Abstract
Autoimmune lymphoproliferative syndrome (ALPS) is characterized by childhood onset of lymphadenopathy, hepatosplenomegaly, autoimmune cytopenias, elevated numbers of double-negative T (DNT) cells, and increased risk of lymphoma. Most cases of ALPS are associated with germline mutations of the FAS gene (type Ia), whereas some cases have been noted to have a somatic mutation of FAS primarily in their DNT cells. We sought to determine the proportion of patients with somatic FAS mutations among a group of our ALPS patients with no detectable germline mutation and to further characterize them. We found more than one-third (12 of 31) of the patients tested had somatic FAS mutations, primarily involving the intracellular domain of FAS resulting in loss of normal FAS signaling. Similar to ALPS type Ia patients, the somatic ALPS patients had increased DNT cell numbers and elevated levels of serum vitamin B12, interleukin-10, and sFAS-L. These data support testing for somatic FAS mutations in DNT cells from ALPS patients with no detectable germline mutation and a similar clinical and laboratory phenotype to that of ALPS type Ia. These findings also highlight the potential role for somatic mutations in the pathogenesis of nonmalignant and/or autoimmune hematologic conditions in adults and children.
Introduction
Autoimmune lymphoproliferative syndrome (ALPS) is typically a heritable disorder of apoptosis, resulting in accumulation of autoreactive lymphocytes.1 It manifests in early childhood as nonmalignant lymphadenopathy, with hepatosplenomegaly and autoimmune multilineage cytopenias.2,3 The laboratory features of ALPS demonstrate lymphocyte dysregulation as evidenced by elevated double-negative T (DNT: T-cell receptor [TCR]-αβ+CD3+CD4−CD8−B220+) cells in peripheral blood and lymphoid tissues,3 diminished CD27+ memory B cells, defective lymphocyte apoptosis in vitro, lymph node biopsies notable for reactive follicular hyperplasia and paracortical expansion,4 autoantibodies, autoimmune cytopenias, and skewed T helper cell responses, including elevated levels of interleukin-10 (IL-10).5 Moreover, patients with mutations affecting the intracellular portion of FAS carry a 51- and 14-fold increased risk of developing Hodgkin and non-Hodgkin lymphoma, respectively, compared with the general population.6
The majority of ALPS patients exhibit a germline mutation in the FAS (TNFRSF6, CD95, APO1) gene, which encodes a receptor protein that is expressed on a wide range of cell types, including B- and T-lymphocytes.3,7 Heterozygous autosomal dominant mutations in FAS account for approximately 65% of all ALPS cases, that is, those classified as ALPS type Ia. Other cases of ALPS have been associated with mutations in the genes encoding FAS ligand (ALPS Ib), caspases 8 or 10 (ALPS type II), or NRAS (ALPS type IV), whereas cases in which no mutations have yet been identified are classified as ALPS type III or ALPS phenotype.2,4,8,9 ALPS type III patients meet all the defining criteria of ALPS: defective in vitro apoptosis, lymphadenopathy and splenomegaly, and increased DNT cells, whereas ALPS phenotype patients have a similar clinical phenotype but lack a demonstrable in vitro apoptosis defect pertaining to the FAS pathway.
Holzelova et al10 previously identified 6 ALPS type III (phenotype by our classification earlier in the “Introduction”) patients with somatic FAS mutations mainly in their DNT cell population, which we currently refer to as somatic ALPS. However, information as to the proportion of ALPS patients with somatic FAS mutations and any differences in their clinical phenotype compared with ALPS type Ia were not presented. Here we report our experience with somatic FAS mutations in our large cohort of ALPS type III and ALPS phenotype patients and characterize their clinical manifestations. Our data support testing for somatic FAS mutations in all ALPS type III and ALPS phenotype patients with a similar clinical and laboratory phenotype to that of ALPS type Ia. The data also highlight a potential role of somatic FAS mutations in the pathogenesis of nonmalignant and autoimmune hematologic conditions in adults and children.
Methods
Patients
DNA isolated from DNT cells from fresh and cryopreserved peripheral blood mononuclear cells (PBMCs) was tested for somatic FAS mutations. Subjects included 15 ALPS type III and 16 ALPS phenotype patients and 18 sex-matched ALPS type Ia patients used as controls. The latter were not absolutely age-matched with the test population because ALPS type Ia patients typically present before the age of 5 years. Samples and clinical data were studied with informed consent under National Institutes of Health Institutional Review Board–approved protocol 93-I-0063, in accordance with the Declaration of Helsinki.
Isolation of DNT and cellular subsets
DNT cells were isolated from PBMCs by magnetic cell separation (StemCell Technologies) by combining CD4+ and CD4− isolation kits according to the manufacturer's instructions. Briefly, 50 μL of CD4+ and CD4− selection beads were added to 5 × 107 cells in phosphate-buffered saline/2% fetal bovine serum. Cells and beads were incubated for 15 minutes at room temperature, followed by 100 μL of magnetic particles for 15 minutes at room temperature. Tubes were placed in a magnetic holder for two 5-minute separations, and nonlabeled cells were collected. Purity was determined by flow cytometry (FACSCalibur; BD Biosciences) of labeled cells: CD4-allophycocyanin, CD8–fluorescein isothiocyanate, and TCR-αβ-phycoerythrin (BD Biosciences). Purities typically exceeded 90% by flow cytometry when starting with greater than 2% DNT cells in the peripheral blood. In addition, single-positive cell populations were also isolated by magnetic separation (Miltenyi Biotec) using CD4−, CD8−, CD14−, and CD19− selection kits according to the manufacturer's instructions. Cells were then stained with phycoerythrin-labeled CD4, CD8, CD14, or CD19 (BD Biosciences) to determine purity by flow cytometry. All flow cytometric data were analyzed using FlowJo, Version 8.8 (TreeStar).
FAS sequencing of DNT cells
DNA was extracted using the Archive Pure DNA kit (5 Prime) according to the manufacturer's instructions. The 9 exons and intron/exon boundaries of the FAS locus were polymerase chain reaction-amplified and sequenced as previously described.11 All identified nucleotide changes were confirmed by sequencing a second polymerase chain reaction product. The sequence of FAS was compared with the genomic reference sequence NG_009089.1. Nucleotides and amino acids were numbered according to the widely used, but nonstandard, notation for FAS where the “A” of the ATG translation initiation codon is designated as “195” and the initiator methionine numbered as “−16.” The predicted amino acid change was written using current Human Genome Variation Society nomenclature.12
Biomarkers
Immunophenotyping and apoptosis assays were performed as previously described.8,13,14 Patient medical and laboratory records were reviewed, with the earliest available result presented. Plasma IL-10 (Pierce Endogen) and soluble FAS-L (R&D Systems) were quantified on thawed plasma samples by ELISA following the manufacturer's instructions. Serum vitamin B12 assays were done in our clinical biochemistry laboratory using a Dimension Vista system (Siemens).
Statistical analysis
Data were analyzed using Prism, Version 5.0 software (GraphPad) for Kruskal-Wallis rank-sum and Dunn multiple comparison tests.
Results
FAS mutations are identified in purified DNT cells, but not in whole PBMCs
DNT cells were isolated by magnetic bead separation from 31 patients (supplemental Table 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). DNA from 15 ALPS type III patients and 16 ALPS phenotype patients was tested for FAS mutations. All 31 patients had previously tested negative for FAS mutations from PBMC DNA preparations. A mutant peak in the chromatogram obtained with the genomic DNA from the patient PBMCs was not distinguishable from baseline noise, even on re-review. This is probably because the mutant cells represented less than 20% of the total lymphocytes, which is, in our experience, below the limit of mutation detection by current sequencing methods. In a series of titration experiments, we found that somatic mutations were reliably detectable by sequencing DNA from preparations of isolated DNTs with purities more than 50% (data not shown). Twelve of 31 (38.7%) patients tested had heterozygous somatic FAS mutations in their DNT cells: 6 of 15 ALPS type III patients (6 males; age range, 1-17 years; median age of disease onset, 10.5 years) and 6 of 16 ALPS phenotype patients (3 males and 3 females; age range, 3 months to 48 years; median age of onset, 1 year). Single-positive mononuclear cells (CD4, CD8, CD14, CD20), DNT cells, and buccal epithelial cells from one patient with a somatic mutation resulting in loss of a restriction endonuclease site (HpyCHIII) were tested for the presence or absence of the mutation observed in the DNT cell population. We confirmed that the mutation was present in less than 10% of PBMCs but not in buccal epithelial cells (data not shown), similar to the findings of Holzelova et al10 In addition, all 12 somatic mutations resulted in known (N = 4) or predicted (N = 8) functional loss of normal FAS signaling (Table 1). Ten of the mutations were predicted to lead to a premature stop codon; 4 of these have been previously observed associated with ALPS as a germline mutation and 6 were newly identified. Although rare mRNAs harboring premature stop codons escape the degradation pathway,15 the nonsense mutations followed by a spliceable intron (E195X, V204GfsX6, N207RfsX3, and L208X) are assumed to generate transcripts that are underexpressed (in the affected cells), as nonsense-mediated mRNA decay is currently observed as a major control mechanism of the mRNA quality in cells.16 Indeed, forms of ALPS Ia with low penetrance have been shown to be the result of haploinsufficiency.17,18 In these cases, heterozygous FAS mutations generated frameshifted transcripts containing premature translation stop codons. Similarly, we have observed 16 cases of ALPS Ia associated with heterozygous truncating FAS mutations leading to haploinsufficiency (A. Hsu, unpublished data, January 2004). The splice mutations that abolish the FAS exon 8 consensus splice sites [846(−9)_847del 11, 846(−1)g→a, 846(−1)g→t, 870G→T, 870(+1)g→a] have been previously shown by our group19 and others20,21 to result in deletion of exon 8 and frameshifting plus premature termination in exon 9. These mutations exert a dominant-negative effect (in the affected cells) as the majority of mutations affecting the intracytoplasmic tail of FAS protein result in defective trimer formation.19 The D244N mutation has been observed in ALPS type Ia and is predicted to lead to diminished FAS-associated death domain binding.22-26 Changes of D244 to V, Y, or G have also been found to result in dominant-negative effects.14,27 The H95R mutation resulted in decreased FAS-L binding as observed and tested in an ALPS type Ia patient with the same mutation (data not shown). The somatic mutations were clustered in an approximately 150-base region of FAS exons 7, 8, and 9, with the exception of H95R, which occurred in FAS exon 3.
Somatic FAS mutations
| Patient . | Nucleotide change . | Location . | Predicted amino acid change . | Effect . |
|---|---|---|---|---|
| 43 | 825G→T | Exon 7 | E195X* | NMD, haploinsuffiency |
| 84 | 846(−9)_847del 11 | Intron 7 | E202MfsX4 (splice mutation)* | Deletion of exon 8 and frameshifting plus premature termination in exon 9; dominant-negative effect |
| 135 | 846(−1)g→t | Intron 7 | E202MfsX4 (splice mutation)* | Deletion of exon 8 and frameshifting plus premature termination in exon 9; dominant-negative effect† |
| 228 | 972G→A | Exon 9 | D244N* | Diminished FADD binding; dominant negative effect† |
| 235 | 870(+1)g→a | Intron 8 | E202MfsX4 (splice mutation)* | Deletion of exon 8 and frameshifting plus premature termination in exon 9; dominant-negative effect† |
| 241 | 862_869del8 | Exon 8 | N207RfsX3* | NMD,‡ haploinsuffiency |
| 108 | 870G→T | Exon 8 | E202MfsX4 (splice mutation)* | Deletion of exon 8 and frameshifting plus premature termination in exon 9; dominant-negative effect§ |
| 164 | 851_852delAG | Exon 8 | V204Gfs6* | NMD,‡ haploinsuffiency |
| 256 | 865T→G | Exon 8 | L208X* | NMD,‡ haploinsuffiency |
| 262 | 526A→G | Exon 3 | H95R* | Decreased FasL binding; dominant-negative effect‖ |
| 263 | 846(−1)g→a | Intron 7 | E202MfsX4 (splice mutation)* | Deletion of exon 8 and frameshifting plus premature termination in exon 9; dominant-negative effect§ |
| 276 | 876_880del5 | Exon 9 | D212EfsX2* | Premature termination in exon 9; dominant-negative effect |
| Patient . | Nucleotide change . | Location . | Predicted amino acid change . | Effect . |
|---|---|---|---|---|
| 43 | 825G→T | Exon 7 | E195X* | NMD, haploinsuffiency |
| 84 | 846(−9)_847del 11 | Intron 7 | E202MfsX4 (splice mutation)* | Deletion of exon 8 and frameshifting plus premature termination in exon 9; dominant-negative effect |
| 135 | 846(−1)g→t | Intron 7 | E202MfsX4 (splice mutation)* | Deletion of exon 8 and frameshifting plus premature termination in exon 9; dominant-negative effect† |
| 228 | 972G→A | Exon 9 | D244N* | Diminished FADD binding; dominant negative effect† |
| 235 | 870(+1)g→a | Intron 8 | E202MfsX4 (splice mutation)* | Deletion of exon 8 and frameshifting plus premature termination in exon 9; dominant-negative effect† |
| 241 | 862_869del8 | Exon 8 | N207RfsX3* | NMD,‡ haploinsuffiency |
| 108 | 870G→T | Exon 8 | E202MfsX4 (splice mutation)* | Deletion of exon 8 and frameshifting plus premature termination in exon 9; dominant-negative effect§ |
| 164 | 851_852delAG | Exon 8 | V204Gfs6* | NMD,‡ haploinsuffiency |
| 256 | 865T→G | Exon 8 | L208X* | NMD,‡ haploinsuffiency |
| 262 | 526A→G | Exon 3 | H95R* | Decreased FasL binding; dominant-negative effect‖ |
| 263 | 846(−1)g→a | Intron 7 | E202MfsX4 (splice mutation)* | Deletion of exon 8 and frameshifting plus premature termination in exon 9; dominant-negative effect§ |
| 276 | 876_880del5 | Exon 9 | D212EfsX2* | Premature termination in exon 9; dominant-negative effect |
Predicted protein based on change in nucleotide sequence.
Protein based on previously observed mutation in germline ALPS and predicted result.
NMD, nonsense-mediated decay.
870G→A and 846(−1)g→t have been in observed in ALPS type Ia.
Previously found to result in decreased FasL binding in patients with ALPS type Ia (A. Hsu, unpublished data, January 2004).
Clinical findings
Clinically, the somatic ALPS patients were similar to ALPS patients with germline FAS gene mutations (type Ia), although they had a slightly lower incidence of splenectomy and lower lymphocyte counts (supplemental Table 1). Somatic ALPS patients showed variable lymph node pathology. Lymph node biopsies were reviewed on 9 somatic ALPS patients. Four patients exhibited characteristic features of ALPS-associated lymphadenopathy, including expansion of DNTs, and in 3 patients, increased S-100-positive cells with emperipolesis resembling Rosai-Dorfman disease were noted.28 The remaining 5 patients showed more nonspecific changes of reactive follicular and paracortical hyperplasia. Other clinical parameters, such as age at presentation, lymph node size, incidence of autoimmune cytopenias, hypergammaglobulinemia, and single-positive lymphocyte percentages, were similar between all groups (supplemental Table 1). As expected, a significant difference was noted in in vitro apoptosis, with ALPS type Ia patients having a more pronounced defect than somatic FAS mutant patients, and type III ALPS patients showing a defect whereas ALPS phenotype patients did not, by definition. Although the apoptotic defects in ALPS type III patients tended to be milder than in the type Ia patients serving as controls for this study, the difference was not statistically significant. The initial age of presentation of the ALPS type III patients who were subsequently found to have a somatic FAS mutation was older than that of the type Ia patients. In addition, the predominance of male type III patients with somatic mutations contrasted with the equal gender distribution of the ALPS phenotype patients with somatic mutations. However, there were no significant differences found comparing all somatic mutation patients with age-matched type Ia patients. The individual clinical and immunologic data of the somatic patients are described in Tables 2 and 3, respectively. Interestingly, those originally classified as the ALPS phenotype with somatic FAS mutations had a greater percentage of DNT cells compared with the remaining somatic FAS mutation-positive patients or the ALPS type III and phenotype patients without a somatic FAS mutation (P < .05; Table 3).
Clinical characteristics of somatic ALPS patients
| Patient . | Original ALPS classification . | Sex . | Age at presentation . | LN size* . | Age at splenectomy, y . | Cytopenias† . | Apoptosis‡ . |
|---|---|---|---|---|---|---|---|
| 43 | Phenotype | M | 3 mo | 3 | 6 | A,T,N | 77 |
| 84 | Phenotype | M | 6 mo | 2 | — | A,N | 61 |
| 135 | Phenotype | F | 1 y | 4 | — | A,T,N | 55 |
| 228 | Phenotype | F | 6 mo | 3 | — | A,AH,N | 102 |
| 235 | Phenotype | M | 48 y | 2 | — | A | 58 |
| 241 | Phenotype | F | 43 y | 4 | — | N | 53 |
| 108 | Type III | M | 1 y | 4 | 5 | A,T,N | 18 |
| 164 | Type III | M | 17 y | 3 | — | N | 39 |
| 256 | Type III | M | 11 y | 4 | — | HSP-AG | 49 |
| 262 | Type III | M | 12 y | 4 | — | A | 23 |
| 263 | Type III | M | 1 y | 3 | 6 (partial) | A,T,N | 18 |
| 276 | Type III | M | 10 y | 4 | — | A | 41 |
| Patient . | Original ALPS classification . | Sex . | Age at presentation . | LN size* . | Age at splenectomy, y . | Cytopenias† . | Apoptosis‡ . |
|---|---|---|---|---|---|---|---|
| 43 | Phenotype | M | 3 mo | 3 | 6 | A,T,N | 77 |
| 84 | Phenotype | M | 6 mo | 2 | — | A,N | 61 |
| 135 | Phenotype | F | 1 y | 4 | — | A,T,N | 55 |
| 228 | Phenotype | F | 6 mo | 3 | — | A,AH,N | 102 |
| 235 | Phenotype | M | 48 y | 2 | — | A | 58 |
| 241 | Phenotype | F | 43 y | 4 | — | N | 53 |
| 108 | Type III | M | 1 y | 4 | 5 | A,T,N | 18 |
| 164 | Type III | M | 17 y | 3 | — | N | 39 |
| 256 | Type III | M | 11 y | 4 | — | HSP-AG | 49 |
| 262 | Type III | M | 12 y | 4 | — | A | 23 |
| 263 | Type III | M | 1 y | 3 | 6 (partial) | A,T,N | 18 |
| 276 | Type III | M | 10 y | 4 | — | A | 41 |
— indicates not applicable.
LN size grading: 1 = presence of few shotty nodes; 2 = presence of multiple 1- to 2-cm nodes; 3 = more than 2-cm nodes; and 4 = visible LN or more than 4 cm by CT.
A = anemia; T = thrombocytopenia; AH = autoimmune hepatitis; N = neutropenia; HSP-AG = Henoch-Schonlein Purpura-acute glomerulonephritis.
Percent of normal control; less than 50% is considered defective.
Immunologic characteristics of somatic ALPS patients
| Patient . | Original ALPS classification . | IgG* . | Lymphocyte* . | % DNT† . | % CD3* . | % CD4* . | % CD8* . | % CD20* . | % CD56+CD3−* . |
|---|---|---|---|---|---|---|---|---|---|
| 43 | Phenotype | 1500 | 1.15 | 5.2 | 55.6 | 22.6 | 29.1 | 26.4 | 13.5 |
| 84 | Phenotype | 650 | 2.73 | 7.1 | 90.3 | 58.8 | 22.7 | 7.4 | 2.2 |
| 135 | Phenotype | 2060 | 0.87 | 4.8 | 64.6 | 16.5 | 46.7 | 12.5 | 22.9 |
| 228 | Phenotype | 2460 | 6.53 | 17.2 | 63.2 | 17.6 | 23.5 | 35.0 | 1.8 |
| 235 | Phenotype | 2520 | 2.00 | 10.6 | 43.7 | 14.6 | 28.7 | 8.6 | 47.7 |
| 241 | Phenotype | 3110 | 1.40 | 18.6 | 83.5 | 32.7 | 31.7 | 10.1 | 6.4 |
| Mean ± SE | 2050 ± 354 | 2.4 ± 0.9 | 10.6 ± 2.5 | 66.8 ± 7.1 | 27.1 ± 6.9 | 30.4 ± 3.6 | 16.7 ± 4.6 | 15.8 ± 7.2 | |
| 108 | Type III | 3800 | 0.81 | 3.5 | 84.6 | 38.3 | 36.8 | 6.0 | 9.4 |
| 164 | Type III | 1230 | 1.55 | 4.8 | 73.3 | 34.2 | 29.4 | 20.3 | 6.4 |
| 256 | Type III | 1680 | 2.11 | 3.9 | 83.0 | 35.3 | 42.3 | 3.5 | 13.5 |
| 262 | Type III | 2020 | 1.23 | 8.1 | 74.2 | 38.9 | 27.2 | 16.6 | 9.2 |
| 263 | Type III | 1690 | 2.10 | 2.7 | 66.3 | 34.5 | 29.9 | 22.6 | 11.1 |
| 276 | Type III | 1840 | 1.20 | 4.0 | 81.1 | 37.6 | 29.3 | 14.6 | 3.9 |
| Mean ± SE | 2043 ± 367 | 1.5 ± 0.2 | 4.5 ± 0.8‡ | 77.1 ± 2.9 | 36.5 ± 0.8 | 32.5 ± 2.4 | 13.9 ± 3.1 | 8.9 ± 1.4 |
| Patient . | Original ALPS classification . | IgG* . | Lymphocyte* . | % DNT† . | % CD3* . | % CD4* . | % CD8* . | % CD20* . | % CD56+CD3−* . |
|---|---|---|---|---|---|---|---|---|---|
| 43 | Phenotype | 1500 | 1.15 | 5.2 | 55.6 | 22.6 | 29.1 | 26.4 | 13.5 |
| 84 | Phenotype | 650 | 2.73 | 7.1 | 90.3 | 58.8 | 22.7 | 7.4 | 2.2 |
| 135 | Phenotype | 2060 | 0.87 | 4.8 | 64.6 | 16.5 | 46.7 | 12.5 | 22.9 |
| 228 | Phenotype | 2460 | 6.53 | 17.2 | 63.2 | 17.6 | 23.5 | 35.0 | 1.8 |
| 235 | Phenotype | 2520 | 2.00 | 10.6 | 43.7 | 14.6 | 28.7 | 8.6 | 47.7 |
| 241 | Phenotype | 3110 | 1.40 | 18.6 | 83.5 | 32.7 | 31.7 | 10.1 | 6.4 |
| Mean ± SE | 2050 ± 354 | 2.4 ± 0.9 | 10.6 ± 2.5 | 66.8 ± 7.1 | 27.1 ± 6.9 | 30.4 ± 3.6 | 16.7 ± 4.6 | 15.8 ± 7.2 | |
| 108 | Type III | 3800 | 0.81 | 3.5 | 84.6 | 38.3 | 36.8 | 6.0 | 9.4 |
| 164 | Type III | 1230 | 1.55 | 4.8 | 73.3 | 34.2 | 29.4 | 20.3 | 6.4 |
| 256 | Type III | 1680 | 2.11 | 3.9 | 83.0 | 35.3 | 42.3 | 3.5 | 13.5 |
| 262 | Type III | 2020 | 1.23 | 8.1 | 74.2 | 38.9 | 27.2 | 16.6 | 9.2 |
| 263 | Type III | 1690 | 2.10 | 2.7 | 66.3 | 34.5 | 29.9 | 22.6 | 11.1 |
| 276 | Type III | 1840 | 1.20 | 4.0 | 81.1 | 37.6 | 29.3 | 14.6 | 3.9 |
| Mean ± SE | 2043 ± 367 | 1.5 ± 0.2 | 4.5 ± 0.8‡ | 77.1 ± 2.9 | 36.5 ± 0.8 | 32.5 ± 2.4 | 13.9 ± 3.1 | 8.9 ± 1.4 |
Percent DNT normal values < 1.5% of total lymphocytes.
Value of P < .05.
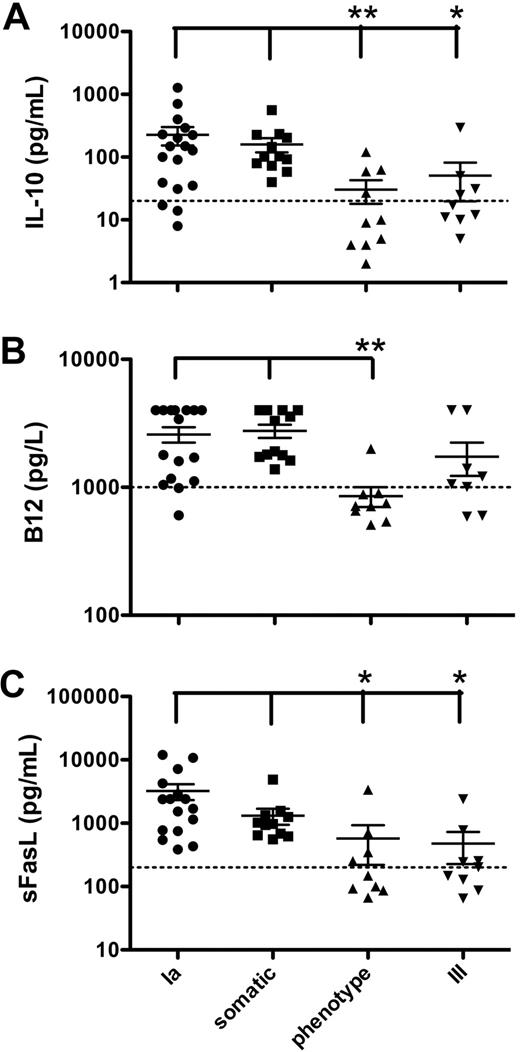
Patients in this study with either a germline or somatic FAS mutation had higher levels of IL-10 than those with ALPS phenotype or type III without FAS mutations (P < .05; Figure 1A). Mean values were 227, 160, 30, and 50 pg/mL in ALPS type Ia, somatic, phenotype, and type III patients, respectively. Similarly, patients with somatic or germline FAS mutations had a greater percentage of DNT cells (supplemental Table 1) compared with patients with unidentified genetic mutations. Furthermore, patients with germline or somatic FAS mutations had higher serum B12 and sFAS-L levels compared with ALPS phenotype (P < .05; Figure 1B-C). In contrast, the cytokine pattern was similar between all groups with respect to interferon-γ, tumor necrosis factor-α, IL-2, IL-6, IL-15, IL-18, and IL-23 (data not shown). Interestingly, the somatic ALPS patients exhibited a lower than normal total and high-density lipoprotein (HDL) cholesterol level, but normal low-density lipoprotein (LDL) levels: 91.1 plus or minus 9.8, 17.3 plus or minus 3.6, and 51.9 plus or minus 9.9 mg/dL, respectively. These data show that patients with somatic FAS mutations have similar clinical features and cytokine levels as those with germline FAS mutations. In addition, those ALPS type III or ALPS phenotype patients with elevated DNT cell numbers, and markedly increased B12, IL-10, and/or sFAS-L, probably have somatic mutations in FAS.
Elevated IL-10, vitamin B12, and sFAS-L associated with FAS mutations. (A) IL-10 levels were determined from plasma samples by ELISA. Dashed line indicates upper normal limit at 20 pg/mL. (B) Vitamin B12 plasma levels were determined by the National Institutes of Health Clinical Center laboratory. Dashed line indicates upper normal limit at 1000 pg/L. Upper limit of assay was 4000 pg/L. Results greater than 4000 pg/L were made to equal 4000 pg/L for analysis. (C) Soluble FAS-L levels were determined from plasma samples by ELISA. Dashed line indicates upper normal limit at 200 pg/mL. Long horizontal bars represent means; short horizontal bars, SE. *P < .05, **P < .01, compared with somatic or type Ia patients.
Elevated IL-10, vitamin B12, and sFAS-L associated with FAS mutations. (A) IL-10 levels were determined from plasma samples by ELISA. Dashed line indicates upper normal limit at 20 pg/mL. (B) Vitamin B12 plasma levels were determined by the National Institutes of Health Clinical Center laboratory. Dashed line indicates upper normal limit at 1000 pg/L. Upper limit of assay was 4000 pg/L. Results greater than 4000 pg/L were made to equal 4000 pg/L for analysis. (C) Soluble FAS-L levels were determined from plasma samples by ELISA. Dashed line indicates upper normal limit at 200 pg/mL. Long horizontal bars represent means; short horizontal bars, SE. *P < .05, **P < .01, compared with somatic or type Ia patients.
Discussion
We sought to confirm and extend the findings of Holzelova et al10 by screening 31 ALPS type III and phenotype patients for somatic mutations in FAS. Both ALPS type III and ALPS phenotype patients are defined as having an unknown underlying genetic mutation, with ALPS phenotype patients lacking a demonstrable in vitro apoptosis defect affecting the FAS pathway. Surprisingly, we found somatic FAS mutations in ALPS type III patients with defective in vitro apoptosis. One would not expect to find this result because the DNT cells do not survive in cell culture, and only 10% to 20% of single-positive mononuclear cells (CD4, CD8, CD14, and CD20) have the mutation.10 Although we have no explanation for these results, we hypothesize that there may be a greater number of mutant single-positive T cells surviving and expanding in the apoptosis assay of patients later classified as ALPS type III. Further experiments need to be conducted to explore this possibility. Although the degree of apoptotic defect observed in the ALPS type III patients was not statistically different from type Ia, there was a 3-fold difference between the groups that may prove significant with increased patient numbers studied. It should also be noted that we use less than 50% of normal control to define defective FAS-mediated apoptosis, but nearly all of the ALPS type Ia (germline) patients in our cohort show less than 15% of control. If 15% were used as a cut-off, virtually all of the ALPS type III patients would be reclassified as ALPS phenotype. These data, along with the higher DNT values in the somatic patients coming from those previously classified as ALPS phenotype, argue that the in vitro FAS-mediated apoptosis results are not meaningful for this group of patients.
We found that 12 of 31 (38.7%) ALPS type III and ALPS phenotype patients had heterozygous somatic FAS mutations in their DNT cell population: 6 of 15 with ALPS type III and 6 of 16 with ALPS phenotype. All but one of the somatic FAS gene mutations clustered to an approximately 200-base region in exons 7, 8, and 9, including the 6 mutations reported by Holzelova et al.10 Overall, the mutations resulted in functional loss of FAS signaling based on previous studies of constitutional mutations of the same or similar type.14,22-27 We confirmed that the somatic mutation was present in a small proportion (< 10%) of lymphocytes and monocytes but was not detected in buccal epithelial cells. The cells with a somatic defect in FAS must have a selective advantage because they would not undergo FAS-mediated apoptosis as readily as normal cells, and thus would accumulate, accounting for the lymphadenopathy/splenomegaly and autoimmunity observed in ALPS. Although rare, patients with somatic FAS mutations now compose the second largest group of known genetic mutations in ALPS, followed by patients with caspase 10, NRAS, caspase 8, and FAS-L mutations, respectively. The type III and phenotype patients still make up a significant portion (nearly 30%) of our ALPS patients and probably have somatic or germline mutations in genes not yet evaluated. Further work in this area should lead to a greater understanding of immune regulation.
Clinically, patients with a somatic FAS mutation were indistinguishable from those with a germline FAS mutation as evidenced by lymphadenopathy, autoimmune cytopenias, high serum vitamin B12, IL-10, and sFAS-L levels, and elevated DNT cell numbers. In addition, data obtained by evaluating our large ALPS cohort demonstrated that IL-18 is elevated in patients with either germline or somatic FAS mutations compared with controls and undefined ALPS patients.31 Elevated IL-18 was not confirmed statistically in the current study, perhaps because of the small number of patients in each group. Of note, patients with somatic FAS mutations had slightly lower rates of splenectomy compared with patients with germline FAS mutations. In general, the ALPS phenotype and ALPS type III were enrolled in our studies more recently than the ALPS type Ia patients; therefore, the younger somatic patients in these groups were more likely to benefit from recent recommendations in management, emphasizing avoidance of splenectomy.32,33
Patients with somatic FAS mutations who were originally classified as ALPS type III were all male and had a later age of onset of disease with the exception of two 1-year olds. This could be the result of the small number of patients studied, and evaluation of additional patients may yield a more balanced gender ratio, similar to the somatic type Ia patients originally classified as ALPS phenotype, with 3 males and 3 females. Based on our results, we suggest that all ALPS type III and ALPS phenotype patients with a similar clinical and laboratory phenotype to that of ALPS type Ia should be tested for the presence of a somatic FAS gene mutation in their isolated DNT cells (> 50% purity). Predictive selection of possible patients with somatic FAS mutations can be accomplished from biomarkers, ie, higher DNT levels and increased IL-10, B12, and/or sFAS-L levels.31,34 In addition, we have noted low total and HDL cholesterol in the somatic ALPS patients, another potential marker that may emerge as a useful finding in guiding the decision as to which patients to test for a somatic FAS defect. However, further work needs to be done to confirm this correlate.
Identification of somatic FAS mutations will allow us, and others, to monitor these patients for development of lymphomas, which are observed at a higher than expected rate in patients with germline FAS mutations.6 However, histologic and immunophenotypic features suspicious for lymphoma have not been identified in any of the somatic ALPS lymph nodes. In addition, in 5 somatic ALPS patients, TCR and immunoglobulin gene rearrangement studies failed to show evidence of a monoclonal population (data not shown). Two patients (nos. 235 and 241) with adult-onset somatic ALPS were suspected of ALPS only after review of lymph node pathology, including TCR and immunoglobulin gene rearrangement analysis, ruled-out malignant lymphoma. Furthermore, somatic ALPS patients have been stable without any clinical intervention besides treatment of their cytopenias. Thus, although the number of patients is relatively small, most of them have been followed for more than 5 years since onset of symptoms; at present, there is no evidence for a neoplastic lymphoproliferative disorder in patients with ALPS and somatic FAS mutations.
The role of DNT cells in ALPS, and whether these cells are pathogenic or merely a marker of disease, remains to be determined. The presence of FAS mutations in 100% of the DNT cell population in somatic ALPS patients10 suggests that these cells could contribute to disease pathogenesis. However, the fact that mutation-positive (germline) relatives of ALPS type Ia patients with elevated DNT cell numbers are often asymptomatic points to the involvement of other factors. ALPS is a multistep process requiring more than a single genetic hit for clinical expression,35 and additional factors must contribute to the increased levels of B12, IL-10, and sFAS-L observed in ALPS patients but not in mutation-positive healthy relatives.31 Further efforts to elucidate mechanisms of disease promotion and/or protection in somatic ALPS, such as the reported protective effect of HLA-B44 in germline autosomal dominant ALPS Ia,36 are needed to provide a better understanding of the factors that contribute to clinical disease. We think that the increased plasma B12 levels are the result of the lymphoproliferation and appear to be a useful marker of disease; we hypothesize that high levels of IL-10 help drive B-cell proliferation and cause autoimmune cytopenias5,37 and thus may be a potential therapeutic target. New data also point to the possible role of soluble FAS-L as being involved in the development of autoimmunity.38 A combination of cell types and soluble factors most probably contributes to the lymphoproliferation in ALPS and thus provides a useful mechanistic framework to explore and develop efficacious treatments for cytopenias resulting from splenic sequestration and autoimmune peripheral destruction.
In conclusion, our findings and those of Holzelova et al10 show that somatic mutations can arise in nonmalignant conditions and induce chronic disease that includes autoimmune cytopenias requiring treatment with immunosuppressive agents in some patients (6 of 12 patients in our study required therapeutic interventions, including one who underwent successful allogenic bone marrow transplantation). Other autoimmune diseases with a potentially pathogenic cellular subset may be worth exploring for somatic mutations. The ALPS patients with somatic FAS mutations are clinically indistinguishable from the patients with germline FAS mutations and, therefore, may also have an increased risk for developing Hodgkin and non-Hodgkin lymphoma.6 Even though no ALPS patient with a somatic FAS mutation has been diagnosed with lymphoma to date, we think that all patients with FAS mutations should be monitored closely for signs of lymphoma and treated appropriately. Evaluation of patients with ALPS based on somatic mutations may provide important information to help elucidate a possible pathophysiologic role of DNT cells that are a result of disordered immune homeostasis. This should also help to clarify whether these cells influence other immune cell types, such as B cells, in the development of autoimmune cytopenias and lymphomas.
Presented at the 51st annual meeting of the American Society of Hematology, New Orleans, LA, December 5-8, 2009.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Janet K. Dale for her valuable contributions related to sample preservation, Mark Raffeld for his assistance with the clonality studies, and Amy P. Hsu for testing the H95R mutation.
This research was initiated by the late Dr Stephen E. Straus.
This work was supported by the Intramural Research Program of the National Institutes of Health, National Institute of Allergy and Infectious Diseases, and Center for Cancer Research, National Cancer Institute, National Institutes of Health (contract HHSN261200800001E).
The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US government.
National Institutes of Health
Authorship
Contribution: K.C.D. performed research, analyzed data, and wrote the paper; J.E.N. performed molecular research and reported mutations; S.P. collected clinical data; J.D. performed genetics counseling; R.L.H. performed research; J.B.O. analyzed data and performed research; J.M.P. made genetic determinations; E.S.J. and S.P. conducted pathology evaluations; J.I.C. supervised research; T.A.F. was involved in diagnostic workups; V.K.R. was responsible for patient care; and all authors reviewed and contributed to the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Kennichi C. Dowdell, Laboratory of Clinical Infectious Diseases, National Institute of Allergy and Infectious Diseases, National Institutes of Health, 10 Center Dr, Rm 11N234, Bethesda, MD 20892-1888; e-mail: kdowdell@niaid.nih.gov.