To the editor:
Southeast Asian ovalocytosis (SAO) is caused by a heterozygous 27-nucleotide deletion in SLC4A1 coding for band 3, the anion-exchange protein of the red cell membrane.1-3 This asymptomatic dominant trait is considered as a host genetic adaptation to malaria in Southeast Asia; homozygozity has not been described and is thought to be lethal.4 We report the first description of homozygous SAO in a child born to asymptomatic Comorian parents. After 22 weeks gestation, the male fetus presented with hydrops and severe anemia (hemoglobin [Hb] 2.9 g/dL) that was treated by in utero transfusion. Common acquired or genetic causes of hydrops were excluded. A second transfusion 7 weeks later triggered bradycardia and emergency delivery. Since birth, a monthly transfusion program has kept the Hb level between 7 and 10 g/dL. At 3 months of age, distal renal tubular acidosis (dRTA) was diagnosed (hyperchloremic metabolic acidosis, increased urinary pH, and Ca2+ urinary excretion, but no nephrocalcinosis) and treated by oral sodium bicarbonate and potassium gluconate. Iron chelation using deferoxamine mesilate was started at 6 months of age because of high ferritinemia.
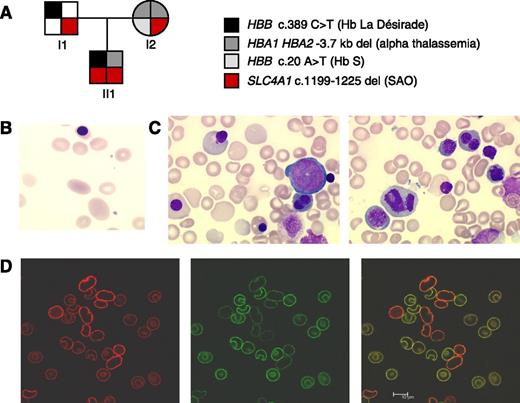
Genetic analyses indicated that the child carried a heterozygous 3.7 kb α-globin deletion, a heterozygous β-globin variant “La Désirade,” and homozygous SAO (Figure 1A).5 Both SAO heterozygote parents showed the typical SAO red cell morphology. Rare large ovalocytes could also be observed in the transfused proband’s blood (Figure 1B). In homozygous SAO, severe anemia resulted from both hemolysis (the haptoglobin level was undetectable) and deficient red cell production. Indeed, bone marrow examination revealed a rich erythroid lineage showing major signs of dyserythropoiesis including bi/multinuclearity, karyorrhexis, abnormal mitosis, and late erythroblasts with enlarged cytoplasm (Figure 1C). Interestingly, dyserythropoiesis was not described in the previously reported human cases of homozygous band 3 mutation but was observed in a zebra fish carrying mutations in the ortholog slc4a1.6,7 Here, dyserythropoiesis was not accounted for by the absence of band 3 because we showed that SAO band 3 was expressed on the proband red cells. Using confocal microscopy and selected antibodies, we could differentiate the proband from the donor cells and demonstrate that homozygous SAO band 3 is folded sufficiently well to insert into the membrane (Figure 1D).8
Characterization of homozygous SAO. (A) Pedigree of the family showing HBA1, HBA2, HBB (coding for α- and β-globin), and SLC4A1 genotype. Genetic analyses indicated that the child (II1) inherited a heterozygous 3.7-kb α-globin deletion from the mother, a heterozygous β-globin variant “La Désirade” (resulting from HBB c.389 C>T) from the father, and homozygous SAO. He has not inherited his mother sickle cell trait (HBB c.20C>T). Hb La Désirade is asymptomatic in heterozygotes and could not account for the early severe anemia because β-globin is not significantly expressed at 22 weeks gestation.5 Informed consent was provided according to the Declaration of Helsinki. Approval was obtained from the Bicêtre institutional review board for these studies. (B) Red cell sample from the transfused child contained donor cells as well as large ovalocytes. (C) May Grunwald Giemsa stain of a proband bone marrow sample showed marked dyserythropoisesis, mostly on late erythroblasts, with binuclearity, carrhyorexis, enlarged erythroblasts, and large reticulocytes and macrocytes. Myeloid and megacaryocyte lineages were normal. (D) Confocal microscopic imaging using selected antibodies could differentiate normal transfused cells and SAO red cells in a proband blood sample. Labeling with a polyclonal anti-band 3 antibody (AE1 pAb, red) that binds an intracellular epitope gave stronger fluorescence in the SAO cells than the donor cells (left). This effect was probably attributable to the fragility of the SAO cells, causing the SAO cells to be more readily permeabilized than the donor cells. In contrast, the donor cells could be seen to fluoresce strongly using the BRIC6 monoclonal antibody and a green fluorescent conjugated secondary antibody (middle). An overlay of the 2 images clearly differentiates the homozygous SAO cells from the donor cells, indicating that some SAO cells could survive in the circulation and that SAO band 3 was present at the red cell membrane, even in the absence of wild-type band 3 (right). Scale bar represents 10 μm.
Characterization of homozygous SAO. (A) Pedigree of the family showing HBA1, HBA2, HBB (coding for α- and β-globin), and SLC4A1 genotype. Genetic analyses indicated that the child (II1) inherited a heterozygous 3.7-kb α-globin deletion from the mother, a heterozygous β-globin variant “La Désirade” (resulting from HBB c.389 C>T) from the father, and homozygous SAO. He has not inherited his mother sickle cell trait (HBB c.20C>T). Hb La Désirade is asymptomatic in heterozygotes and could not account for the early severe anemia because β-globin is not significantly expressed at 22 weeks gestation.5 Informed consent was provided according to the Declaration of Helsinki. Approval was obtained from the Bicêtre institutional review board for these studies. (B) Red cell sample from the transfused child contained donor cells as well as large ovalocytes. (C) May Grunwald Giemsa stain of a proband bone marrow sample showed marked dyserythropoisesis, mostly on late erythroblasts, with binuclearity, carrhyorexis, enlarged erythroblasts, and large reticulocytes and macrocytes. Myeloid and megacaryocyte lineages were normal. (D) Confocal microscopic imaging using selected antibodies could differentiate normal transfused cells and SAO red cells in a proband blood sample. Labeling with a polyclonal anti-band 3 antibody (AE1 pAb, red) that binds an intracellular epitope gave stronger fluorescence in the SAO cells than the donor cells (left). This effect was probably attributable to the fragility of the SAO cells, causing the SAO cells to be more readily permeabilized than the donor cells. In contrast, the donor cells could be seen to fluoresce strongly using the BRIC6 monoclonal antibody and a green fluorescent conjugated secondary antibody (middle). An overlay of the 2 images clearly differentiates the homozygous SAO cells from the donor cells, indicating that some SAO cells could survive in the circulation and that SAO band 3 was present at the red cell membrane, even in the absence of wild-type band 3 (right). Scale bar represents 10 μm.
As expected, the child presented with dRTA. In the kidney, an N-terminally truncated band 3 (kAE1) is expressed in α-intercalated distal tubular cells and contributes to the acidification of urine. Total loss of band 3 results in dRTA, as reported in homozygous band 3 Coimbra.6 Homozygous SAO has a similar effect because SAO band 3 cannot exchange anions.9,10
The child is now 3 years old. His psychomotor development is normal, as well as his height and weight growth. He does not present neurologic complications, increased susceptibility to infection, or thromboembolic disorder; however, the prognosis is uncertain. Bone marrow transplantation can be considered because the disease is as severe as thalassemia major. Finally, a prenatal diagnosis can be proposed for a future pregnancy in this couple, and in case of homozygous SAO, medical termination of pregnancy remains an alternative.
Authorship
Acknowledgments: The authors thank the family for their interest and support and all members of involved laboratories for technical skill. This work was supported by the UK National Health Service R & D Directorate (J.F.F., L.J.B.).
Contribution: V.P., C.T., S.P., and L.J.B. designed the study; C.T., M.-L.C., and G.C. treated the patient and collected and interpreted clinical and biological data; V.P., A.P., M.E., S.P., M.F.-T., M.R., and A.F. designed, performed, and interpreted biochemical, hematologic, or genetic analyses; J.F.F. and L.J.B. designed, performed, and interpreted confocal microscopy analyses; and V.P., J.D., L.J.B., and C.T. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Véronique Picard, Service d’Hématologie Biologique, Hôpital Bicêtre, 78 rue du Général Leclerc, 94275 Le Kremlin Bicêtre, France; e-mail: veronique.picard@bct.aphp.fr.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal