Abstract
Transfer of tumor antigen–specific T-cell receptors (TCRs) into human T cells aims at redirecting their cytotoxicity toward tumors. Efficacy and safety may be affected by pairing of natural and introduced TCRα/β chains potentially leading to autoimmunity. We hypothesized that a novel single-chain (sc)TCR framework relying on the coexpression of the TCRα constant α (Cα) domain would prevent undesired pairing while preserving structural and functional similarity to a fully assembled double-chain (dc)TCR/CD3 complex. We confirmed this hypothesis for a murine p53-specific scTCR. Substantial effector function was observed only in the presence of a murine Cα domain preceded by a TCRα signal peptide for shuttling to the cell membrane. The generalization to a human gp100-specific TCR required the murinization of both C domains. Structural and functional T-cell avidities of an accessory disulfide-linked scTCR gp100/Cα were higher than those of a dcTCR. Antigen-dependent phosphorylation of the proximal effector ζ-chain–associated protein kinase 70 at tyrosine 319 was not impaired, reflecting its molecular integrity in signaling. In melanoma-engrafted nonobese diabetic/severe combined immunodeficient mice, adoptive transfer of scTCR gp100/Cα transduced T cells conferred superior delay in tumor growth among primary and long-term secondary tumor challenges. We conclude that the novel scTCR constitutes a reliable means to immunotherapeutically target hematologic malignancies.
Introduction
Presentation of many tumor- and leukemia-associated antigens at low copy numbers by normal cells and tissues such as thymus, spleen, and lymphohemopoietic cells results in the elimination of high-avidity, tumor-reactive T cells from the peripheral T-cell repertoire.1 A promising approach to overcome the limitations imposed by self-tolerance is the adoptive transfer of T cells genetically modified with tumor-associated antigen (TAA)–specific T-cell receptors (TCRs).2,3 In this study, we focused on a tumor suppressor protein p53-specific TCR,4 whose wild-type (WT) antigen specificity was recently prioritized as one of the most “ideal” cancer antigens5 and was proven to target both cell lines derived from solid tumors and hematologic malignancies6 and, respectively, a melanoma/melanocyte differentiation antigen gp100-specific TCR.5,7
Because of the heterodimeric nature of a TCR, pairing of introduced TCR chains with endogenous TCR chains resulting in expression of mixed TCR dimers might generate TCRs with unknown and potential autoimmune reactivities. Efforts to experimentally prove these “off-target” reactions in a mouse model have provided evidence to support the occurrence of such events. In line with that, the observed coexistence of 2 unrelated TCRs in reprogrammed T cells indicated that neither TCR was entirely suppressed in surface expression and effector function,8,9 their relative levels of expression being dependent on the intrinsic qualities of those particular TCR subfamilies.10 Strategies to avoid mispairing involved the molecular design of the TCRαβ interface11 or the transfer of TCRαβ into γδ T cells.12
We sought to improve efficacy and the general feasibility of this aim. For this, we designed murine as well as human (Hu) 3-domain single-chain (sc) TCRs13 of the domain order signal peptide–variable α domain (Vα)–linker–variable β domain (Vβ)–constant β (Cβ) domain that recognize the Hu leukocyte antigen (HLA)–A2-restricted TAAs 264 to 272 of p536 or 280 to 288 of gp100,7 respectively. Recent approaches relied on the fusion to signaling molecules to initiate antigen-specific effector function.14 However, the chimeric molecules are suspected to irregularly modify the signaling threshold of a cell and to give rise to increased immunogenicity. In this study, we hypothesized that coexpression of the missing constant α (Cα) domain originating from TCRα should confer both stability and signaling of a 3-domain scTCR devoid of, eg, CD3ζ. This may yield a 4-domain scTCR that structurally and functionally resembles a native double-chain (dc) TCR and eventually results in normal T-cell signaling (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). The signal peptide of a MDM2(81-88)–specific TCRα15 was spliced in front of the murine (Mu) Cα ectodomain for proper export to the cell membrane. Our data show that this strategy rendered scTCR constructs that mediated effective antitumor reactivity in vitro as well as in a Hu tumor-bearing nonobese diabetic/severe combined immunodeficient (NOD/SCID) mouse model. On a molecular level, substantial coupling to the proximal downstream effector ζ-chain–associated protein kinase 70 (ZAP-70) was documented. Thus, we present herein a novel approach for the construction and functional expression of potent and safe scTCRs.
Methods
Peptides, antibodies, and tetrameric pA2.1 complexes
Peptides p53(264-272), gp100(280-288), MDM2(81-88), and tyrosinase-related protein 2 were synthesized by Affina. The monoclonal antibodies (mAbs) used were anti–Hu CD8–fluorescein isothiocyanate, CD4–fluorescein isothiocyanate (Beckman Coulter), anti–mouse-TCR-phycoerythrin (PE), Vβ3-PE (BD Biosciences), and anti–Hu–interferon γ (IFNγ)–Mab1–D1K, Mab2-7B61 (Mabtech AB). PE- or phycoerythrin-cyanin 5–labeled tetrameric pA2.1 complexes were synthesized as described elsewhere.16
Cells
Bulk CD4+/CD8+ T cells were obtained from peripheral blood mononuclear cells isolated from buffy coats of Hu A2.1+/− donors. Retroviral packaging cells were Phoenix-Ampho (ATCC). A2.1+MDM2+/p53+ cells were Uoc B11, BV173 (leukemia), MZ1851 (renal cancer), IM-9, and JY (Epstein-Barr virus [EBV] lymphoblastoid cell line). An A2.1+ and MDM2/p53-deficient cell line was Saos-2 (osteosarcoma), Saos-2/143 was a p53-transfectant, and Saos-2 Cl6 was a MDM2 transfectant cell line; A2.1− cell lines were Uoc B1 (leukemia) and natural killer (NK)–sensitive K562 (chronic myeloid leukemia) cells.6,15 A HLA-A2 transfectant of K562 was used in IFNγ enzyme-linked immunospot (ELISPOT) assays. A2.1+gp100+ cells were Mel526 and MeWo (melanomas)17 and A2.1−gp100+ was Mel397 (melanoma). A2.1+ cells were transporter associated with antigen processing deficient T2 cells. Jurkat-76 is a T-cell leukemia devoid of endogenous TCRs.
Molecular cloning of TCR gene constructs
Transduction, selection, and expansion of Hu T cells
Retroviral transduction was performed as described elsewhere18 with the exception of using Fugene (Roche) as transfection agent. Twenty-five percent transduction efficiencies necessitated normalized TCRα/TCRβ expression which was accomplished by drug selection.11,18 T cells were expanded by weekly stimulation with anti–Hu CD3/CD28 Dynabeads (Invitrogen) and recombinant Hu interleukin-2 (50 U/mL). They underwent magnetic cell sorting (Miltenyi Biotec) to obtain pure and untouched CD4+ and CD8+ T-cell subsets.
Transfection of TCR RNA
We used RNA electroporation as outlined elsewhere19 with the Bio-Rad GenePulser Xcell system applying a square wave pulse of 500 V, 4 milliseconds, to 4 × 106 Jurkat-76 cells resuspended in 200 μL of OPTI-MEM medium (Invitrogen) to 10 μg of pGEM-4Z encoded in vitro transcribed TCR RNA (Ambion).
Flow cytometry, Scatchard analysis, and T-cell assays
Recombinant TCR expression was determined in flow cytometric analysis on a FACSCalibur (BD Biosciences) device. Dose-escalating equilibrium tetramer binding data were plotted as Scatchard plot of mean fluorescence intensity (MFI)/concentration of tetramer against MFI. The dissociation constant KD equals −1/slope.6 Standard 51Cr-release and IFN-γ ELISPOT assays were performed in duplicate wells as reported.15 KD and EC50 were determined with Prism version 3.0 (GraphPad Software Inc).
Total and phospho–ZAP-70 (Y319) enzyme-linked immunosorbent assay
Total ZAP-70 or Y319 phosphorylated ZAP-70 on TCR stimulation was measured by the sandwich enzyme-linked immunosorbent assay PathScan Total or Phospho-ZAP-70 (New England Biolabs) slightly modified from the manufacturer's instructions. Briefly, 0.5 × 106 transduced T cells were washed and unspecifically stimulated with 1.5 μg of anti-MuVβ3 (KJ25; BD Biosciences) as isotype control or 0.1 μg of irrelevant tetramer p53(264-272)A2.1. T cells were TCR-specifically stimulated by cross-linking of murinized TCR gp100 with 1.5 μg of anti-Mu TCRβ (H57-597; BD Biosciences) or antigen specifically with 0.1 μg of the cognate gp100(280-288)A2.1 tetramer and incubated for 4 minutes at 37°C. Stimulation was stopped by the addition of ice-cold cell lysis buffer supplemented with phosphotyrosyl phosphatase and protease inhibitors. Total and Phospho (Y319) ZAP-70 of cell extract were quantified with the use of specific capture and detection antibodies, a horseradish peroxidase–linked secondary antibody and TMB as substrate at 450 nm on a Dynex microplate reader.
Adoptive T-cell transfer in vivo
For each independent experiment, NOD/SCID mice (7-9 weeks old; strain NOD/NCrCrl-Prkdcscid) were obtained from Charles River. They were engrafted with the melanoma cell line MeWo17 intradermally (2.5 × 106 cells in 50 μL of matrigel basement membrane matrix [BD Biosciences]) on the right flank and adoptively transferred intravenously with T cells retrovirally transduced with different TCR gp100 constructs (mean, 2.4 × 106 cells in 100 μL of phosphate-buffered saline [PBS]) at day 7. In second tumor challenge experiments, primary local tumors were removed from the right flank under anesthesia. A secondary tumor inoculation of MeWo (2.5 × 106 cells in 50 μL of matrigel) was performed intradermally on the left flank. The tumor growth was assayed by determination of tumor volume according to the formula [TV (mm3) = π/6 × 0.5 × length × (width)2].20 To quantify the kinetics of tumor growth the mean and the standard error of the mean of tumor volume was calculated for the indicated number of mice that received the identical TCR in serial experiments.
Results
Cα increased surface expression of a Mu p53(264-272)A2.1-specific scTCR construct
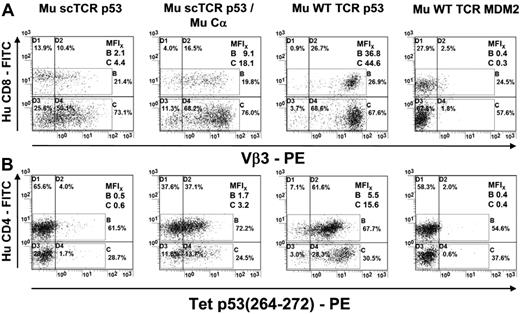
We took advantage of the CD8-independent p53(264-272) HLA-A2.1–specific Mu TCR generated in A2-transgenic mice6 and retrovirally introduced into Hu T lymphocytes the WT receptor or the scTCR expressing the glycine/serine-rich peptide linker SL7 (scTCR p53; supplemental Figure 1A-B).21 After retroviral transduction of Mu WT TCR p53 and normalized TCR expression provided by drug selection,11,18 almost all Hu T cells stained positive for the Vβ3 subfamily domain of this receptor (MFI: CD8+, 36.8/CD4+, 44.6; Figure 1A). In contrast, the transfer of the scTCR p53 into Hu T lymphocytes resulted only in a low Vβ3 staining (MFI: CD8+, 2.1/CD4+, 4.4). Next, we improved the expression of the scTCR by coexpression of a Mu Cα domain (MFI: CD8+, 9.1/CD4+, 18.1). The intensity of tetramer staining of scTCR p53/Cα-transduced CD8+ (MFI, 3.2) and CD4+ T cells (MFI, 1.7) was lower relative to those expressing Mu WT TCR p53 (MFI: CD8+, 15.6/CD4+, 5.5; Figure 1B), consistent with the differences in the intensity of their TCR Vβ3 expression. T cells solely transduced with scTCR p53 bound very few tetramers compared with the negative control: T cells transduced with a Mu MDM2-specific TCR (Mu WT TCR MDM2, Mu Vβ6)15 did not show specific Vβ3 or Tet p53 staining. Thus, Cα confers a robust and stable scTCR expression in CD8+ as well as in CD4+ T cells.
Expression of a CD8-independent p53(264-272)A2.1-specific scTCR in Hu T lymphocytes compared with WT TCR-transduced T cells. (A) TCR p53 or MDM2-transduced human T cells (Mu scTCR p53, Mu scTCR p53/Mu Cα, Mu WT TCR p53, Mu WT TCR MDM2) were tested by flow cytometry for expression of Hu CD4 and the Mu subfamily Vβ3 of the p53-specific TCR. MFIs of TCR expression were given for CD4+/CD8+ T cells. (B) MFIs of p53(264-272)A2.1 tetramer–labeled p53- or MDM2-specific scTCR (Mu scTCR p53, Mu scTCR p53/Mu Cα) and dcTCR (Mu WT TCR p53 or MDM2) transduced Hu CD8+ and CD4+ T lymphocytes.
Expression of a CD8-independent p53(264-272)A2.1-specific scTCR in Hu T lymphocytes compared with WT TCR-transduced T cells. (A) TCR p53 or MDM2-transduced human T cells (Mu scTCR p53, Mu scTCR p53/Mu Cα, Mu WT TCR p53, Mu WT TCR MDM2) were tested by flow cytometry for expression of Hu CD4 and the Mu subfamily Vβ3 of the p53-specific TCR. MFIs of TCR expression were given for CD4+/CD8+ T cells. (B) MFIs of p53(264-272)A2.1 tetramer–labeled p53- or MDM2-specific scTCR (Mu scTCR p53, Mu scTCR p53/Mu Cα) and dcTCR (Mu WT TCR p53 or MDM2) transduced Hu CD8+ and CD4+ T lymphocytes.
Specific cytotoxicity requires the coexpression of the Mu Cα domain in scTCR-transduced T lymphocytes
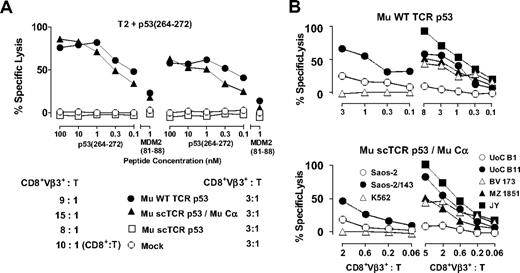
The differences in the intensity of tetramer binding suggested that expression of the Cα domain would trigger specific cytotoxic function in scTCR-transduced Hu T cells. Indeed, p53(264-272)A2.1-specific scTCR p53/Cα cotransduced cytotoxic T lymphocytes (CTLs) efficiently recognized p53 peptide–loaded T2 cells (EC50, 0.34nM), showing a nearly equivalent cytolytic activity compared with WT TCR p53–transduced CTLs (EC50, 0.12nM) especially for lower CD8+Vβ3+:T ratios (all 3:1; Figure 2A). T2 cells loaded with irrelevant MDM2 peptide were not recognized, and lysis of peptide-loaded T2 cells by scTCR p53 and Mock-transduced T cells was not observed. CTLs equipped with scTCR p53/Cα efficiently killed a variety of malignant HLA-A2.1 (A2.1)+ p53+ targets, among them the leukemia cell lines BV 1736 and Uoc-B11,22 and the EBV-transformed lymphoblastoid cell line JY,6 but not p53-deficient Saos-2, NK-sensitive K562 or A2.1− leukemia UoC-B1 cells (Figure 2B) comparable with WT TCR p53+ CTLs.
Cytolysis in T2 peptide titration and specific tumor recognition of Hu CD8+ CTLs transduced with a p53(264-272)A2.1-specific scTCR. (A) Cytotoxicity of p53-specific Mu WT TCR p53 (●), Mu scTCR p53 (□), Mu scTCR p53/Mu Cα (▴), or Mock (○) transduced Hu CD8+ T cells in response to peptide-pulsed T2 targets at the indicated effector to target ratios (CD8+Vβ3+:T). (B) p53(264-272)A2.1-specific, TCR-transduced Hu CD8+ CTLs were tested at the indicated CD8+Vβ3+ to target ratios (CD8+Vβ3+:T) for cytolysis in response to p53+A2.1+ (Saos-2/143, UoC B11, BV 173, MZ 1851, and JY), p53−A2.1+ (Saos-2), p53+A2.1− (Uoc B1), and p53−A2.1− (K562) human tumor targets. The percentages of lysis are calculated as means from replicates and are shown for a representative chromium release assay of 2 experiments.
Cytolysis in T2 peptide titration and specific tumor recognition of Hu CD8+ CTLs transduced with a p53(264-272)A2.1-specific scTCR. (A) Cytotoxicity of p53-specific Mu WT TCR p53 (●), Mu scTCR p53 (□), Mu scTCR p53/Mu Cα (▴), or Mock (○) transduced Hu CD8+ T cells in response to peptide-pulsed T2 targets at the indicated effector to target ratios (CD8+Vβ3+:T). (B) p53(264-272)A2.1-specific, TCR-transduced Hu CD8+ CTLs were tested at the indicated CD8+Vβ3+ to target ratios (CD8+Vβ3+:T) for cytolysis in response to p53+A2.1+ (Saos-2/143, UoC B11, BV 173, MZ 1851, and JY), p53−A2.1+ (Saos-2), p53+A2.1− (Uoc B1), and p53−A2.1− (K562) human tumor targets. The percentages of lysis are calculated as means from replicates and are shown for a representative chromium release assay of 2 experiments.
Transfer of the CD8-independent p53(264-272)A2.1-specific WT TCR p53 into Hu CD4+ T cells would reprogram them into p53A2.1-specific T helper (Th) cells.6 Consistent with this, we found that purified scTCR p53/Cα–cotransduced CD4+ Th cells were able to specifically produce IFN-γ in ELISPOT assays toward peptide-loaded target cells or leukemia cells and EBV–lymphoblastoid cell lines, respectively (supplemental Figure 2A-B).
The state-of-the-art approach for the functional expression of Hu scTCRs is their elaborate chimerization to the T-cell signaling component, the Hu CD3ζ chain.23 However, fusion of the Mu p53(264-272)–specific scTCR to the Hu CD3ζ-signaling domain led to a substantial surface expression but decreased killing of peptide-loaded target cells compared with scTCR p53/Cα-transduced Hu T cells (supplemental Figure 3A-B). Thus, the coexpression of Cα outperformed the classical approach of linking scTCR p53 to the transmembrane and signaling moiety of CD3ζ.
To confirm our approach for an antigen- and TCR subfamily–unrelated TCR we tested a CD8-dependent Mu MDM2(81-88)A2.1-specific scTCR (supplemental Figure 1A-B),15 whose target operates as a negative regulator of p53.22 Consistently, surface expression and cytolytic activity was not observed until we coexpressed the Mu Cα domain (data not shown). Furthermore, reducing versus nonreducing sodium dodecyl sulfate–polyacrylamide gel electrophoresis and subsequent immunoblot studies showed that the exported Cα molecule corresponded to the theoretical molecular weight (supplemental Figure 4B) and was able to heterodimerize with the scTCR by the native disulfide bond (supplemental Figure 4A-C).
Expression of a Hu gp100(280-288)A2.1-specific scTCR/Cα progressively depends on the murinization of its constant domains and accessory disulfide linkage
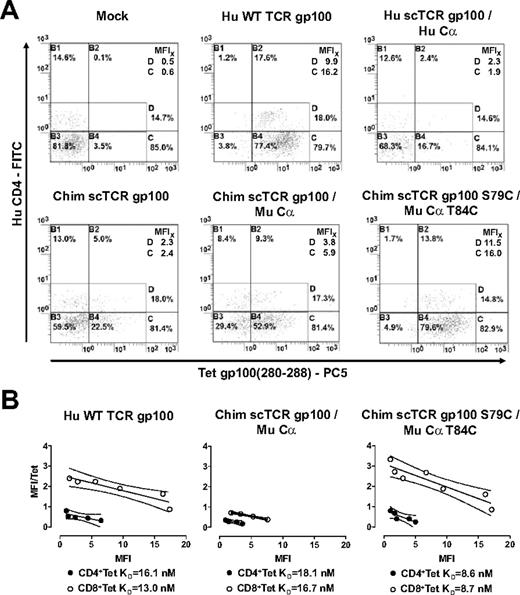
Next, we investigated whether this concept could be applied to a Hu CD8-independent gp100(280-288)–specific TCR7 as well (supplemental Figure 1A). Most of the Hu WT TCR gp100–transduced CD8+ and CD4+ T cells bound tetrameric gp100(280-288)A2.1 complexes (Figure 3A). Again, T cells bearing Hu scTCR gp100 without Cα failed to bind tetrameric gp100(280-288)A2.1 complexes (data not shown). However, Hu scTCR/Hu Cα cotransduced T lymphocytes only marginally stained with the cognate tetramer (MFI, 1.9/2.3). Because TCRs chimerized to Mu constant domains exhibited marked expression and function in Hu T lymphocytes,24,25 we replaced the Hu for the Mu Cβ domain in Hu scTCR gp100 and coexpressed this chimeric (Chim) receptor with the Mu Cα domain (supplemental Figure 1C) just as applied to Mu scTCR p53. Indeed, T lymphocytes transduced with Chim scTCR gp100 alone (MFI, 2.4/2.3) and progressively in combination with Mu Cα (MFI, 5.9/3.8) exhibited elevated tetramer binding.
Expression and tetramer avidity of a murinized Hu gp100(280-288)A2.1-specific scTCR in Hu T lymphocytes. (A) Mock, Hu WT TCR gp100, Hu scTCR gp100/Hu Cα, Chim scTCR gp100, Chim scTCR gp100/Mu Cα, and Chim scTCR gp100 S79C/Mu Cα T84C transduced Hu T cells were tested for binding tetrameric gp100(280-288)A2.1 complexes by flow cytometry. (B) Avidity (KD) of tetramer binding to T cells determined by Scatchard analysis. Hu WT TCR gp100, Chim scTCR gp100/Mu Cα, and Chim scSL7TCR gp100 S79C/Mu Cα T84C transduced Hu CD8+ (○) and CD4+ (●) T lymphocytes were quantitatively tested for binding to tetrameric gp100(280-288)A2.1 complexes by flow cytometry. Linear regression analysis was accomplished with GraphPad Prism Software version 3.0. FITC indicates fluorescein isothiocyanate; and PC5, phycoerythrin-cyanin 5.
Expression and tetramer avidity of a murinized Hu gp100(280-288)A2.1-specific scTCR in Hu T lymphocytes. (A) Mock, Hu WT TCR gp100, Hu scTCR gp100/Hu Cα, Chim scTCR gp100, Chim scTCR gp100/Mu Cα, and Chim scTCR gp100 S79C/Mu Cα T84C transduced Hu T cells were tested for binding tetrameric gp100(280-288)A2.1 complexes by flow cytometry. (B) Avidity (KD) of tetramer binding to T cells determined by Scatchard analysis. Hu WT TCR gp100, Chim scTCR gp100/Mu Cα, and Chim scSL7TCR gp100 S79C/Mu Cα T84C transduced Hu CD8+ (○) and CD4+ (●) T lymphocytes were quantitatively tested for binding to tetrameric gp100(280-288)A2.1 complexes by flow cytometry. Linear regression analysis was accomplished with GraphPad Prism Software version 3.0. FITC indicates fluorescein isothiocyanate; and PC5, phycoerythrin-cyanin 5.
We sought to tighten the interaction of the Mu C-domains in the scTCR/Cα scaffold by introducing a second interchain disulfide bond,26 yielding Chim scTCR gp100 S79C and Mu Cα T84C (supplemental Figure 1D).27 T-cell avidity to the cognate tetramer equaled (MFI, 16.0/11.5) that of WT TCR gp100–expressing T cells (MFI, 16.2/9.9; Figure 3A) and, notably, was considerably better than chimerized scTCR/Cα (MFI, 5.9/3.8). In contrast, cotransduction of a Hu scTCR gp100 with Mu Cα or vice versa, Chim scTCR gp100 with Hu Cα, resulted in minute amounts of TCR gp100 expression (data not shown). From this, it became obvious that peculiar Mu Cα/β domain interactions account for the beneficial effect on expression.
We quantified the structural avidities to tetramers in Scatchard analysis (Figure 3B). The introduction of the accessory disulfide bond into Chim scTCR gp100 S79C/Mu Cα T84C substantiated a 2-fold increase of their avidities, read out as the inverse of their equilibrium dissociation constants (KD) in CD4+ (8.6nM) and CD8+ (8.7nM) T cells versus Chim scTCR gp100/Mu Cα transduced T-cell subsets (18.1nM, 16.7nM). The avidities of WT TCR gp100 (16.1nM, 13.0nM) were slightly better than those for Chim scTCR/Mu Cα but fell behind by nearly a factor of 2 compared with the disulfide-bonded scTCR construct.
Mu Cα barely interacts with Hu TCRβ chains
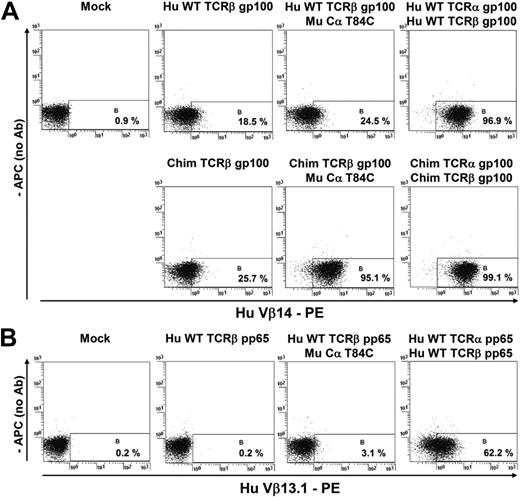
Cα might interact with endogenous TCRβ and thereby might either alter the fraction of correctly paired Mu Cα/scTCRs or even lead to a side reaction product (ie, Mu Cα/TCRβ) of unknown reactivity. For this, we aimed at assessing pairing of an introduced Mu Cα domain bearing the cysteine for disulfide formation (Mu Cα T84C) with introduced arbitrary TCRβ chains of unrelated subfamilies (TCR gp100 [Figure 4A], TCR pp65 [Figure 4B]) in a Jurkat cell line (Jurkat-76) devoid of natural TCRs.11 Detectable surface expression of this heterodimer is highly likely because of successful Mu Cα/TCRβ chain pairing. The TCR chains were transfected by RNA electroporation.19 From these experiments it emerged that the positive controls (Hu WT or Chim TCR gp100, Hu WT TCR pp65; Figure 4A-B outmost right) elicited productive chain pairing as opposed to those samples when a Hu WT TCRβ chain (Hu WT TCRβ gp100, Hu WT TCRβ pp65) was expressed in the absence (Figure 4A-B left) or most notably, in the presence (Figure 4A-B right) of Mu Cα T84C. Conclusively, a Mu Cα domain hardly pairs with a Hu TCRβ chain in Jurkat-76. We postulate that the interchain affinity of an autonomous Mu Cα to a Hu WT TCRβ chain is too weak to accomplish substantial chain pairing.
Coexpression of a Mu Cα and different Hu TCRβ chains in Jurkat-76 lacking endogenous TCRs. To elucidate the propensity of Mu Cα to pair with arbitrary endogenous TCRβ chains (A-B right; 24.5%/3.1%), we introduced the TCRβ chain alone as a negative control (A-B left; 18.5%/0.2%) or the WT Hu TCRα/β chains of the gp100(280-288) specificity (Vβ14) or the Hu CMV pp65(495-503) specificity (Vβ13.1) as positive controls (A-B outmost right; 96.9%/62.2%). In addition, a partially murinized (ie, chimerized in C domains) dcTCR gp10025 was assayed to confirm that Mu Cα T84C pairs with a murinized TCRβ with higher efficacy than with a Hu full-length TCRβ chain (A bottom right; 95.1% vs 24.5%). We used TCR RNA transfection by electroporation as described elsewhere.19 Surface expression of any monodimer or heterodimer was already monitored next day in flow cytometric analysis with the use of the related anti-TCR Vβ antibody. Mu Cα marginally pairs with an unrelated Hu TCRβ chain (24.5% vs 18.5% and 3.1% vs 0.2%, respectively). In a clinical situation, the tiny fraction of hybrid Mu Cα T84C/Hu TCRβ should be theoretically outcompeted by endogenous TCRα chains which contribute with 2 domains (ie, Vα + Cα) to chain pairing.
Coexpression of a Mu Cα and different Hu TCRβ chains in Jurkat-76 lacking endogenous TCRs. To elucidate the propensity of Mu Cα to pair with arbitrary endogenous TCRβ chains (A-B right; 24.5%/3.1%), we introduced the TCRβ chain alone as a negative control (A-B left; 18.5%/0.2%) or the WT Hu TCRα/β chains of the gp100(280-288) specificity (Vβ14) or the Hu CMV pp65(495-503) specificity (Vβ13.1) as positive controls (A-B outmost right; 96.9%/62.2%). In addition, a partially murinized (ie, chimerized in C domains) dcTCR gp10025 was assayed to confirm that Mu Cα T84C pairs with a murinized TCRβ with higher efficacy than with a Hu full-length TCRβ chain (A bottom right; 95.1% vs 24.5%). We used TCR RNA transfection by electroporation as described elsewhere.19 Surface expression of any monodimer or heterodimer was already monitored next day in flow cytometric analysis with the use of the related anti-TCR Vβ antibody. Mu Cα marginally pairs with an unrelated Hu TCRβ chain (24.5% vs 18.5% and 3.1% vs 0.2%, respectively). In a clinical situation, the tiny fraction of hybrid Mu Cα T84C/Hu TCRβ should be theoretically outcompeted by endogenous TCRα chains which contribute with 2 domains (ie, Vα + Cα) to chain pairing.
Cysteine-modified chimeric scTCR/Cα elicits improved cytolytic activity in vitro
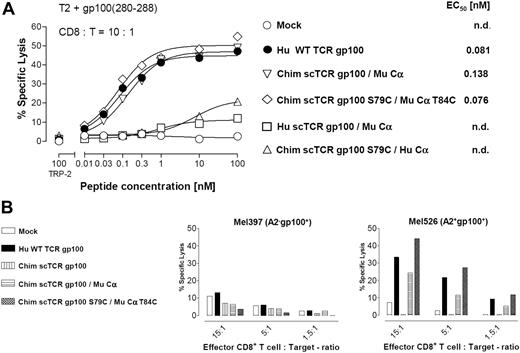
We asked whether increased tetramer avidities of Cys-modified scTCR and Cα constructs translated into enhanced cytolysis in a 51Cr-release assay (Figure 5A). CD8+ T cells transduced with the Cys-modified Chim scTCR gp100 S79C/Mu Cα T84C recognized T2 cells pulsed with titrated amounts of gp100(280-288) peptide down to the subnanomolar range (EC50, 0.076nM), but cytotoxicity did not markedly exceed that of WT TCR+ CTL (EC50, 0.081nM). Lysis efficiency of Chim scTCR gp100/Mu Cα–transduced CTLs was reduced by almost a factor of 2 (EC50, 0.138nM). These data correlated with the observed tetramer avidities (Figure 3). No relevant cytotoxicity emerged against T2 cells loaded with an irrelevant A2-binding tyrosinase-related protein 2 peptide, Mock-transduced Hu T cells (Figure 5A) or those expressing Hu scTCR gp100/Hu Cα, or solely Chim scTCR gp100 (data not shown). Interspecies combinations of Hu scTCR gp100/Mu Cα or vice versa, Chim scTCR gp100/Hu Cα, transduced T cells were also unable to appropriately lyse and underlined the conclusions drawn from tetramer binding as outlined before.
Cytolytic effector function of a murinized Hu gp100(280-288)A2.1-specific scTCR in Hu CD8+ T cells. (A) Cytotoxicity of Hu CD8+ T cells transduced with Hu WT TCR gp100, Chim scTCR gp100 coexpressed with Mu Cα, Chim scTCR S79C coexpressed with Mu Cα T84C, Hu scTCR gp100 with Mu Cα, and Chim scTCR gp100 with Hu Cα toward peptide-pulsed T2 cells at the indicated CD8+:T ratio. (B) Cytolytic recognition mediated by a panel of TCR gp100 constructs as described in panel A toward the Hu gp100+A2.1+ melanoma cell line Mel526 and as a negative control toward gp100+A2.1− Mel397 at the indicated CD8+: T ratios. The percentages of lysis are calculated as means from replicates and shown for a representative chromium release assay of 2 experiments.
Cytolytic effector function of a murinized Hu gp100(280-288)A2.1-specific scTCR in Hu CD8+ T cells. (A) Cytotoxicity of Hu CD8+ T cells transduced with Hu WT TCR gp100, Chim scTCR gp100 coexpressed with Mu Cα, Chim scTCR S79C coexpressed with Mu Cα T84C, Hu scTCR gp100 with Mu Cα, and Chim scTCR gp100 with Hu Cα toward peptide-pulsed T2 cells at the indicated CD8+:T ratio. (B) Cytolytic recognition mediated by a panel of TCR gp100 constructs as described in panel A toward the Hu gp100+A2.1+ melanoma cell line Mel526 and as a negative control toward gp100+A2.1− Mel397 at the indicated CD8+: T ratios. The percentages of lysis are calculated as means from replicates and shown for a representative chromium release assay of 2 experiments.
To assess their potency to lyse naturally processed gp100(280-288) peptides, we used the HLA-A2.1−gp100+ melanoma cell line Mel397 and the HLA-A2.1+gp100+ cell line Mel526 as targets (Figure 5B). Here, CD8+ T cells transduced with the disulfide bonded Chim scTCR gp100 S79C/Mu Cα T84C lysed somewhat better (45% at CD8+:T = 15:1) Mel526 tumor cells than Hu WT TCR gp100+ CD8+ T cells (34%) such as shown for different CD8+:T ratios. Chim scTCR gp100/Mu Cα CD8+ T cells were less efficient in gp100-specific tumor cell lysis (24%). Chim scTCR gp100 or Mock-transduced T cells did not specifically lyse at all. The HLA-A2.1−gp100+ tumor cell line Mel397 was barely recognized by any TCR gp100 construct.
Tetramer positivity of TCR gp100–transduced CD4+ T cells let us assume to reprogram them to gp100-specific Th cells. Indeed, purified WT TCR gp100– and Chim scTCR gp100/Mu Cα–transduced CD4+ Th cells secreted equal amounts of IFN-γ against gp100(280-288) peptide–loaded T2 cells and recognized gp100+A2.1+ melanoma cells (Mel526; supplemental Figure 5).
ScTCR/Cα efficiently coupled to the proximal downstream effector molecule ZAP-70
Next, we were interested to verify on a molecular level whether the scTCR/Cα construct is capable of efficiently coupling to the CD3 ζ-chain–associated protein kinase ZAP-70. On antigen encounter the TCR/CD3 complex is phosphorylated at the immunoreceptor tyrosine-based activation motifs (ITAMs) in CD3ζ by the protein tyrosine kinase Lck. This recruits ZAP-70 which in turn becomes phosphorylated by Lck at multiple sites. Phosphotyrosine 319 (pY319) confers a stimulatory signal to phosphorylation of the T-cell membrane scaffold linker for activation of T cells and phospholipase Cγ1 and eventually leads to Ca2+-mobilization, nuclear factor of activated T cells transcription, Ras activation, and interleukin-2 production.28,29
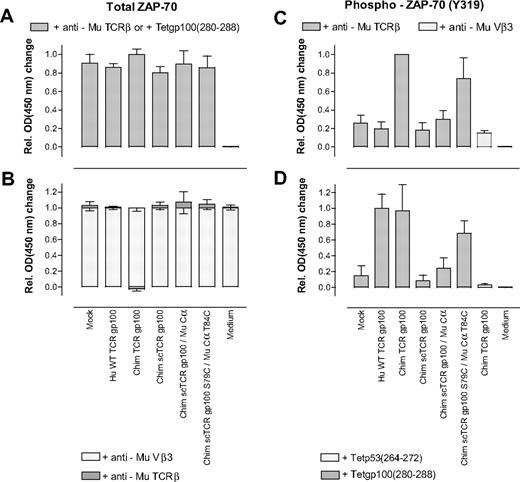
We kinetically assayed TCR gp100–transduced T cells in Phospho–ZAP-70 enzyme-linked immunosorbent assay, based on the detection of phosphorylated tyrosine 319 after short-term anti–TCR-directed stimulation (ie, 4 minutes). In general, a murinized dcTCR gp100 (Chim TCR gp100),25 yielded the highest OD450 readings and served as an alternative reference to WT TCR gp100 in anti-Mu TCRβ cross-linking experiments.
Initially, we verified that anti–Mu TCRβ- or gp100(280-288)A2.1 tetramer–stimulated TCR gp100 or Mock-transduced T cells expressed equal amounts of total ZAP-70 as a prerequisite to compare normalized changes in Y319 phosphorylation (Figure 6A). The amount of total ZAP-70 did not substantially vary, most OD450-changes were ranging between 0.80 (± 0.07) (Chim scTCR gp100) and 0.86 (± 0.04) (WT TCR gp100).
Quantification of total ZAP-70 and phosphorylated ZAP-70 after antigen–(in)dependent stimulation of TCR gp100-transduced T cells. (A) Hu T cells, transduced with a panel of indicated TCR gp100(280-288)–specific constructs, were stimulated with anti-Mu TCRβ or gp100(280-288)A2.1 tetramers in 6 independent experiments and cell extracts were submitted to enzyme-linked immunosorbent assay (ELISA) to quantify total ZAP-70. (B) TCR gp100-transduced T cells were treated with either a nonstimulating Mu Vβ3-specific antibody as isotype control or specifically stimulated with anti-Mu TCRβ by Cβ-domain cross-linking and analyzed for their amounts of total ZAP-70. For this, the mean OD450 changes for each unspecifically, that is, anti-Mu Vβ3, stimulated, and TCR gp100-transduced T-cell population were set to one. The relative changes in absorbance on specific ( ) versus unspecific stimulation (
) versus unspecific stimulation ( ) are indicated as stacked bars. Results were from 2 independent experiments. (C) Hu T cells transduced with the enumerated TCR gp100(280-288)–specific constructs were specifically stimulated with anti-Mu TCRβ in 6 independent experiments. In addition, murinized dcTCR gp100 was treated with irrelevant Mu Vβ3-specific antibody. Amounts of phosphorylated Y319 in ZAP-70 were quantified by ELISA. (D) TCR gp100-transduced Hu T cells were antigen-specifically stimulated with gp100(280-288)A2.1 tetramers or Hu Chim TCR gp100 was treated with an irrelevant p53(264-272)A2.1 tetramer. The indicated amounts of phosphorylated Y319 of ZAP-70 were the mean of 4 independent experiments. (A-D) The measured absorbances were converted to relative OD450 changes normalized to the sample with the highest absorbance in each experiment to merge replicate experiments conducted on subsequent days. Error bars represent SD.
) are indicated as stacked bars. Results were from 2 independent experiments. (C) Hu T cells transduced with the enumerated TCR gp100(280-288)–specific constructs were specifically stimulated with anti-Mu TCRβ in 6 independent experiments. In addition, murinized dcTCR gp100 was treated with irrelevant Mu Vβ3-specific antibody. Amounts of phosphorylated Y319 in ZAP-70 were quantified by ELISA. (D) TCR gp100-transduced Hu T cells were antigen-specifically stimulated with gp100(280-288)A2.1 tetramers or Hu Chim TCR gp100 was treated with an irrelevant p53(264-272)A2.1 tetramer. The indicated amounts of phosphorylated Y319 of ZAP-70 were the mean of 4 independent experiments. (A-D) The measured absorbances were converted to relative OD450 changes normalized to the sample with the highest absorbance in each experiment to merge replicate experiments conducted on subsequent days. Error bars represent SD.
Quantification of total ZAP-70 and phosphorylated ZAP-70 after antigen–(in)dependent stimulation of TCR gp100-transduced T cells. (A) Hu T cells, transduced with a panel of indicated TCR gp100(280-288)–specific constructs, were stimulated with anti-Mu TCRβ or gp100(280-288)A2.1 tetramers in 6 independent experiments and cell extracts were submitted to enzyme-linked immunosorbent assay (ELISA) to quantify total ZAP-70. (B) TCR gp100-transduced T cells were treated with either a nonstimulating Mu Vβ3-specific antibody as isotype control or specifically stimulated with anti-Mu TCRβ by Cβ-domain cross-linking and analyzed for their amounts of total ZAP-70. For this, the mean OD450 changes for each unspecifically, that is, anti-Mu Vβ3, stimulated, and TCR gp100-transduced T-cell population were set to one. The relative changes in absorbance on specific ( ) versus unspecific stimulation (
) versus unspecific stimulation ( ) are indicated as stacked bars. Results were from 2 independent experiments. (C) Hu T cells transduced with the enumerated TCR gp100(280-288)–specific constructs were specifically stimulated with anti-Mu TCRβ in 6 independent experiments. In addition, murinized dcTCR gp100 was treated with irrelevant Mu Vβ3-specific antibody. Amounts of phosphorylated Y319 in ZAP-70 were quantified by ELISA. (D) TCR gp100-transduced Hu T cells were antigen-specifically stimulated with gp100(280-288)A2.1 tetramers or Hu Chim TCR gp100 was treated with an irrelevant p53(264-272)A2.1 tetramer. The indicated amounts of phosphorylated Y319 of ZAP-70 were the mean of 4 independent experiments. (A-D) The measured absorbances were converted to relative OD450 changes normalized to the sample with the highest absorbance in each experiment to merge replicate experiments conducted on subsequent days. Error bars represent SD.
) are indicated as stacked bars. Results were from 2 independent experiments. (C) Hu T cells transduced with the enumerated TCR gp100(280-288)–specific constructs were specifically stimulated with anti-Mu TCRβ in 6 independent experiments. In addition, murinized dcTCR gp100 was treated with irrelevant Mu Vβ3-specific antibody. Amounts of phosphorylated Y319 in ZAP-70 were quantified by ELISA. (D) TCR gp100-transduced Hu T cells were antigen-specifically stimulated with gp100(280-288)A2.1 tetramers or Hu Chim TCR gp100 was treated with an irrelevant p53(264-272)A2.1 tetramer. The indicated amounts of phosphorylated Y319 of ZAP-70 were the mean of 4 independent experiments. (A-D) The measured absorbances were converted to relative OD450 changes normalized to the sample with the highest absorbance in each experiment to merge replicate experiments conducted on subsequent days. Error bars represent SD.
Next, we assessed whether T-cell stimulation may change the amount of total ZAP-70. The mean OD450 changes for all TCR gp100–transduced T cells slightly increased in most cases on specific anti-Mu TCRβ stimulation but, importantly, remained below (± 0.08) (Figure 6B). Thus, stimulation did not substantially change the amount of total ZAP-70 for any TCR gp100–transduced T-cell population.
Then, we stimulated TCR gp100–transduced T cells by cross-linking the Mu C-domains with anti-Mu TCRβ and detected phosphorylated Y319. Expectedly, the amount of pY319 for WT TCR gp100+ T cells ranged within the negative controls Mock and unspecifically (Mu Vβ3-) stimulated Chim TCR gp100+ T cells (Figure 6C). Chim TCR gp100+ T cells exhibited the highest level of phosphorylation in all experiments and was set to one. Cys-modified Chim scTCR gp100 S79C/Mu Cα T84C–transduced T cells induced phosphorylation of Y319 to 0.74 (± 0.22) to that of Chim TCR gp100. OD450 changes of Chim scTCR/Mu Cα+ T cells lay around 0.30 (± 0.09) and were slightly above background (Mock, 0.26 ± 0.08; anti-Mu Vβ3 added to Hu Chim TCR gp100, 0.15 ± 0.03). T cells transduced with Chim scTCR devoid of Mu Cα missed phosphorylation at Y319 (0.18 ± 0.08).
Eventually, to antigen specifically stimulate TCR gp100–expressing T cells we repeated the former experiment with gp100(280-288)A2.1 tetramers instead of anti-Mu TCRβ. As opposed to C-domain cross-linking (Figure 6C), Hu WT TCR gp100–transduced T cells responded to antigen-specific stimulation and yielded along with its murinized counterpart (Chim TCR gp100) the highest pY319 levels (1.00 ± 0.18; 0.97 ± 0.33; Figure 6D). Chim scTCR gp100 (0.09 ± 0.07) was around the negative controls Mock (0.15 ± 0.13) or irrelevantly p53(264-272)A2.1-tetramer stimulated Chim TCR gp100 (0.03 ± 0.01). Strikingly, Chim scTCR gp100 S79C/Mu Cα T84C achieved phosphorylation to 0.68 (± 0.16) to that of WT TCR gp100 or Chim TCR gp100. Chim scTCR gp100/Mu Cα ranked with a moderate stimulation potency (0.24 ± 0.13).
Conclusively, equal efficacies in tyrosine 319 phosphorylation triggered by either antigen-independent (0.74 ± 0.22) or -dependent (0.68 ± 0.16) TCR cross-linking suggested the structural and functional integrity of the scTCR/Cα scaffold.
Control of melanoma growth by scTCR gp100/Cα–transduced T cells in NOD/SCID mice
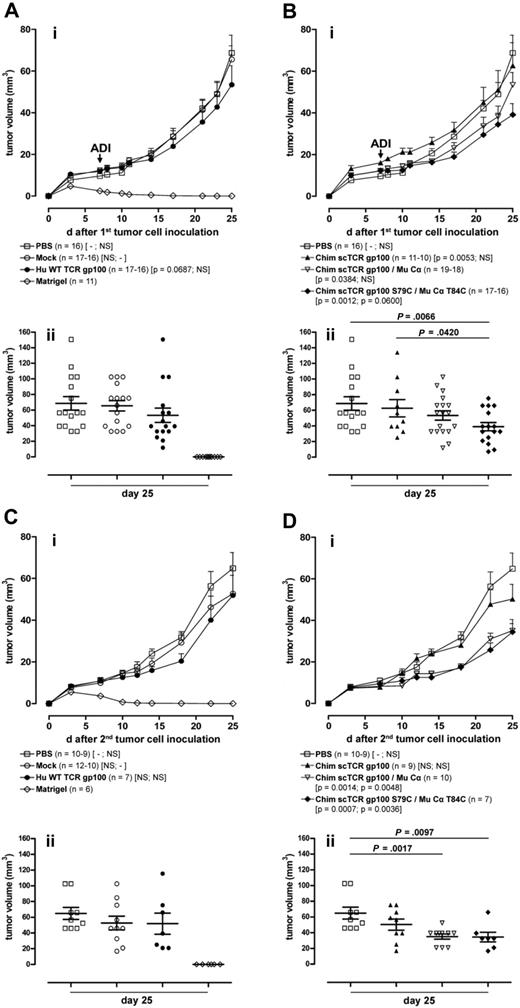
We evaluated the ability of Hu T cells transduced with different gp100(280-288)–specific TCRs to control the outgrowth of the Hu HLA-A2.1+gp100+ melanoma cell line MeWo17 in NOD/SCID mice.30 Seven days after intradermal tumor cell inoculation, mice were intravenously treated with ex vivo–expanded pure CD3+/TCR gp100+ effector memory-like T (CD45RA− CD62Ldim/−) cells31 in a nonconditioned regimen. In independent adoptive transfer experiments, the CD4+/CD8+ ratio of the T-cell graft shifted toward CD8+ (70%-85%) during ex vivo expansion and was approximately the same for all TCR gp100 constructs.
The tumor volumes in mice treated with Mock-transduced T cells and the PBS control were not significantly different (ie, P > .1 = NS; Mock compared with PBS: P = .149; PBS compared with Mock: P = .158; Figure 7Ai). Adoptive transfer of T cells bearing Hu WT TCR gp100 (compared with PBS: P = .069) and Chim scTCR gp100/Mu Cα (PBS: P = .038), respectively, led to a modest statistically significant reduction in tumor growth (Figure 7Ai,Bi). Control of tumor growth was maximal on transfer of T cells transduced with the Cys-modified Chim scTCR gp100 S79C/Mu Cα T84C (PBS: P = .001; Mock: P = .06).
Growth regression of melanoma in NOD/SCID mice treated with TCR gp100-transduced T cells after primary and secondary tumor challenges. (A-B) NOD/SCID mice were engrafted with the MeWo melanoma cell line intradermally (2.5 × 106 cells in 50 μL of matrigel) at the right flank (first tumor cell inoculation, day 0) and adoptively transferred intravenously with T cells transduced with different TCR gp100 constructs (as indicated by symbols) (mean 2.4 × 106 cells in 100 μL of PBS; ADI day 7). (Ai) Effect of ADI on primary tumor outgrowth of animals treated with PBS, Mock, Hu WT TCR gp100, or matrigel. Curves show the cumulative mean and standard error of the mean (SEM) tumor volume of 5 independent experiments (□, ○, ●, ◇). Animals with an intradermal injection of matrigel (50 μL) without tumor cells and animals adoptively transferred intravenously with 100 μL of PBS without T cells were used as controls. P values in brackets indicate the statistical significance of differences in tumor progression over time with the use of a linear regression model with mixed effects (fixed and random) against PBS, Mock. (Bi) Effect of ADI on primary tumor outgrowth of animal treated with PBS, Chim scTCR gp100, Chim scTCR gp100/Mu Cα, and Chim scTCR gp100 S79C/Mu Cα T84C. Curves show the cumulative mean and SEM tumor volume of 5 (□, ▿, ♦) or 2 (▴) independent experiments. The tumor volume of individual mice, their mean, and SEM from experimental groups on day 25 after primary tumor challenge is depicted in panels Aii or Bii. (C-D) Primary local tumors from mice treated with a single injection of TCR gp100-transduced T cells (from A-B) were safely removed after day 25 from the right flank, and 2 days later a secondary tumor inoculation (reset to day 0) with MeWo cell line (2.5 × 106 cells in 50 μL of matrigel) was performed intradermally at the left flank of the same animals. (Ci) Effect of a long-term T-cell response of animal treated with PBS, Mock, Hu WT TCR gp100, or matrigel. Curves show the cumulative mean and SEM tumor volume of 3 (□,○, ◇) or 2 (●) independent experiments. (Di) Effect of a long-term T-cell response of animals treated with PBS, Chim scTCR gp100, Chim scTCR gp100/Mu Cα, and Chim scTCR gp100 S79C/Mu Cα T84C. Curves show the cumulative mean and SEM tumor volume of 3 (□) or 2 (▴, ▿, ♦) independent experiments. The tumor volume of individual mice, their mean, and SEM from experimental groups on day 25 after secondary tumor challenge is given in panels Cii or Dii.
Growth regression of melanoma in NOD/SCID mice treated with TCR gp100-transduced T cells after primary and secondary tumor challenges. (A-B) NOD/SCID mice were engrafted with the MeWo melanoma cell line intradermally (2.5 × 106 cells in 50 μL of matrigel) at the right flank (first tumor cell inoculation, day 0) and adoptively transferred intravenously with T cells transduced with different TCR gp100 constructs (as indicated by symbols) (mean 2.4 × 106 cells in 100 μL of PBS; ADI day 7). (Ai) Effect of ADI on primary tumor outgrowth of animals treated with PBS, Mock, Hu WT TCR gp100, or matrigel. Curves show the cumulative mean and standard error of the mean (SEM) tumor volume of 5 independent experiments (□, ○, ●, ◇). Animals with an intradermal injection of matrigel (50 μL) without tumor cells and animals adoptively transferred intravenously with 100 μL of PBS without T cells were used as controls. P values in brackets indicate the statistical significance of differences in tumor progression over time with the use of a linear regression model with mixed effects (fixed and random) against PBS, Mock. (Bi) Effect of ADI on primary tumor outgrowth of animal treated with PBS, Chim scTCR gp100, Chim scTCR gp100/Mu Cα, and Chim scTCR gp100 S79C/Mu Cα T84C. Curves show the cumulative mean and SEM tumor volume of 5 (□, ▿, ♦) or 2 (▴) independent experiments. The tumor volume of individual mice, their mean, and SEM from experimental groups on day 25 after primary tumor challenge is depicted in panels Aii or Bii. (C-D) Primary local tumors from mice treated with a single injection of TCR gp100-transduced T cells (from A-B) were safely removed after day 25 from the right flank, and 2 days later a secondary tumor inoculation (reset to day 0) with MeWo cell line (2.5 × 106 cells in 50 μL of matrigel) was performed intradermally at the left flank of the same animals. (Ci) Effect of a long-term T-cell response of animal treated with PBS, Mock, Hu WT TCR gp100, or matrigel. Curves show the cumulative mean and SEM tumor volume of 3 (□,○, ◇) or 2 (●) independent experiments. (Di) Effect of a long-term T-cell response of animals treated with PBS, Chim scTCR gp100, Chim scTCR gp100/Mu Cα, and Chim scTCR gp100 S79C/Mu Cα T84C. Curves show the cumulative mean and SEM tumor volume of 3 (□) or 2 (▴, ▿, ♦) independent experiments. The tumor volume of individual mice, their mean, and SEM from experimental groups on day 25 after secondary tumor challenge is given in panels Cii or Dii.
A significant reduction in tumor volume for individual mice at day 25 was in particular evident for Cys-modified Chim scTCR gp100/Mu Cα (Figure 7Aii,Bii; P < .007) compared with PBS. The differences in tumor volumes between scTCR gp100 and scTCR gp100/Cα–transduced T cells were only significant for Cys-modified scTCR (P = .042).
Enumeration of Hu T cells in different tissue samples (each 0.5 × 106 tissue cells) by flow cytometry at day 6 after adoptive immunotherapy (ADI) showed that the frequency of Hu CD45+ or CD3+ T cells was increased in tumor tissue on a per mille level for representative mice that received T cells transduced with highly potent TCR gp100 constructs (data not shown). This correlation could not be observed for samples taken from spleen and bone marrow.
However, the onset of tumor growth emerged for all TCR gp100 constructs. This might result from insufficient gp100(280-288)A2.1 presentation on melanoma because specific lysis of MeWo cells in vitro did not exceed 50% for any TCR gp100–transduced T cells even at high effector/target ratios (data not shown; see also “Discussion”).
Subsequently, we analyzed whether transferred T cells might circulate or latently reside in memory niches of secondary lymphatic organs and provide for an antigen-specific recall response after a secondary tumor challenge.32
After removal of the tumor at the right flank we freshly engrafted MeWo tumor cells at the opposite flank. Here, unmodified and Cys-modified Chim scTCR gp100/Mu Cα constructs significantly delayed tumor growth compared with both controls, PBS (P < .001 and P < .001, respectively) and Mock (P < .005 and P < .004; Figure 7Ci,Di) with the same order as observed during the primary response. Strikingly, differences in tumor growth were more pronounced in the course of the secondary immune response than in the primary response although mice received T cells only once at the beginning of the consecutive experiments. However, this might be also assigned to some extent to a better accessibility of injected tumor suspension cells. Tumor sizes at day 25 of the secondary tumor challenge experiment were significantly reduced for Chim scTCR gp100/Mu Cα and Chim scTCR gp100 S79C/Mu Cα T84C compared with the PBS control (P < .002 and P < .001; Figure 7Cii,Dii). Strikingly, the Hu WT TCR gp100 group did not exhibit any significant recall response.
Discussion
Adoptive immunotherapy of cancer based on TAA-redirected T cells demands a clinically safe regimen to assure a sustained treatment without adverse reactions.33 The formation of mixed TCR dimers by pairing of the introduced and the natural TCR chains is one potential mechanism for the induction of “off-target” reactions that may provoke autoimmunity.34 To address this concern, we developed a novel scTCR approach that relied on the coexpression of a Cα domain and obviates the need to fuse T-cell signaling molecules such as CD3ζ.14
Importantly, we generalized this strategy to a p53- and an MDM2-specific TCR that holds promise for eradicating both solid tumors and hematologic malignancies such as lymphoma, leukemia, and multiple myeloma.6 Strong in vivo evidence of aberrant p53 expression in a multitude of hematologic and visceral cancer diseases22 makes it an ideal general tumor antigen.4,5
The major effect mediated by the truncated Cα domain was assumed to reside in the structural stabilization of the scTCR35 and/or in improving T-cell signaling by the CD3 complex. The latter argument is in line with a TCR/CD3 assembly model that has recently been suggested.36,37 The Cα domain interacts exclusively with the CD3 subunits CD3δϵ and the CD3ζ by charged residues in the transmembrane region and its highly conserved DE loop, respectively. The absence of Cα may implicate the failure to recruit CD3δ and CD3ζ interfering with T-cell function: partial tyrosine phosphorylation of the CD3ζ ITAMs is shown to evoke aberrant signaling.38 It is speculated, that ITAMs located on separate CD3 subunits can be assigned to different signaling pathways,39 and hereditary CD3δ deficiency is prone to cause severe combined immunodeficiency during T-cell maturation in humans.40
On TCR engagement the TCR and the costimulatory molecule CD28 colocalize in “TCR-CD28 microclusters” assorting a set of downstream adapters to bolster T-cell signaling.41 The hypothesized cooperativity of CD28 and the TCR/CD3 complex is supported by the observation that chimeric antigen receptors of the scFv-CD3ζ T body design that were additionally endowed with CD28 signaling domains were functionally superior to chimeric antigen receptor–CD3ζ.42
The α-chain connecting peptide motif is a highly conserved sequence in the membrane proximal region of the TCR α-chain. This region mediates coreceptor CD8 approximation, notably also by CD3δ, followed by a higher peptide–major histocompatibility complex affinity.43
However, the scTCR/Cα concept was not readily applicable to a Hu A2.1-restricted gp100-specific scTCR and a Hu Cα domain. From previous results, murinized TCRα/β associated to each other much stronger to preferentially form heterodimers.24,25 The same observation applies to scTCR/Cα; the beneficial effect is supposed to be primarily caused by a few charged residues that strengthen pairing of the C-domains and interaction with CD3.24,44 Efforts are in progress to minimize the fraction of Mu amino acid residues in Cα/β-domains while retaining function of the scTCR approach. We are also in favor of introducing a set of disulfides customized to full-length Hu scTCR/Cα that comparable with murinization may functionalize the scTCR/Cα format.45 Nevertheless, it appears feasible to use C-domain murinized or even full-length Mu TCRs because under lymphodepleting conditions that facilitate the engraftment of T cells the suppressed immune system would be unable to respond to the xenogeneic moieties as shown in a recent clinical trial.3 Moreover, the need for 2 coding sequences (ie, scTCR/ Cα) can be successfully circumvented by genetically linking their genes in frame by a short 2A element to yield a nascent protein being processed in situ.46
Moreover, scTCRs in contrast to scFv's are prone to misfolding and aggregation caused by thermal instability of Vα and weak interactions between Vα and Vβ. Keeping in mind that productive pairing is largely affected by the intrinsic qualities of TCR Vα/Vβ interchain affinities,10 the broad applicability of the scTCR/Cα approach to arbitrary TCR Vα/Vβ combinations may require additionally structural fine-tuning.35
In cellular assays, insertion of an accessory disulfide bond at the interface of the Cα and Cβ domain in scTCR gp100/Cα raised its structural avidity substantially. The cystine bridge increases the occupancy of scTCR with Cα and, thus, the stability and surface expression of a TCR/CD3 complex. In contrast, in a cell-free system, the amount of site-specific ZAP-70 tyrosine 319 phosphorylation on antigen encounter increased but did not entirely match that of the Hu WT TCR gp100. However, Cys-modified scTCR/Cα was equally expressed as deduced from flow cytometric analysis. Most importantly, both antigen-independent as well as antigen-mediated ligation yielded the same amount of Y319 phosphorylation. This strongly suggests that antigen recognition and signal transfer to the proximal effector molecule is not compromised in scTCR/Cα. As opposed to the results obtained from the kinetic resolution (ie, 4 minutes) of Y319 phosphorylation, T cells may accumulate signaling over time (ie, 4-24 hours of incubations) as long as a threshold of TCR-mediated T-cell avidity is exceeded.47
The biologic relevance of Hu T lymphocytes transduced with gp100(280-288)–specific scTCR constructs was verified in an immunodeficient, melanoma-engrafted NOD/SCID mouse model that allows one to study the control of tumor growth by T-cell subsets48 or TCR-redirected T cells.49 In analogy to in vitro results, T cells transduced with Chim scTCR gp100 S79C/Mu Cα T84C showed the highest reactivity and triggered a considerable delay in tumor growth. In addition, the observation of a secondary immune response after excision of the primary tumor and reinoculation of tumor cells suggests the persistence of these scTCR gp100–transduced T cells in mice. Both the reduction of the mean tumor volumes and the narrower standard errors of the mean at day 25 of the secondary response argue for a robust antitumor reaction.
Nonetheless, tumors eventually grew with various kinetics in all groups of T cell–engrafted mice. This most likely resulted from clonal exhaustion of ex vivo–expanded T cells or residual Mu NK-cell activity.33,50 Experimentally, the period for recovering adoptively transferred T cells in the NOD/SCID state depends on their T-cell subset and phenotype.30,48
However, we assume that a fraction of transferred T cells persists as a less-differentiated, peripheral effector memory-like T-cell subset31 or reshaped, highly reactive central memory-like T cells preferentially homing to lymphatic memory niches.32 This might account for the more pronounced secondary immune response of Cys-modified scTCR gp100–transduced T cells (P < .004 versus Mock) compared with the primary response (P = .060 versus Mock).
Recently, it has been shown that the transfer of 2 unrelated melanoma-specific dcTCRα/β into autologous T cells resulted in remarkable objective antitumor responses.3 However, the induction of “off-target” immunity cannot be excluded over a longer period especially with regard to the envisioned presence of long-living memory T cells in cancer therapy. The scTCR/Cα framework may serve as a safeguard to avoid mispairing of introduced and natural TCR chains which otherwise might take place with a reasonable frequency in reprogrammed bulk T-cell populations of a patient.10 In this study, the reduction of hybrid TCR formation in turn may enlarge the fraction of tumor-reactive TCRs in T cells and, hence, explain the observed superior effector function of a scTCR in comparison to the introduced WT TCR in vivo.
In summary, our experiments characterize a novel scTCR/Cα framework that clearly confirms the feasibility of TCR design for adoptive immunotherapy to realize a profound therapeutical recovery from both hematologic and solid malignancies and as a further perspective from viral diseases in vivo.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Edite Antunes-Ferreira, Diana Stolle, and Ratna S. Intan-Poendl for expert technical contributions and Cedrik Britten (University Medical Center, Mainz) for the delivery of K562-A2. Jurkat-76 was kindly given by Shao-An Xue (Royal Free Hospital, University College London). We thank Ralph Willemsen (Erasmus University of Rotterdam) for providing the gp100(280-288)–specific TCR, Mirjam Heemskerk (University Medical Center, Leiden) for generously forwarding TCR pp65(495-503), and Niels Schaft (University Hospital of Erlangen) for his advice and support in TCR RNA transfection. We thank Axel Benner from the Department of Biostatistics of the German Cancer Research Center, Heidelberg, for help with statistical analyses.
This work was mainly supported by the Deutsche Forschungsgemeinschaft (DFG) and the Faculty of Medicine, Mainz (grant KFO 183-TP1; R.-H.V. and M.T.); DFG (grant SFB-432-A3), the European Commission, and the Wilhelm-Laupitz foundation (M.T.); the Initiative and Networking Fund of the Helmholtz Association on Immunotherapy of Cancer (P.B.).
Authorship
Contribution: R.-H.V. designed and performed experiments, analyzed data, and wrote the paper; S.T. contributed to the design of experiments, performed experiments, and wrote the paper; C.P. performed and analyzed the in vivo experiments and wrote the paper; B.H. and S.K. performed experiments; J.K. performed experiments and analyzed data; M.G. and R.E. performed experiments; P.G. prepared tetramers for all experiments; P.R. contributed to the design of tetramer experiments, analyzed data, and wrote the paper; C.H. analyzed data; P.B. designed in vivo experiments, analyzed data, and wrote the paper; and M.T. contributed to the design of experiments, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for J.K. is Department of Hematology and Van Creveld Clinic, University Medical Center, Utrecht, The Netherlands. The current affiliation for R.E. is The European Institute for Research and Development of Transplantation Strategies, Idar-Oberstein, Germany.
Correspondence: Ralf-Holger Voss, Department of Hematology and Oncology, University Medical Center Mainz, Langenbeckstr 1, 55101 Mainz, Germany; e-mail: hvoss@uni-mainz.de.
References
Author notes
R.-H.V. and S.T. contributed equally to this study.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal