Abstract
Somatic gain-of-function mutations in members of the RAS subfamily of small guanosine triphosphatases are found in > 30% of all human cancers. We recently described a syndrome of chronic nonmalignant lymphadenopathy, splenomegaly, and autoimmunity associated with a mutation in NRAS affecting hematopoietic cells, and initially we classified the disease as a variant of the autoimmune lymphoproliferative syndrome. Here, we demonstrate that somatic mutations in the related KRAS gene can also be associated with a nonmalignant syndrome of autoimmunity and breakdown of leukocyte homeostasis. The activating KRAS mutation impaired cytokine withdrawal–induced T-cell apoptosis through the suppression of the proapoptotic protein BCL-2 interacting mediator of cell death and facilitated proliferation through p27kip1 down-regulation. These defects could be corrected in vitro by mitogen-activated protein kinase/extracellular signal-regulated kinase kinase 1 or phosphatidyl inositol-3 kinase inhibition. We suggest the use of the term RAS-associated autoimmune leukoproliferative disease to differentiate this disorder from autoimmune lymphoproliferative syndrome.
Introduction
The autoimmune lymphoproliferative syndrome (ALPS) is characterized by childhood onset chronic lymphadenopathy, splenomegaly, multilineage cytopenias secondary to sequestration and autoimmune destruction, and an increased risk of B-cell lymphoma.1 Laboratory findings include polyclonal hypergammaglobulinemia and expansion of a unique population of circulating T-cell receptor αβ+B220+CD4−CD8− T (αβ+-DNT) lymphocytes.2,3 Most patients with ALPS harbor heterozygous autosomal-dominant germline mutations in FAS, with somatic FAS mutations representing the second most common genetic cause.4-7 Germline mutations in the genes encoding FAS ligand and caspase 10 have been identified in a small minority of patients.8-12 In our cohort, approximately one-third of the patients with ALPS have an undetermined genetic basis. In addition, there is a group of genetically undetermined ALPS-like patients without αβ+-DNT cell elevation.
We recently reported one person among these latter patients with a syndrome of lymphoproliferation, autoimmunity, and minimally increased αβ+-DNT cells caused by a somatic mutation in the NRAS gene, resulting in defective lymphocyte apoptosis.13 Here, we demonstrate that somatic mutations in the homologous KRAS gene can also be associated with a syndrome consisting of autoimmune phenomena and dysregulated leukocyte homeostasis, with normal αβ+-DNT cells. The activating KRAS mutation, like the previously described NRAS mutation, impaired intrinsic T-cell apoptosis through the suppression of the proapoptotic protein BCL-2 interacting mediator of cell death (BIM) and facilitated cellular proliferation by repression of p27kip1.
Methods
Cells and treatments
All patients were studied at the National Institutes of Health (NIH) under protocols approved by the institutional review board (93-I-0063 and 95-I-0066). DNA sequencing, apoptosis assays, immunoblotting, and active RAS pull down were performed as previously described.13
Plasmids and transfection
The plasmids pCEFL-KZ-AU5-KRAS-wt and pCEFL-KZ-AU5-KRAS-V12 were kindly provided by Silvio Gutkind (National Institute of Dental and Craniofacial Research, NIH). AU5-tagged KRAS G13C plasmid was constructed by site-directed mutagenesis with the use of the QuickChange kit (Stratagene) according the manufacturer's instructions. Transient transfections in human 293T and Jurkat cells were performed with the TransIT-LT1 reagent (MirusBio) and Lonza solution V kit (Lonza), respectively. Assays were performed 48 hours after transfection.
Results and discussion
Somatic gain-of-function KRAS mutation in 2 patients with ALPS-like symptoms
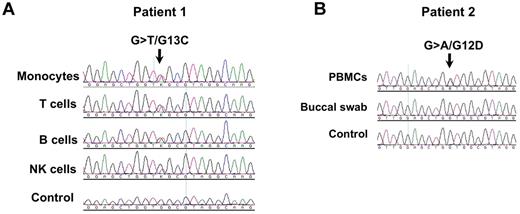
To identify novel genes linked to persistent lymphadenopathy, splenomegaly, and autoimmunity, we sequenced patients with ALPS-like syndromes for candidate genes and identified activating KRAS mutations in 2 patients. Patient 1 showed a c.37G > T, p.G13C mutation, present in lymphoid and myeloid cell types (Figure 1A) but not in heart tissue (data not shown). Patient 2 showed a c.35G > A, p.G12D mutation in peripheral blood mononuclear cells but not in buccal swab cells (Figure 1B). This indicated a somatic origin probably at the hematopoietic stem cell level. No mutations in FAS, NRAS, or HRAS were detected in either patient. Gain of function for G13C was confirmed in patient 1 by the increased amount of active RAS present in cells after transfection with a plasmid-encoding mutant G13C versus wild-type KRAS (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). KRAS G12D is already described to produce a gain of function.14
Gain-of-function somatic KRAS mutations. (A) Cell subsets were sorted by flow cytometry and used for DNA sequencing; (B) Peripheral blood mononuclear cells (PBMCs) were lysed and used for DNA sequencing; a buccal swab was also sequenced to rule out a germline mutation. The small mutant peak seen in the buccal sample probably reflects the presence of hematopoietic cells in the cell mixture.
Gain-of-function somatic KRAS mutations. (A) Cell subsets were sorted by flow cytometry and used for DNA sequencing; (B) Peripheral blood mononuclear cells (PBMCs) were lysed and used for DNA sequencing; a buccal swab was also sequenced to rule out a germline mutation. The small mutant peak seen in the buccal sample probably reflects the presence of hematopoietic cells in the cell mixture.
Clinical history and laboratory findings
Patient 1.
This white female patient had a history of lymphadenopathy and splenomegaly first noted at 4 years of age at the time of a tonsillectomy and adenoidectomy for recurrent upper respiratory tract infections. Splenomegaly, autoimmune hemolytic anemia, and thrombocytopenia (Evan syndrome) were diagnosed at follow-up. She was evaluated at the NIH at 9 years of age and was found to have positive serology for several autoantibodies and polyclonal hypergammaglobulinemia as well as persistent splenomegaly and lymphadenopathy (supplemental Table 1). Flow cytometry did not show the hallmark elevation of αβ+-DNTs seen in ALPS but did document B-cell lymphocytosis and monocytosis (supplemental Table 1). Lymph node biopsy showed plasmacytosis but no paracortical infiltration by αβ+-DNTs (supplemental Figure 2). The patient had a history of recurrent infections, including bronchitis, otitis media (several episodes), cellulitis, lymphadenitis, and pneumonia (one episode each), without documentation of an infectious agent. She died at the age of 13 after an episode of fever followed 48 hours later by acute loss of consciousness and cardiac arrest at home. Autopsy studies could not determine the cause of death but ruled out malignancy.
Patient 2.
This patient is a white female of Italian ancestry who was first noted to have splenomegaly with neutropenia, monocytosis, reticulocytosis, thrombocytopenia, and hyperuricemia when evaluated for a routine respiratory tract infection with facial rash at 5 years of age. Serologies were positive for Parvovirus B19 and Epstein-Barr virus, and the findings were attributed to acute viral infection, with hematologic parameters returning to near normal levels 5 months later. The patient remained well with the exception of periodic, painful, erythematous bilateral swelling of the ankles progressing to ecchymosis over a short period, which occurred once to twice yearly and was associated with swimming and beach activities. After an abrupt increase in the frequency and severity of these symptoms at 7 years of age, the patient was referred for further evaluation and was found to have pancytopenia, massive splenomegaly with bulky intra-abdominal and inguinal lymphadenopathy, marked hypergammaglobulinemia, and anticardiolipin and anti-β2-glycoprotein-I antibodies. Bone marrow examination showed only a hyperreactive state. Hematologic malignancy and lysosomal storage diseases were excluded. A diagnosis of Evan syndrome was considered on the basis of the presence of antineutrophil and antiplatelet antibodies. She had normal cognitive and motor development throughout these episodes. Weight and height were normal at birth, decreasing progressively to ∼ 3 percentile currently. She continues to the present with rash of the lower extremities presumed to represent a vasculopathy secondary to antiphospholipid syndrome, but she is otherwise well.
KRAS mutation impaired lymphocyte apoptosis and increased proliferation
We evaluated lymphocyte apoptosis and proliferation in samples from patient 1 and found that the KRAS G13C mutation did not impair FAS-mediated apoptosis but induced resistance to apoptosis triggered by interleukin-2 (IL-2) withdrawal of activated T lymphocytes (Figure 2A-B). Because BIM, a proapoptotic protein of the BCL-2 family, is the main mediator of this form of apoptosis,15 we checked the patient's cells and found markedly reduced BIM levels compared with healthy controls or patients with ALPS-FAS (Figure 2C). These findings are reminiscent of our previous work that described a patient with a mutation in NRAS.13 We speculate that low BIM levels underlie the accumulation of B cells and monocytes in these patients, given the critical role for this protein in leukocyte homeostasis in murine models.15
Functional evaluation of patient 1 lymphocytes. Activated peripheral blood mononuclear cells (PBMCs) from normal volunteers (NL), a patient with an inactivating FAS mutation (FAS mut), a patient with a gain-of-function somatic NRAS mutation (NRAS mut), and from patient 1 (KRAS mut) were treated for 18 hours with anti-Fas (Apo1.3) antibody (A) or cultured in media without IL-2 for the indicated periods of time (B). (C) Analysis by BIM immunoblotting under IL-2 rich “+” (100 IU/mL) or low “-”(1 IU/mL) conditions in PBMCs from a normal control (NL), a patient with a FAS mutation (FASm), a patient with an NRAS mutation (NRASm), and patient 1 (KRASm). β-actin is a loading control. (D) Activated lymphocytes from a normal control (NL) and patient 1 (KRASm) were cultured in media without IL-2 and treated with dimethyl sulfoxide (DMSO), PD98059 (PD; 20μM), or LY294002 (LY; 10μM) for the indicated periods of time. Apoptosis was measured daily by flow cytometry. (E) Activated PBMCs from normal volunteers (NLs), a patient with an inactivating FAS mutation (FAS mut), a patient with a gain-of-function NRAS mutation (NRAS mut), and from patient 1 (KRAS mut) were cultured in media the indicated concentrations of IL-2, and total cell counts were determined at baseline and 72 hours later; (F) p27kip1 expression was interrogated by immunoblotting in PBMCs (top) under IL-2 rich “+” (100 IU/mL) or low “-” (1 IU/mL) conditions and also in Jurkat T-cell lines (bottom) transfected with 1 or 6 μg of plasmids encoding GFP-only (negative control), or wild-type, G13C, or G12V (positive control) KRAS. Error bars represent SEs. Data shown are representative of 2 (A-E) independent experiments.
Functional evaluation of patient 1 lymphocytes. Activated peripheral blood mononuclear cells (PBMCs) from normal volunteers (NL), a patient with an inactivating FAS mutation (FAS mut), a patient with a gain-of-function somatic NRAS mutation (NRAS mut), and from patient 1 (KRAS mut) were treated for 18 hours with anti-Fas (Apo1.3) antibody (A) or cultured in media without IL-2 for the indicated periods of time (B). (C) Analysis by BIM immunoblotting under IL-2 rich “+” (100 IU/mL) or low “-”(1 IU/mL) conditions in PBMCs from a normal control (NL), a patient with a FAS mutation (FASm), a patient with an NRAS mutation (NRASm), and patient 1 (KRASm). β-actin is a loading control. (D) Activated lymphocytes from a normal control (NL) and patient 1 (KRASm) were cultured in media without IL-2 and treated with dimethyl sulfoxide (DMSO), PD98059 (PD; 20μM), or LY294002 (LY; 10μM) for the indicated periods of time. Apoptosis was measured daily by flow cytometry. (E) Activated PBMCs from normal volunteers (NLs), a patient with an inactivating FAS mutation (FAS mut), a patient with a gain-of-function NRAS mutation (NRAS mut), and from patient 1 (KRAS mut) were cultured in media the indicated concentrations of IL-2, and total cell counts were determined at baseline and 72 hours later; (F) p27kip1 expression was interrogated by immunoblotting in PBMCs (top) under IL-2 rich “+” (100 IU/mL) or low “-” (1 IU/mL) conditions and also in Jurkat T-cell lines (bottom) transfected with 1 or 6 μg of plasmids encoding GFP-only (negative control), or wild-type, G13C, or G12V (positive control) KRAS. Error bars represent SEs. Data shown are representative of 2 (A-E) independent experiments.
Apoptosis resistance in patient 1 was at least partially mediated by downstream extracellular signal-regulated kinase (ERK) and phosphatidyl inositol-3 kinase hyperactivation, because the addition of mitogen-activated protein kinase/ERK kinase 1 (PD98059; 20μM) or phosphatidyl inositol-3 kinase (LY294002; 10μM) inhibitors almost completely abrogated the apoptotic defect in vitro (Figure 2D).16 Myeloid cells from patients with somatic KRAS mutations associated with juvenile myelomonocytic leukemia, unlike normal myeloid cells, expand in the presence of low concentrations of granulocyte-macrophage colony-stimulating factor.17 We hypothesized that a similar phenomenon could occur in this patient's cells and cultured activated primary lymphocytes under limiting IL-2 concentrations and demonstrated increased proliferation compared with controls (Figure 2E). This was linked to low levels of the cell cycle inhibitor protein p27kip1 in primary cells and transfected cell lines, as previously described for cancer cells (Figure 2F).18,19
Because missense mutations at certain RAS codons, including 12, 13, and 61, are known prevent interactions with guanosine triphosphatase (GTPase)–activating proteins and thus greatly decrease GTP hydrolysis, resulting in constitutively active RAS,20 it is reasonable to speculate that the KRAS G12D mutation in patient 2 also impairs apoptosis and proliferation. Moreover, the G12V control showed repressed p27kip1 levels.
Spectrum of clinical manifestations associated with somatic RAS mutations
KRAS is a member of the p21 small GTPase family of proteins that also includes HRAS and NRAS. Germline RAS mutations are associated with specific developmental disorders, including Noonan, Costello, and cardio-facio-cutaneous syndromes.21 Somatic RAS mutations are seen in > 30% of all human cancers.16 In the hematopoietic system, somatic KRAS and NRAS mutations are commonly observed in aggressive tumors such as multiple myeloma or juvenile myelomonocytic leukemia.22
This report and our previous work13 suggest that activating somatic mutations in NRAS or KRAS can be associated with a nonmalignant hematologic syndrome, which we refer to as RAS-associated autoimmune leukoproliferative disease (RALD).23 The factors determining which patients with somatic RAS mutations develop hematologic malignancy versus RALD are unknown, but a previous report also showed that patients with somatic NRAS or KRAS mutations could follow a more benign clinical course.24
The clinical entity described here seems distinct from ALPS in several respects: the characteristic αβ+-DNT cells are present at normal levels or only marginally elevated in the peripheral blood and absent in lymph nodes; there is no defect in the FAS pathway of apoptosis; biomarkers typically associated with ALPS, such as IL-10 and sFASL25,26 are normal; and monocytosis, reminiscent of juvenile myelomonocytic leukemia, was seen in all the patients evaluated to date (supplemental Table 2).
We suggest that RALD should be considered in the differential diagnosis of patients with autoimmune cytopenias, monocytosis, and lymphoid organ expansion. These patients may in the future benefit from therapies directed at blocking ERK and/or RAS that are under development as cancer therapeutics.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank the patients and their families for participating in the study. We also thank Silvio Gutkind for plasmids and Jack Bleesing for patient referral.
This work was supported by the Intramural Research Program of the National Institutes of Health Clinical Center.
National Institutes of Health
Authorship
Contribution: J.E.N. designed and performed candidate gene research and genetic analysis; L.L. performed experiments and analyzed data; T.A.F. supervised the research; V.K.R., J.D., and M.N. contributed patient samples and provided clinical care; L.A.B. performed mutation analysis; K.C.D. provided patient cells; I.C. performed experiments; S.P. performed histopathologic studies; M.R. performed mutation analysis by pyrosequencing; J.B.O. designed and supervised the project, performed experiments, and wrote the manuscript; and all authors revised the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: João Bosco Oliveira, Department of Laboratory Medicine, Clinical Center, National Institutes of Health, 10 Center Dr, Bethesda, MD; e-mail: oliveirajb@cc.nih.gov.
References
Author notes
J.E.N. and L.L. contributed equally to this study.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal