating compartment and the extravascular compartment. CD49d expression correlated with (A) MMP-9 (r2, 0.5; n = 28) and (B) CD38 (r2, 0.8; n = 27). In addition, (C) MMP-9 and CD38 expression correlated with each other (r2, 0.5; n = 25) and (D) MMP-9 and CXCR4 expression levels correlated with each other (r2, 0.2; n = 24). (E) CD49d expression on circulating CLL cells after 48 hours showed the strongest correlation with the percentage of migration of CLL cells into the EVS (r2, 0.47; n = 12). (F) To establish a functional role for CD49d in CLL cell migration, CLL cells were pretreated with the anti-CD49d monoclonal antibody and then introduced into the circulating system. Natalizumab-treated CLL cells showed a significant reduction in their capacity to migrate into the EVS at 48 hours.
Inhibition of CD49d function with natalizumab inhibited CLL cell migration
The correlation observed between CD49d expression and the migratory potential of CLL cells suggested it might be a therapeutic target in CLL. Given that normal B-lymphocytes do not appear to require this integrin for transendothelial migration,33 blockade of α4β1 may selectively inhibit CLL cell trafficking. The anti-CD49d antibody natalizumab has been shown to prevent the interaction of CD49d and VCAM-1 on endothelial cells,34 so we assessed the effects of this antibody into our model system. The model can operate 2 separate cartridges in tandem, which allowed us to compare cells isolated from the same patient at the same time under different conditions. In 1 cartridge, CLL cells were circulated without natalizumab, and in the other cartridge, CLL cells were pretreated with natalizumab for 30 minutes before introduction into the system. CLL cells treated with natalizumab showed a significant decrease in their ability to migrate out of circulation into the EVS after 48 hours (Figure 4F; P = .01).
Normal PBMCs showed similar phenotypic changes and migrate in our circulating model
To determine whether our findings were restricted to CLL lymphocytes, we introduced PBMCs from 4 healthy donors in our dynamic model system. Normal B cells were identified by the expression of CD19, and T cells were identified as CD5+/CD19−. Cells recovered from the circulating compartment and the EVS circulating normal B cells showed significant increases in CD62L (Figure 5A) and CXCR4 (Figure 5B; normal B and T cells) expression. No significant differences were seen in CD5 expression in normal B or T cells over the course of 48 hours in circulation (Figure 5C). In addition, we showed time-dependent increases in CD49d and CD69 in normal B cells recovered from the circulation, but these were not significant (Figure 5D-E). Furthermore, both normal B and T cells migrated into the EVS (Figure 5F). Consistent with our findings with CLL B cells, CD19 expression was increased on normal B cells that migrated compared with B cells in circulation (P = .016; Figure 5G). In contrast to CLL B cells, normal B and T cells showed significantly decreased expression of CD49d and CD69 in cells that migrated into the EVS (Figure 5G). In addition, small but nonsignificant increases in MMP-9 and CD80 expression were observed on normal B cells that migrated into the EVS. The lack of induction of both CD49d and CD38 in migrated normal B cells when compared with CLL B cells is consistent with these molecules being important in the survival of CLL cells,32 and previous reports have shown that CD49d is essential for CLL cell migration, but not normal B-cell migration.33,35 Despite these apparent molecular differences, we found no significant difference in B-cell migration in normal and CLL samples at 1 hour (P = .31). However, at 48 hours, a higher percentage of CLL B cells migrated compared with B cells from normal samples (CLL, 1.37% ± 2.14%; normal B cells, 0.32% ± 0.05%; P = .03; Figure 5H).
Normal B and T cells showed similar phenotypic changes when compared with CLL cells. PBMCs from 4 normal healthy donors were introduced into the circulating model in the same way as CLL PBMCs. Samples were then taken from the circulating compartment and the EVS at 48 hours and labeled with the same panel of cell surface markers used for the CLL samples. B cells were identified as CD19+ and T cells as CD5+/CD19−. Expression (MFI) of (A) CD62L, (B) CXCR4, (C) CD5, (D) CD49d, and (E) CD69 in circulating B and T cells is shown. (F) Normal B and T cells also migrated into the EVS, and the percentage of B-cell and T-cell migration is shown. (G) Expression of MMP-9, CD49d, CD80, CD38, CD69, and CD19 in normal B and T cells was compared in cells recovered from the EVS and cells remaining in the circulation compartment. (H) B cells from normal samples migrated significantly less than CLL B cells at 48 hours. Error bars indicate ± standard deviation. *P < .05.
Normal B and T cells showed similar phenotypic changes when compared with CLL cells. PBMCs from 4 normal healthy donors were introduced into the circulating model in the same way as CLL PBMCs. Samples were then taken from the circulating compartment and the EVS at 48 hours and labeled with the same panel of cell surface markers used for the CLL samples. B cells were identified as CD19+ and T cells as CD5+/CD19−. Expression (MFI) of (A) CD62L, (B) CXCR4, (C) CD5, (D) CD49d, and (E) CD69 in circulating B and T cells is shown. (F) Normal B and T cells also migrated into the EVS, and the percentage of B-cell and T-cell migration is shown. (G) Expression of MMP-9, CD49d, CD80, CD38, CD69, and CD19 in normal B and T cells was compared in cells recovered from the EVS and cells remaining in the circulation compartment. (H) B cells from normal samples migrated significantly less than CLL B cells at 48 hours. Error bars indicate ± standard deviation. *P < .05.
Discussion
Despite advances in our knowledge of the pathology of CLL, there are still significant gaps in our understanding of the key mechanisms that modulate the clinical course of this heterogenous disease. There is a growing appreciation that the complex interactions between CLL cells and their microenvironments hold the key to improving clinical outcomes in this disease. This is perhaps best exemplified by the success of the Bruton’s tyrosine kinase inhibitor ibrutinib.36 Although the molecular target of this agent is clear, its profound effects on ejecting CLL cells from the lymphoid tissues was unexpected. This, in turn, has reinforced the importance of tissue homing and retention within prosurvival, pro-proliferative sites as pathological drivers in CLL. It is therefore important to dissect the key molecular events that underpin these processes to explain the variation in the natural pathology of this disease and develop more rational therapeutic strategies.
None of the existing in vitro coculture systems of CLL provide an accurate proxy for the peripheral vasculature of CLL patients and, hence, cannot provide meaningful insights into the process of extravasation and migration. To address this, we have developed a dynamic model system in which CLL cells were circulated through endothelium-lined hollow fibers under shear forces comparable to those present in the capillary beds and high endothelial venules.37 Under these conditions, we showed that HUVEC cells flattened against the walls of the hollow fibers and formed pseudovessels. They also took on the phenotype of endothelial cells under shear.21,22 Shear force promotes tight junction formation between HUVEC endothelial cells38 and is required for peripheral blood lymphocyte adhesion to39 and migration across the endothelium.40
Next we characterized the effects of introducing CLL cells into our hollow-fiber system. CLL cell viability was maintained at levels equivalent to that achieved in static coculture with endothelial cells. Furthermore, circulation of CLL cells in our system induced specific phenotypic changes; the extent of these changes was sample-dependent, implying that some CLL cells are more predisposed to responding to the costimuli of shear and transient interaction with endothelial cells. The changes that were induced were consistent with priming CLL cells for migration; we observed an early increase in the expression of the selectin CD62L, as well as increased expression of CD49d, CXCR4, CD5, and CD69. CD62L is necessary for the initial tethering of the lymphocytes to endothelial cells via its ligand, PNAd41 , and CD49d, which is necessary for CLL cell adhesion to endothelial cell walls.32 In addition, circulating CLL cells also showed a significant increase in the expression of CXCR4, the receptor of stromal cell-derived factor 1, which has chemotactic activity for lymphocytes and results in homing to protective microenvironments.42 CD69 has been shown to be a strong predictor of CLL prognosis and to be upregulated by microenvironmental contact.5,11 The increase in expression of these markers on CLL cells under shear force implicates the action of shear force in the control of expression of these regulators of CLL cell motility.
Consistent with these phenotypic changes, we were able to demonstrate that CLL cells underwent transendothelial migration into the EVS of our model system. The accumulation of CLL cells in the EVS was associated with higher expression of MMP-9, CD38, CD49d, CD80, and CD69. Previous reports have indicated that these molecules may physically interact31 and that CD38 and CD49d are associated with worse prognosis in CLL14 and lymph node infiltration.8,32 Furthermore, correlations between CD38 and CD49d expression have previously been reported,43 as have interactions among CD38, CD49d, and MMP-931 and CXCR4.10 In addition, interactions between CD49d on the CLL cells and VCAM-1 on endothelial cells are crucial for the adhesion of CLL cells to stromal cells44 and in stroma-mediated protection from apoptosis.45 The level of migration in our circulating system was strongly associated with the expression of CD49d on the circulating CLL cells. This is consistent with the concept that CD49d is a critical regulator of CLL cell migration33,37 and may explain its prognostic effect on patients with CLL.15 We therefore set out to inhibit the function of this integrin by using the blocking antibody natalizumab. Pretreatment of CLL cells with natalizumab resulted in reduced migratory ability in our model system, highlighting the importance of CD49d to CLL cell homing and migration. However, we were not able to abolish CLL cell migration under these conditions, suggesting either that the inhibition of CD49d was incomplete or that other molecules also modulate the capacity of CLL cells to migrate in our system. These possibilities are not mutually exclusive, and we are currently evaluating of the role of other key molecular targets, using multiple inhibition strategies.
Finally, we established that our model system could also be used to investigate the behavior of nonmalignant lymphocytes. In keeping with our CLL cell data, normal B cells and T cells showed phenotypic evidence of activation when they were recovered from the circulating compartment of our system. Subsequently, both lymphocyte subsets were recovered from the EVS, suggesting that our model system is suitable for investigating normal lymphocyte transendothelial migration.
In summary, we have built and characterized a dynamic in vitro model system of the peripheral vasculature that is suitable for investigating the key molecular drivers of transendothelial migration. In the context of CLL, this represents a significant advance, as it provides a physiologically relevant means of understanding how CLL cells interact with and traffic across endothelial cell barriers. It is also ideally suited to the study of the effects of new therapeutic agents targeting molecules involved in lymphocyte migration and homing.
There is an Inside Blood Commentary on this article in this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Jan Hobot for his expert assistance with all electron microscopy undertaken in the preparation of this manuscript and John Cadwell for interesting discussions about the adaptation of the bioreactor.
This work was supported in part by Leukaemia and Lymphoma Research and the Leukaemia Research Appeal for Wales. C.P. is supported by the National Institute for Social Care and Health Research through the Cancer Genetics Biomedical Research Unit.
Authorship
Contribution: E.W. carried out the experimental work, analyzed the data, and wrote the manuscript; A.B. carried out the experimental work, analyzed the data, and revised the manuscript; S.D., C.J., and G.P. provided clinical samples, analyzed data, and revised the manuscript; P.B. analyzed data and revised the manuscript; C. F. provided clinical samples and data and revised the manuscript; and C.P. conceived the work and revised the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Chris Pepper, Cardiff CLL Research Group, Institute of Cancer & Genetics, School of Medicine, Cardiff University, Heath Park, Cardiff, UK, CF14 4XN; e-mail: peppercj@cf.ac.uk.

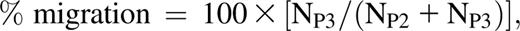
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal