To the editor:
In juvenile myelomonocytic leukemia (JMML), activating RAS mutations are responsible for a hyperactive RAS/ERK signaling.1 Somatic codons 12-13-61 RAS mutations are described in cases of RAS-associated lymphoproliferative disease (RALD),2-4 believed to be a benign entity distinct from JMML. RALD features autoimmune cytopenias and lymphoproliferation secondary to a T-cell apoptosis defect, similar to autoimmune lymphoproliferative syndrome (ALPS). In RALD, the apoptosis defect is caused by a RAS/ERK-mediated downregulation of the proapoptotic BIM protein,2-4 whereas in ALPS, the defect is caused by mutations of the death receptor FAS.5 Thus, the same oncogenic hematopoietic RAS mutations are associated with different phenotypes, considered as distinct pathologies. We report a case of G13C KRAS mutation firstly leading to RALD and then evolving from an indolent to an aggressive form of leukemia.
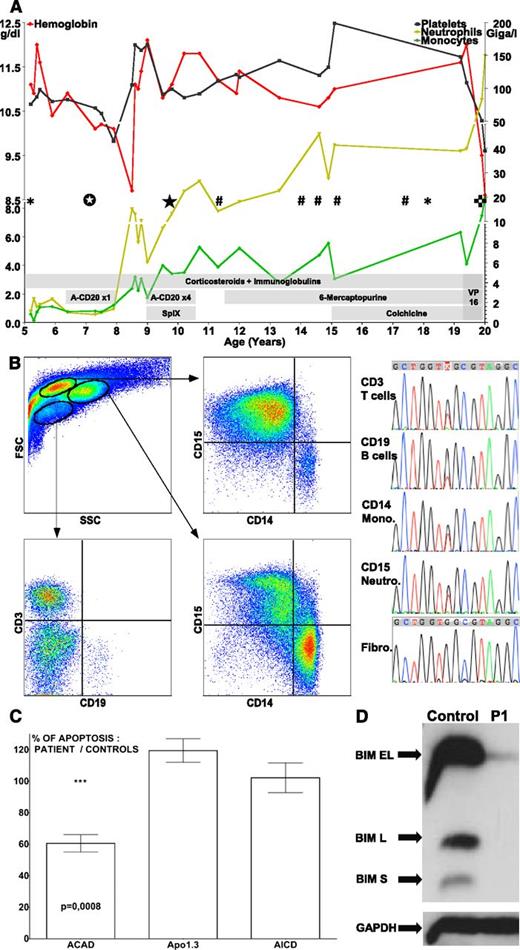
The patient presented with autoimmune hemolytic anemia and thrombocytopenia, hepatosplenomegaly, and multifocal lymphadenopathy at age 5. He developed a colic B-cell lymphoproliferation at age 7.5. At age 9.5, he underwent splenectomy and received 4 anti-CD20 infusions. From then on, the Evans syndrome declined and the leukocyte numbers rose. At age 20, he presented with chest pain and leucocytosis. The situation evolved into fatal acute respiratory distress syndrome secondary to pulmonary leucostasis (Figure 1A).
Biological, phenotypical, molecular and functional analyses. (A) Hematobiological evolution of the patient with treatment courses. A-CD20, rituximab; SplX, splenectomy; VP16, etoposide; *, pneumopathy, #, pericarditis associated to arthritis; ☆, colic B-cell proliferation; ★, Evans syndrome aggravation with spleen enlargement crisis and circulating B-cell proliferation; ✜, acute myeloproliferation with pulmonary infiltration leading to death. (B) Cell sorting and sequencing of circulating leukocytes populations and primary derived fibroblasts. Fibro., fibroblasts; Mono., monocytes; Neutro, neutrophils. (C) In vitro apoptosis assays on superantigen stimulated T cells from the patient; results are compared with controls. ACAD, activated cells autonomous death using cytokines withdrawal; AICD, activation-induced cell death by T-cell receptor restimulation; Apo1.3, direct Fas stimulation with an agonist antibody. (D) Western blot of patient’s (P1) Bim expression on activated T cells. GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Biological, phenotypical, molecular and functional analyses. (A) Hematobiological evolution of the patient with treatment courses. A-CD20, rituximab; SplX, splenectomy; VP16, etoposide; *, pneumopathy, #, pericarditis associated to arthritis; ☆, colic B-cell proliferation; ★, Evans syndrome aggravation with spleen enlargement crisis and circulating B-cell proliferation; ✜, acute myeloproliferation with pulmonary infiltration leading to death. (B) Cell sorting and sequencing of circulating leukocytes populations and primary derived fibroblasts. Fibro., fibroblasts; Mono., monocytes; Neutro, neutrophils. (C) In vitro apoptosis assays on superantigen stimulated T cells from the patient; results are compared with controls. ACAD, activated cells autonomous death using cytokines withdrawal; AICD, activation-induced cell death by T-cell receptor restimulation; Apo1.3, direct Fas stimulation with an agonist antibody. (D) Western blot of patient’s (P1) Bim expression on activated T cells. GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
The patient initially displayed classical RALD features. ALPS was ruled out after eliminating a FAS mutation. A somatic KRAS G13C mutation was found in all circulating hematopoietic cells subsets but not on primary derived fibroblasts (Figure 1B). Consistent with published data,2-4 BIM was downregulated in activated T cells (Figure 1C), leading to an in vitro defect of activated cell autonomous death (Figure 1D). Interestingly, lymph node histology showed Rosai-Dorfman–like features when biopsied at age 6, an aspect not yet described in RALD (supplemental Figure 1A-C on the Blood Web site).
After splenectomy, the patient started displaying classical JMML features with increasing peripheral myeloid cell numbers. In addition to ALPS features, the spleen showed an important immature myeloid colonization (supplemental Figure 1D-E). The blood smear showed circulating dysplastic neutrophils and abnormal monocytes (supplemental Figure 1F-G). The bone marrow was hypercellular with granulocytic hyperplasia without blast excess at ages 5 and 19 (supplemental Figure 1H-I). Spontaneous growth and hypersensitivity to granulocyte macrophage–colony-stimulating factor of the bone marrow progenitors were found. Rearrangement of the BCR-ABL fusion gene was always negative. The JMML evolved during a 10-year period as a “long survivor,” but the final stage was a classical feature seen in severe JMML.6
Autoimmunity has recently been reported in spontaneous remission cases of JMML with persistent RAS-mutated clones.7 We now show that RALD can be the initial presentation of a severe JMML. Moreover, the patient’s history shows that 1 RAS mutation associates with the 3 different possible phenotypes (RALD and indolent and severe JMML). We thus postulate that RALD and JMML are not distinct entities but a continuum with additional genetic or epigenetic events contributing to the clinical phenotype and evolution. Therefore, close monitoring of such patients is recommended, and further efforts are needed to elucidate the additional factors.
The online version of this article contains a data supplement.
Authorship
Acknowledgments: This work was funded by grants from INSERM (to F.R.-L.), The European Research Council (ERC PIDIMMUN N°249816) (to A.F.), Association pour la Recherche contre le Cancer (SFI20121205717) (to A.M.-C.). This program has received a state subsidy managed by the National Research Agency under the “Investments for the Future” program bearing the reference ANR-10-IAHU-01 and IDEX Sorbonne Paris Cité (SPC/JFG/2013-031) (to F.R.-L.). The authors are grateful to Prof L. Lament for providing the patient's biological specimen.
Contribution: N.L. designed research, performed research, analyzed data, and wrote the paper; J.B., A.T., and M.-C.S. performed research and analyzed data; B.N. designed research and analyzed data; J.F., E.L., and N.J. performed research; F.S., N.M., and A.F. participated in clinical care; A.M.-C. designed research and analyzed data; and H.C. and F.R.-L. designed research and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Frédéric Rieux-Laucat, Institut Imagine, INSERM UMR 1163, 24 Bd du Montparnasse, 75015 Paris, France; e-mail: frederic.rieux-laucat@inserm.fr.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal