Key Points
Clonotypic B cells, long suspected to represent circulating stem-like cells, are consistently absent in the blood of myeloma patients.
Malignant plasma cells frequently circulate in the peripheral blood, show evidence for clonal evolution, and may spread the disease.
Abstract
The identity of the proliferative compartment of myeloma progenitor cells remains a matter of debate. Polymerase chain reaction-based studies suggested pre-switch “clonotypic” B cells sharing the immunoglobulin (Ig) rearrangement of the malignant plasma cell (M-PC), to circulate in the blood and possess stem cell-like properties. Here, we disprove this hypothesis. We screened peripheral blood IgM, IgG, and IgA repertoires of myeloma patients for the clonotypic rearrangement by next-generation sequencing. None of 12 cases showed pre-switch clonotypic transcripts. In the post-switch IgG/IgA repertoires, however, the clonotypic rearrangement was detected at high frequency in 6 of 8 patients with active disease, whereas it was undetectable after treatment, correlating with flow cytometric presence or absence of circulating M-PCs. Minor subclones with alternative post-switch isotypes suggested ongoing switch events and clonal evolution at the M-PC level. Our findings consistently show an absence of pre-switch clonotypic B cells, while M-PCs circulate in the peripheral blood and may contribute to spreading of the disease.
Introduction
Improvement of long-term outcomes in multiple myeloma critically relies on a better understanding of the tumor-initiating cell in this disease. As in other malignancies, the cancer stem cell concept incriminates drug-resistant myeloma stem cells with tumor-initiating, self-renewing properties to feed the malignant plasma cell (M-PC) compartment in disease relapse and progression.1,2 However, it remains a matter of controversy if the clonogenic population resides within the pool of terminally differentiated post-switch M-PCs or within a less differentiated (surface) immunoglobulin (Ig)-positive pre-switch B-cell compartment. The latter hypothesis has been fueled by the description of so-called clonotypic pre-switch (IgM+) B cells postulated to express the same patient-individual variable region Ig rearrangement as the M-PC.3-7 This finding even provided the rationale for therapeutic targeting of this postulated CD20+ population with the monoclonal antibody rituximab.8,9 However, inconsistent results of xenotransplantation experiments,10-15 data on intraclonal evolution at the PC level,16-22 the analysis of class switch junctions,23 and the lack of benefit from CD20-directed targeted therapy8,9 challenged the concept of such feeder cells in myeloma. Moreover, a recent study entirely failed to detect clonotypic rearrangements in highly purified B-cell populations devoid of contaminating M-PCs,24 and our own data also pointed in this direction, because we could not phenotypically detect clonotypic B cells in the majority of patients with patient-individual Ig ligands as tracers.25
Here we used next-generation sequencing to definitively confirm or disprove the existence of this highly controversial cell population.
Materials and methods
Patients and samples
Blood and bone marrow samples of 12 myeloma patients (Table 1) visiting the Freiburg and Hamburg University Medical Centers were obtained after written informed consent as approved by the institutional review boards. This study was conducted in accordance with the Declaration of Helsinki.
Clinical features and PCR detection of clonotypic rearrangement in the study cohort of 12 myeloma patients
| Patient code . | Treatment . | Remission status at date(s) of sample acquisition . | PCR-positivity for clonotypic rearrangement in peripheral blood . |
|---|---|---|---|
| Patients in remission after treatment | |||
| MM001 | auto-HSCT, Rd | PR | — |
| MM020 | auto-SCT | PR | — |
| MM023 | Vel/Dex | PR | — |
| MM036 | Vel/Dex | PR | — |
| Patients with active disease | |||
| MM032 | — | First diagnosis | — |
| MM050 | Rd, Vel/Dex | Relapse | — |
| MM021 | auto-HSCT, Rd, Vel/Dex | Relapse | + |
| MM024 | auto-HSCT, Vel/Dex | Relapse (+ FU sample in CR) | + (CR sample: −) |
| MM031 | allo-HSCT | Relapse (+ FU samples in CR) | + (CR sample: −) |
| MM034 | — | First diagnosis | + |
| MM048 | — | First diagnosis | + |
| MM081 | — | First diagnosis | + |
| Patient code . | Treatment . | Remission status at date(s) of sample acquisition . | PCR-positivity for clonotypic rearrangement in peripheral blood . |
|---|---|---|---|
| Patients in remission after treatment | |||
| MM001 | auto-HSCT, Rd | PR | — |
| MM020 | auto-SCT | PR | — |
| MM023 | Vel/Dex | PR | — |
| MM036 | Vel/Dex | PR | — |
| Patients with active disease | |||
| MM032 | — | First diagnosis | — |
| MM050 | Rd, Vel/Dex | Relapse | — |
| MM021 | auto-HSCT, Rd, Vel/Dex | Relapse | + |
| MM024 | auto-HSCT, Vel/Dex | Relapse (+ FU sample in CR) | + (CR sample: −) |
| MM031 | allo-HSCT | Relapse (+ FU samples in CR) | + (CR sample: −) |
| MM034 | — | First diagnosis | + |
| MM048 | — | First diagnosis | + |
| MM081 | — | First diagnosis | + |
allo-HSCT, allogeneic hematopoietic stem cell transplant; auto-HSCT, melphalane high-dose chemotherapy followed by autologous stem cell transplant; CR, complete remission; FU, follow-up; MM, multiple myeloma; PR, partial remission; Rd, Revlimid (Lenalidomide) + Dexamethasone; Vel/Dex, Velcade (Bortezomib) + Dexamethasone.
Detection and Sanger sequencing of clonotypic Ig rearrangements
M-PC heavy-chain Ig rearrangements were determined as previously described from bone marrow and are shown in supplemental Table 1.25 For detection of circulating clonotypic cells, peripheral blood mononuclear cells were used. Polymerase chain reaction (PCR) approaches for the qualitative detection of clonotypic rearrangements of all isotypes, of IgM isotype only, and for isotype subclass determination by Sanger sequencing are shown in supplemental Figure 1A-B,D and supplemental Table 2, available on the Blood Web site.
NGS of Ig repertoires
Ig transcripts were amplified for next-generation sequencing (NGS) from peripheral blood as described in the supplemental Detailed Methods section. The amplification strategy and primer sequences are shown in supplemental Figure 1C and supplemental Table 2. Amplicons were multiplex-sequenced on a MiSeq Illumina sequencer. Data were plotted using ggplot2 for R statistical programming by display on a matrix providing a specific position for each potential V-D-J rearrangement.
Multiparametric flow cytometry
Peripheral blood cells were stained with fluorescently labeled antibodies (CD45-ECD, CD138-PC5, CD38-FITC, CD19-PC7) to discriminate B-lineage cell populations.
Results and discussion
PCR-based detection of clonotypic peripheral blood cells
Peripheral blood clonotypic rearrangements were qualitatively detectable with HCDR3-specific primers in 6 of 8 patients with active disease, suggesting the presence of circulating clonotypic B-lineage cells (data not shown). None of the cases in remission after treatment was PCR-positive. All HCDR3 PCR-positive cases were also positive in an established semi-nested PCR approach using IgM-specific primers. Surprisingly, however, Sanger sequencing of PCR products revealed post-switch IgG/IgA transcripts despite the use of IgM-specific primers, indicating that the PCR may yield false-positive results in the presence of a dominant clonotypic rearrangement of alternative isotype (data not shown).
Targeted NGS of peripheral blood IgM, IgG, and IgA repertoires
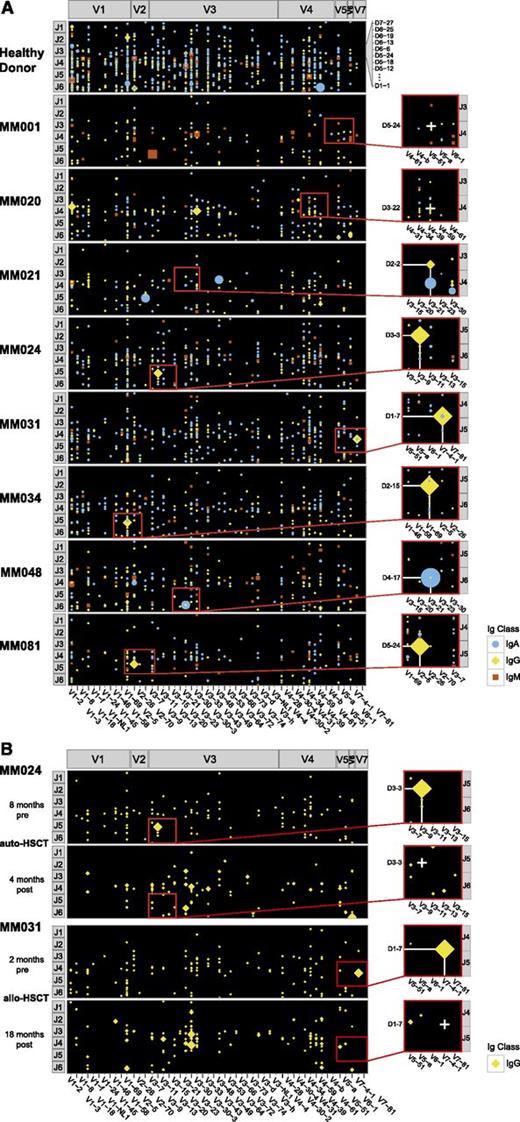
This observation prompted us to comprehensively screen Ig repertoires of circulating cells expressing pre- and post-switch isotypes for the presence of the clonotypic rearrangement by NGS. Sequencing data were displayed on a matrix providing a specific position for each potential V-D-J rearrangement (Figure 1). We found highly skewed peripheral Ig repertoires in myeloma patients compared with the polyclonal repertoires of a healthy donor (Figure 1A), reflecting the suppression of healthy polyclonal B-lineage cells by the M-PC clone in the bone marrow. None of the patients showed evidence for IgM+ clonotypic rearrangements, suggesting absence of pre-switch clonotypic B cells in the peripheral blood. The clonotypic rearrangement could, however, be detected within the post-switch repertoire of the M-PC clone (IgG or IgA), indicating that M-PC circulate in the peripheral blood of these patients. In the majority of cases, the clonotypic rearrangement was the most abundant rearrangement detectable within the repertoire with a median of 48% of reads of the respective isotype (supplemental Table 3). PCR-negative control cases MM001 and MM020 (Figure 1A) as well as blood samples of patients MM024 and MM031 in complete remission after treatment (Figure 1B) were negative for the clonotypic rearrangement. In patients MM031 and MM048, we found, apart from the dominant clonotypic rearrangement of the M-PC, an identical rearrangement of alternative post-switch isotype (nonclinical rearrangement). Sequencing of the exact isotype subclass revealed a dominant IgG3 clone with a small IgA1 nonclinical isotype in patient MM031 and a dominant IgA1 clone with a small IgG1 nonclinical isotype in patient MM048. Considering the architecture of the Ig gene locus, this suggested that the MM031 IgG3 clone emerged from the preexisting IgA1 clone, while the small MM048 IgG1 clone had to be considered as precursor of the dominant IgA1 clone. This finding suggested ongoing sequential switch events in the progeny of post-switch myeloma cells and therefore clonal evolution at the PC level.
Targeted NGS of heavy-chain Ig repertoires from peripheral B-lineage cells of myeloma patients. (A) Clonotypic rearrangements in the peripheral blood of the myeloma patient cohort. Panel A shows patients MM001 and MM020, who were negative for the clonotypic rearrangement by a qualitative PCR approach. In patients MM021, MM024, MM031, MM034, MM048, and MM081, the clonotypic rearrangement was qualitatively detectable by PCR. For NGS, Ig transcripts were amplified with isotype-specific primers and multiplex sequenced on a MiSeq Illumina sequencer. Data were plotted using ggplot2 for R statistical software assigning a position to each potential V-D-J rearrangement. In patient MM020, a small clone was plotted at the expected site of the clonotypic rearrangement. However, this rearrangement was different from the clonotypic rearrangement (HCDR3 sequence and somatic hypermutation pattern). (B) Absence of clonotypic rearrangements in posttreatment follow-up samples of patients MM024 and MM031. Only IgG repertoires are shown. allo-HSCT, allogeneic hematopoietic stem cell transplant; auto-HSCT, melphalane high-dose chemotherapy followed by autologous stem cell transplant.
Targeted NGS of heavy-chain Ig repertoires from peripheral B-lineage cells of myeloma patients. (A) Clonotypic rearrangements in the peripheral blood of the myeloma patient cohort. Panel A shows patients MM001 and MM020, who were negative for the clonotypic rearrangement by a qualitative PCR approach. In patients MM021, MM024, MM031, MM034, MM048, and MM081, the clonotypic rearrangement was qualitatively detectable by PCR. For NGS, Ig transcripts were amplified with isotype-specific primers and multiplex sequenced on a MiSeq Illumina sequencer. Data were plotted using ggplot2 for R statistical software assigning a position to each potential V-D-J rearrangement. In patient MM020, a small clone was plotted at the expected site of the clonotypic rearrangement. However, this rearrangement was different from the clonotypic rearrangement (HCDR3 sequence and somatic hypermutation pattern). (B) Absence of clonotypic rearrangements in posttreatment follow-up samples of patients MM024 and MM031. Only IgG repertoires are shown. allo-HSCT, allogeneic hematopoietic stem cell transplant; auto-HSCT, melphalane high-dose chemotherapy followed by autologous stem cell transplant.
Flow cytometry-based detection of circulating PCs
To confirm our assumption that the clonotypic post-switch rearrangements may derive from circulating M-PCs, we analyzed peripheral blood cells by multiparametric flow cytometry. We found evidence of circulating M-PCs with the classical immunophenotype in all but one patient with a clonotypic rearrangement in ∼1% of leukocytes (supplemental Figure 2A). In the control cases MM001 and MM020 and posttreatment samples of patients MM024 and MM031 (supplemental Figure 2B), no circulating M-PCs could be detected. Patient MM021, who had a low burden of clonotypic rearrangements (only 2% of all IgG reads), was the only NGS-positive case with flow cytometric negativity for circulating M-PCs, most likely due to the different sensitivity levels of these assays.
Discussion of data in the context of previously published work
The hypothesis that pre-switch clonotypic B cells act as tumor-initiating and -propagating cells in myeloma has caused considerable debate for over decades. The results presented here should terminate this controversy. Using highly sensitive and highly specific state-of-the-art technology, we disprove the existence of such cells in the blood. This is in accordance with a recently growing body of evidence by other studies.8-23
Two methodological aspects most likely account for the discrepancy between prior reports3-7 and our study. First, PCR with HCDR3- and isotype-specific primers may lack specificity in the presence of abundant clonotypic transcripts of alternative isotype. Without confirmation by direct sequencing of such transcripts, this finding may have falsely suggested the existence of IgM-positive clonotypic B cells, although the transcripts derived from IgG- or IgA-positive M-PCs. Second, many of the previous studies were primarily based on B-cell subpopulations isolated by fluorescence-activated cell sorting. Because M-PCs can circulate in the peripheral blood, such B-cell populations may easily have been contaminated by circulating M-PCs, which may then have provided the clonotypic sequences falsely attributed to B cells. Consequently, when highly purified B-cell populations devoid of contaminating M-PCs were investigated, no clonotypic transcripts were found.24
Taken together, our data show that pre-switch clonotypic B cells are inexistent in the blood of myeloma patients, while circulating M-PCs frequently occur in active disease and may contribute to spreading the disease.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the Wilhelm Sander Foundation (grant 2009.035.02 to M.B.).
Authorship
Contribution: B.T., M.K., M.A., and D.I. performed experiments; M.B., B.T., M.K., M.T., and A.G. interpreted data; and M.B., B.T., and M.K. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Mascha Binder, University Medical Center Hamburg-Eppendorf, Department of Oncology, Hematology, BMT with section Pneumology, Martinistrasse 52, D-20246 Hamburg, Germany; e-mail: m.binder@uke.de.
References
Author notes
B.T. and M.K. contributed equally to this study.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal