Abstract
The impact of human leukocyte antigen (HLA) mismatch after reduced-intensity conditioning allogeneic hematopoietic stem cell transplantation (RIT) using unrelated donors (UD) is unclear, and may be modulated by T-cell depletion. We therefore examined outcomes of 157 consecutive patients undergoing RIT after uniform conditioning with fludarabine, melphalan, and alemtuzumab (FMC). Donors were 10/10 HLA-matched (MUDs, n = 107) and 6 to 9/10 HLA-matched (MMUDs, n = 50), with no significant differences in baseline characteristics other than increased cytomegalovirus seropositivity in MMUDs. Rates of durable engraftment were high. Graft failure rates (persistent cytopenias with donor chimerism) were similar (8% vs 3%, P = .21), though rejection (recipient chimerism) was more frequent in MMUDs (8% vs 0%, P < .01). There were no significant differences between donors in the incidences of acute graft-versus-host disease (GVHD; 20% vs 22% grade 2-4, respectively, P = .83), chronic extensive GVHD (3-year cumulative incidence [CI] 23% vs 24%, P = .56), or treatment-related mortality (1-year CI 27% vs 27%, P = .96). Furthermore, there was no difference in 3-year overall survival (OS; 53% vs 49%, P = .44). Mismatch occurred at the antigenic level in 40 cases. The outcome in these cases did not differ significantly from the rest of the cohort. We conclude that RIT using HLA-mismatched grafts is a viable option using FMC conditioning.
Introduction
Allogeneic hematopoietic stem cell transplantation after reduced-intensity conditioning (RIT) is an important treatment modality for patients with a range of hematologic malignancies,1-5 as well as several benign conditions.6 A sibling matched at 10/10 human leukocyte antigen (HLA) loci remains the preferred donor, but only approximately one-third of patients will have such a donor available. Improved HLA-typing techniques allow more stringent matching of unrelated donors (UD) at the molecular level, potentially improving outcomes after transplantation in this setting. However, the likelihood of identifying a “fully matched” UD (MUD) has decreased as a result of using these more stringent criteria. Consequently, despite considerable expansion of international stem cell registries,7 only approximately two-thirds of Caucasian patients will find a 10/10 HLA allele-matched donor. For other ethnic groups, the probability is often much lower.8
When a MUD is not available, mismatched UDs (MMUD) matching at 8 to 9/10 HLA loci have been used as alternatives. Registry studies reporting data in the myeloablative setting have observed that mismatches at 1 or 2 HLA alleles increase risk of graft failure, graft-versus-host disease (GVHD), and treatment-related mortality (TRM), particularly with mismatches involving multiple loci.9-11 The degree to which HLA mismatch increases toxicity in the RIT setting and how this is influenced by different regimens is unclear. It is possible that persistence of host antigen presenting cells may lead to an increase in the incidence and severity of GVHD associated with HLA mismatch to unacceptably high levels. It is therefore noteworthy that 2 studies have demonstrated high TRM rates in patients receiving class I HLA-mismatched RIT with T cell–replete grafts after conditioning with either busulfan and fludarabine or with fludarabine and total body irradiation.12,13 Furthermore, a 2- to 3-loci HLA mismatch has been reported to be associated with increased GVHD, graft failure, and a markedly adverse prognosis in the RIT setting.14
Incorporation of alemtuzumab (anti–CD52 monoclonal antibody) into the RIT conditioning effectively reduces GVHD in the UD setting.15,16 It may be informative, therefore, to examine how alemtuzumab-based T-cell depletion may modulate the impact of HLA mismatch. The availability of alternative stem cell sources, such as mismatched related donors, or umbilical cord blood,17 as well as an ever-increasing number of nontransplantation-based salvage regimens makes it important to clearly define such risks. To address this issue, we retrospectively analyzed the outcome of 157 consecutive patients who underwent a uniformly conditioned alemtuzumab-based RIT using an UD in University College London (UCL) Medical School over a 10-year period, and stratified the cohort according to the presence or absence of an HLA mismatch.
Methods
Patients
All patients received transplants at UCL Medical School (comprising 2 clinical sites with a joint transplantation program) between October 1998 and September 2008, and receiving identical conditioning with a T cell–depleted RIT from an UD were included in this analysis (n = 157). Clinical characteristics of these patients are shown in Table 1. Patients were excluded from transplantation if they were older than 70 years of age, had an Eastern Cooperative Oncology Group performance score greater than 1, a left ventricular ejection fraction less than 40%, a creatinine clearance less than 40 mL/min, a serum bilirubin level greater than 30μM, or liver transaminases more than 3 times the upper limit of the normal range.
Clinical characteristics of the MMUD and MUD cohorts
| . | MUD, n (%) . | Disease status (S/R) . | MMUD, n (%) . | Disease status (S/R) . |
|---|---|---|---|---|
| Diagnosis | ||||
| LG-NHL | 23 (21) | 23/0 | 8 (16) | 6/2 |
| Transformed LG-NHL | 2 (2) | 2/0 | 2 (4) | 2/0 |
| INT/HG-NHL | 13 (12) | 12/1 | 11 (22) | 11/0 |
| Chronic lymphocytic leukemia | 5 (5) | 5/0 | 2 (4) | 2/0 |
| Hodgkin lymphoma | 21 (20) | 16/5 | 5 (10) | 3/2 |
| Plasma cell disorders | 10 (9) | 9/1 | 6 (12) | 5/1 |
| Acute myeloid leukemia | 17 (16) | 16/1 | 6 (12) | 6/0 |
| Acute lymphoblastic leukemia | 1 (1) | 1/0 | 1 (2) | 1/0 |
| Chronic phase CML | 3 (3) | 3/0 | 1 (2) | 1/0 |
| Idiopathic myelofibrosis | 5 (5) | N/A | 5 (10) | N/A |
| Myelodysplasia | 3 (3) | N/A | 3 (6) | N/A |
| Other | 4 (4) | N/A | 0 (0) | N/A |
| Seattle risk group19 | ||||
| Low | 41 (38) | 20 (40) | ||
| Standard | 23 (21) | 10 (20) | ||
| High | 39 (36) | 20 (40) | ||
| Not applicable | 4 (4) | 0 (0) | ||
| Median age, y (range) | 48 (13-65) | 48 (18-67) | ||
| Sex | ||||
| Female | 41 (38) | 22 (44) | ||
| Male | 66 (62) | 28 (56) | ||
| Lines of treatment | ||||
| Median (range) | 4 (0-9) | 3 (1-8) | ||
| Prior autograft | 46 (43) | 19 (38) | ||
| Chemosensitive disease* | 87 (92) | 37 (88) |
| . | MUD, n (%) . | Disease status (S/R) . | MMUD, n (%) . | Disease status (S/R) . |
|---|---|---|---|---|
| Diagnosis | ||||
| LG-NHL | 23 (21) | 23/0 | 8 (16) | 6/2 |
| Transformed LG-NHL | 2 (2) | 2/0 | 2 (4) | 2/0 |
| INT/HG-NHL | 13 (12) | 12/1 | 11 (22) | 11/0 |
| Chronic lymphocytic leukemia | 5 (5) | 5/0 | 2 (4) | 2/0 |
| Hodgkin lymphoma | 21 (20) | 16/5 | 5 (10) | 3/2 |
| Plasma cell disorders | 10 (9) | 9/1 | 6 (12) | 5/1 |
| Acute myeloid leukemia | 17 (16) | 16/1 | 6 (12) | 6/0 |
| Acute lymphoblastic leukemia | 1 (1) | 1/0 | 1 (2) | 1/0 |
| Chronic phase CML | 3 (3) | 3/0 | 1 (2) | 1/0 |
| Idiopathic myelofibrosis | 5 (5) | N/A | 5 (10) | N/A |
| Myelodysplasia | 3 (3) | N/A | 3 (6) | N/A |
| Other | 4 (4) | N/A | 0 (0) | N/A |
| Seattle risk group19 | ||||
| Low | 41 (38) | 20 (40) | ||
| Standard | 23 (21) | 10 (20) | ||
| High | 39 (36) | 20 (40) | ||
| Not applicable | 4 (4) | 0 (0) | ||
| Median age, y (range) | 48 (13-65) | 48 (18-67) | ||
| Sex | ||||
| Female | 41 (38) | 22 (44) | ||
| Male | 66 (62) | 28 (56) | ||
| Lines of treatment | ||||
| Median (range) | 4 (0-9) | 3 (1-8) | ||
| Prior autograft | 46 (43) | 19 (38) | ||
| Chemosensitive disease* | 87 (92) | 37 (88) |
UD cohorts stratified according to 10/10 HLA match (MUD) or 6 to 9/10 HLA match (MMUD). All P values for heterogeneity (χ2 test) were not significant between MUD and MMUD groups.
NHL indicates non-Hodgkin lymphoma; CML, chronic phase leukemia; N/A, not applicable; LG, low grade; INT/HG, intermediate/high grade; S, chemosensitive disease; and R, chemoresistant disease.
Excluding “not applicable” cases.
Donors
Donor selection was performed according to standard criteria, including molecular typing for HLA-A, -B, -C, DRB1, and DQB1. Donors were 10/10 HLA-matched (MUD) in 107 cases. For those patients in whom a 10/10 HLA-matched donor was not available, a search was performed based on a HLA-A mismatch in the first instance. Donors mismatched at up to 2 HLA loci were deemed routinely acceptable, and 3 transplantations using donors mismatched at 3 to 4 loci were also performed (MMUD; n = 50). Further details of recipient-donor matching are shown in Table 2.
Recipient-donor matching characteristics
| . | MUD, n (%) . | MMUD, n (%) . | Level of mismatch . |
|---|---|---|---|
| Blood group | |||
| Matched | 51 (48) | 18 (36) | |
| Major incompatibility | 21 (20) | 16 (32) | |
| Minor incompatibility | 28 (26) | 15 (30) | |
| Bidirectional | 7 (7) | 1 (2) | |
| Sex | |||
| Matched | 57 (53) | 28 (56) | |
| Male recipient, female donor | 20 (19) | 11 (22) | |
| Female recipient, male donor | 29 (27) | 11 (22) | |
| Missing data | 1 (1) | ||
| HLA match | |||
| Single class I mismatch | N/A | 18 (36) | |
| -A | N/A | 5 (10) | 5 antigenic |
| -B | N/A | 0 (0) | |
| -C | N/A | 13 (26) | 12 antigenic, 1 allelic |
| Single class II mismatch | N/A | 14 (28) | |
| DQB1 | N/A | 9 (18) | 6 antigenic, 3 allelic |
| DRB1 | N/A | 5 (10) | 5 allelic |
| 2 loci mismatch | N/A | 15 (30) | |
| Both class I | N/A | 7 (14) | 4 antigenic, 3 mixed |
| Both class II | N/A | 0 (0) | |
| Class I and II | N/A | 8 (16) | 3 antigenic, 1 allelic, 4 mixed |
| 3+ loci mismatch | N/A | 3 (6) | 3 mixed* |
| CMV status (recipient/donor)† | |||
| −/− | 61 (57) | 16 (32) | |
| −/+ | 11 (10) | 3 (6) | |
| +/− | 11 (10) | 13 (26) | |
| +/+ | 24 (22) | 18 (36) | |
| Stem cell source | |||
| BM | 42 (39) | 16 (32) | |
| PBSC | 65 (61) | 34 (68) | |
| Stem cell dose | |||
| CD34 ×106/kg (range) | 5.2 (0.6-18.5) | 5.7 (0.7-29) | |
| CFU × 104/kg (range) | 181 (24-465) | 171 (51-331) |
| . | MUD, n (%) . | MMUD, n (%) . | Level of mismatch . |
|---|---|---|---|
| Blood group | |||
| Matched | 51 (48) | 18 (36) | |
| Major incompatibility | 21 (20) | 16 (32) | |
| Minor incompatibility | 28 (26) | 15 (30) | |
| Bidirectional | 7 (7) | 1 (2) | |
| Sex | |||
| Matched | 57 (53) | 28 (56) | |
| Male recipient, female donor | 20 (19) | 11 (22) | |
| Female recipient, male donor | 29 (27) | 11 (22) | |
| Missing data | 1 (1) | ||
| HLA match | |||
| Single class I mismatch | N/A | 18 (36) | |
| -A | N/A | 5 (10) | 5 antigenic |
| -B | N/A | 0 (0) | |
| -C | N/A | 13 (26) | 12 antigenic, 1 allelic |
| Single class II mismatch | N/A | 14 (28) | |
| DQB1 | N/A | 9 (18) | 6 antigenic, 3 allelic |
| DRB1 | N/A | 5 (10) | 5 allelic |
| 2 loci mismatch | N/A | 15 (30) | |
| Both class I | N/A | 7 (14) | 4 antigenic, 3 mixed |
| Both class II | N/A | 0 (0) | |
| Class I and II | N/A | 8 (16) | 3 antigenic, 1 allelic, 4 mixed |
| 3+ loci mismatch | N/A | 3 (6) | 3 mixed* |
| CMV status (recipient/donor)† | |||
| −/− | 61 (57) | 16 (32) | |
| −/+ | 11 (10) | 3 (6) | |
| +/− | 11 (10) | 13 (26) | |
| +/+ | 24 (22) | 18 (36) | |
| Stem cell source | |||
| BM | 42 (39) | 16 (32) | |
| PBSC | 65 (61) | 34 (68) | |
| Stem cell dose | |||
| CD34 ×106/kg (range) | 5.2 (0.6-18.5) | 5.7 (0.7-29) | |
| CFU × 104/kg (range) | 181 (24-465) | 171 (51-331) |
Recipient-donor matching for blood group, sex, HLA, and CMV stratified according to 10/10 HLA match (MUD) or 6 to 9/10 HLA match (MMUD). BM indicates bone marrow; CFU, colony-forming unit; N/A, not applicable; and PBSC, peripheral blood stem cell.
Two mismatched at 2 antigens + 1 allele, and one at 3 antigens + 1 allele.
P < .01. P values (χ2 test) for all other factors were not significant.
Conditioning regimen
All patients received uniform conditioning with alemtuzumab (20 mg/d) from day −8 to −4 (total dose, 100 mg), fludarabine (30 mg/m2 daily) from day −7 to −3 (total dose, 150 mg/m2), and melphalan (140 mg/m2) on day −2 (FMC conditioning). Cyclosporine was commenced as an intravenous infusion at 3 mg/kg daily starting on day −1, with a target level of 200 to 300 ng/mL. On day 0, patients received the bone marrow or peripheral blood stem cell infusion. In the absence of GVHD, cyclosporine was tapered from 3 months after transplantation.
Supportive care
All patients received standard nursing and supportive care protocols. Infection prophylaxis always included antifungal agents (fluconazole or itraconazole), low-dose acyclovir, and prophylaxis against both Streptococcus pneumoniae and Pneumocystis (carinii) jiroveci. Patients at risk for cytomegalovirus (CMV) infection (CMV-seropositive recipients or those who received grafts from CMV-seropositive donors) were monitored weekly by quantitative polymerase chain reaction and treated on the basis of 2 consecutive positive results and increasing titers with ganciclovir- or foscarnet-dependent on the blood counts. Blood products were universally depleted of white blood cells and irradiated. CMV-seronegative recipients received CMV-seronegative blood products.
Analysis of donor chimerism and donor lymphocyte infusions
Whole blood and lineage-specific chimerism (in T-cell and myeloid lineages) were assessed as previously described.16 Patients who had mixed chimerism or residual disease 6 months after transplantation were eligible to receive donor lymphocyte infusions (DLI) if there was no evidence of active GVHD. Escalating doses of CD3+ lymphocytes were administered starting at a dose of 1 × 106 T cells/kg. Increasing doses were administered at 3-month intervals (3 × 106, 1 × 107, 3 × 107, and 1 × 108 T cells/kg) in the absence of GVHD if mixed chimerism persisted or if there was no evidence of disease response. The dose administered for disease progression was determined by the individual physician on a case by case basis according to the disease type and the interval since transplantation.
Outcome measures and statistical evaluation
End points examined were engraftment, CMV infection, acute or chronic GVHD, and cause-specific mortality. Neutrophil engraftment was defined as neutrophil count of 0.5 × 109/L or more and platelet engraftment was defined as an unsupported platelet count of 50 × 109/L or more on the first of 2 consecutive days. Graft failure was defined as persistent severe cytopenia (absolute neutrophil count of < 0.5 × 109/L) with more than 90% donor chimerism in T-cell and granulocyte lineages. Graft rejection was defined as loss of donor chimerism with or without autologous hematopoietic reconstitution. GVHD was assessed according to consensus guidelines.18
Actuarial curves were estimated using the Kaplan-Meier method for overall survival (OS). Time-to-event outcomes with competing risks (TRM and GVHD) were estimated by cumulative incidence (CI) analyses. TRM was defined as death from any cause in the absence of disease relapse. Relapse was considered a competing event for TRM analyses, and death without relapse a competing risk in the GVHD analyses. GVHD analyses were performed both with patients censored at the time of DLI or last follow-up, and with censoring only at last follow-up to differentiate the impact of DLI. Outcomes were compared using the log-rank test for Kaplan-Meier analyses, and Gray test was used for TRM and GVHD. A value for P of .05 or less was considered significant.
Results
Clinical characteristics
Clinical characteristics of the patients, stratified according to the presence or absence of an HLA mismatch, are shown in Table 1. There were no significant differences between MUD and MMUD groups in terms of age, sex, indication for transplantation, prior autograft, number of treatment lines, chemosensitivity of disease at transplantation or Seattle risk group.19 Patients had received a median of 4 previous lines of therapy and the majority had chemosensitive disease at the time of transplantation. Median follow-up in surviving patients did not differ significantly between the groups (2.1 years in MUD vs 2.3 years in MMUD, P = .67 by Mann-Whitney test, 2-tailed).
Donors
Details of donors and recipient-donor matching stratified according to the presence or absence of an HLA mismatch are shown in Table 2. There was no difference between the donors used for MUD or MMUD transplantations with regards to the presence of a sex mismatch or blood group mismatch. The distribution of CMV groups differed because of the increased incidence of CMV-seropositive recipients in the MMUD group. There was no significant difference between the MUD and MMUD groups with regards to stem cell source or dose.
Engraftment, graft failure, and rejection
Neutrophil engraftment (> 0.5 × 109/L) occurred at a median of 12 days (range 9-57 days) and platelet engraftment (> 50 × 109/L) at 14 days (8-349 days). There were no significant differences in engraftment rates between MUD and MMUD groups (median 11 vs 12 days for neutrophils, and 14 vs 15 days for platelets). Three patients (1 MMUD, in whom cyclosporine was withdrawn early because of development of microangiopathic hemolytic anemia, and 2 MUDs), failed to engraft by 28 days after stem cell infusion. These 3 patients all demonstrated donor chimerism. Two engrafted after a further infusion of CD34-selected stem cells without additional conditioning, and one (MMUD) died of gastrointestinal bleeding before a repeat donor harvest could be performed. Late graft failure occurred in 4 cases, 1 MUD, and 3 MMUDs (P < .01 for MUD vs MMUD). In one case (MUD), the patient was documented to have infection with human herpesvirus 6. All 4 received at least one CD34-selected stem cell top-up. Two had subsequent count regeneration (donor hematopoiesis). One died shortly after infusion of cells and 1 remained thrombocytopenic and died of hemorrhage associated with underlying gastric angiodysplasia 4 years after the transplantation. Of the 3 MMUDs with late graft failure, all were mismatched at class I HLA (1 bidirectional HLA-A, 1 HLA-C in the host-versus-graft [HVG] direction, and 1 HLA-A [HVG] plus HLA-C [bidirectional]).
Four patients, all MMUDs, rejected the graft after initial engraftment, (P < .01 for MUD vs MMUD). Underlying diagnoses were chronic myeloid leukemia (CML) in first chronic phase, idiopathic myelofibrosis, follicular lymphoma, and small lymphocytic lymphoma. These patients all had single class II HLA mismatches (2 bidirectional allelic DRB1, 1 bidirectional antigenic DQB1, and 1 HvG antigenic DQB1). Two of these patients remained alive after 841 and 2644 days follow-up with autologous reconstitution and 2 patients died, 1 due to invasive fungal infection after a second allograft and the other after disease relapse.
CMV infection
There was a higher frequency of CMV-seropositive recipients in MMUDs versus MUDs (P < .01; Table 2). Consequently, CMV infection was more frequent in MMUDs (28/50; 56%) than in MUDs (31/107; 30%, P < .01) despite similar infection rates in ‘at risk’ patients (28/33 vs 31/42, P = .27, excluding those surviving < 40 days without reactivation). Of 31 MUDs that experienced at least one episode of CMV infection, none developed graft failure, as opposed to 3 of 28 (11%) MMUDs (P = .10). One of 3 patients experiencing early graft failure developed CMV infection. She received 10 days of foscarnet with viral clearance. Of the patients experiencing late graft failure, 2 of 4 had treatment for CMV infection. Neither received ganciclovir. In 1 patient, the fall in counts began before treatment (18 days of foscarnet, single episode). The other patient experienced 2 episodes of infection treated with 21 and 7 days of foscarnet, respectively.
GVHD incidence
The incidence of acute GVHD (aGVHD) was similar between MUDs and MMUDs for all grades (45% vs 36%, P = .31), and grade 2-4 aGVHD (20% vs 22%, P = .83). Before DLI, only 5 cases of grade 3 (3 MUD and 2 MMUD) and no cases of grade 4 aGVHD occurred. Excluding cases that occurred post-DLI, there was a trend for an increased incidence of chronic GVHD (cGVHD) in MMUDs with a 1-year CI of cGVHD of 26% for MUDs (95% confidence interval 18%-38%) versus 39% for MMUDs (28%-58%, P = .12), as shown in Figure 1A. The CI of chronic extensive GVHD (ceGVHD) was relatively low with no significant difference between MUDs and MMUDs (1-year CI of ceGVHD 8% for MUDs [4%-16%] vs 15% for MMUDs [7%-31%], P = .29; Figure 1B).
CI of cGVHD stratified according to 10/10 HLA match (MUD) or 6 to 9/10 HLA match (MMUD). (A) cGVHD with follow-up censored at the time of DLI. (B) ceGVHD with follow-up censored at the time of DLI. (C) ceGVHD including cases occurring post-DLI.
CI of cGVHD stratified according to 10/10 HLA match (MUD) or 6 to 9/10 HLA match (MMUD). (A) cGVHD with follow-up censored at the time of DLI. (B) ceGVHD with follow-up censored at the time of DLI. (C) ceGVHD including cases occurring post-DLI.
DLI administration
DLIs were administered in 44 cases, 29 for disease relapse and 15 for mixed chimerism alone, with a median dose of 3 × 106/kg. There was no significant difference in the frequency of patients receiving DLI between MUD (n = 31; 29%) and MMUD (n = 13; 26%, P = .85). Of those who received DLI for progressive disease, 10 (34%) re-entered complete remission (7 of 19 MUDs [37%] and 3 of 10 MMUDs [30%]; P = 1.0), which was sustained in the long term (median follow-up, 4.3 years).
Post-DLI GVHD occurred in 19 cases with 6 cases of grade 3/4 aGVHD (4 MUDs and 2 MMUDs). CI curves for ceGVHD, including cases that occurred post-DLI are shown in Figure 1C with no evidence of a difference between MUD and MMUD groups. At 3 years, the CI of ceGVHD was 23% for MUDs (15%-36%) and 24% for MMUDs (13%-42%; P = .56). It should be noted that when post-DLI GVHD is included in the analysis, the aforementioned trend for an increased incidence of cGVHD in MMUDs versus MUDs is lost by 2-year follow-up.
TRM and OS analysis
With a median follow-up of 2.3 years, there was no significant difference in the CI of TRM between MUDs and MMUDs either at 100 days (17% [11%-26%] vs 16% [8%-30%]; Figure 2A) or at 1 year (27% [20%-37%] vs 27% [17%-43%], P = .96). OS at 3 years was 52% (43%-60%) for the whole cohort with no significant difference between MUDs (53% [42%-64%]) and MMUDs (49% [33%-64%], P = .44; Figure 2B).
Survival of patients stratified according to 10/10 HLA match (MUD) or 6 to 9/10 HLA match (MMUD). (A) CI curves of TRM. (B) Kaplan-Meier curves for OS.
Survival of patients stratified according to 10/10 HLA match (MUD) or 6 to 9/10 HLA match (MMUD). (A) CI curves of TRM. (B) Kaplan-Meier curves for OS.
Outcomes according to mismatch at the antigenic level
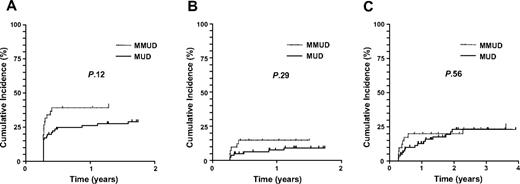
Forty of the mismatched donors differed from the recipient at the antigenic level at one or more HLA locus (Table 2). Comparison with cases either fully matched or mismatched at only the allelic level (n = 117) showed no significant differences in incidence of aGVHD (grade 2-4 20% vs 23% in matched vs mismatched cases, P = .82), chronic GVHD (Figure 3A) or TRM (Figure 3B). Graft rejection was not restricted to those with mismatch at the antigenic level. It occurred in 2 cases mismatched at 1 antigen, and in 2 cases mismatched at 1 allele. Furthermore, OS was not significantly different (52% [42%-63%] vs 49% [31%-66%] at 3 years, respectively, P = .49; Figure 3C).
Outcome according to presence of antigenic mismatch. (A) CI of ceGVHD including cases occurring post-DLI. (B) CI of TRM (28% [21%-38%] vs 23% [13%-41%] at 1 year). (C) Kaplan-Meier curves of OS.
Outcome according to presence of antigenic mismatch. (A) CI of ceGVHD including cases occurring post-DLI. (B) CI of TRM (28% [21%-38%] vs 23% [13%-41%] at 1 year). (C) Kaplan-Meier curves of OS.
One versus 2 or more HLA mismatches
An exploratory analysis of the outcomes of 1 locus (n = 32) versus 2 or more loci (n = 18) HLA mismatch was also performed. There was no significant difference in the occurrence of grade 2-4 aGVHD for 1 locus versus 2 or more loci MMUD (22% vs 22%). Furthermore, there was no difference between 1 locus and 2 or more loci mismatch groups for ceGVHD (4-year CI 29% [15%-55%] for 1 locus MMUD vs 23% [9%-63%] for ≥ 2 loci MMUD, P = .64; supplemental Figure 1A, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). TRM and OS were also comparable for 1 locus and 2 or more loci MMUDs (supplemental Figure 1B and 1C, respectively). For example, the 4-year OS was 43% (23%-62%) for 1 locus and 42% (16%-68%) for 2 or more loci MMUD (P = .88).
Discussion
Despite considerable expansion in donor registries, patients in whom an RIT is indicated but for whom a suitable HLA-matched donor is lacking are frequently identified. Possible management options in these cases include experimental nontransplantation strategies, umbilical cord blood transplantation, haploidentical-related donor transplantation, or the use of mismatched UDs.17 It is essential to clearly define outcomes associated with the use of HLA-mismatched UDs to define the role of MMUD transplantation. The degree of HLA mismatch might impact on both engraftment rates and GVHD incidence, both of which could influence survival outcomes.9-12,14,20 Alemtuzumab has been shown to be very effective at reducing GVHD and, when given in vivo as part of the conditioning regimen, at enhancing engraftment.15,21 We therefore reasoned that an alemtuzumab-containing regimen might overcome any adverse impact of HLA mismatch in the UD setting. To address this question, we retrospectively examined the outcomes of 157 consecutive UD transplant recipients after a uniform RIT regimen including 100 mg alemtuzumab that were performed at our center over the last 10 years. The procedural toxicity and long-term outcome of patients stratified according to the presence of an HLA mismatch were strikingly similar with no evidence of a clinically meaningful increased incidence of aGVHD or cGVHD in the MMUD versus MUD group.
These data differ from large registry studies of myeloablative transplantation regimens, which have shown an adverse impact of a high-resolution HLA mismatch. For example, the Japan Marrow Donor Program demonstrated that single disparities of the HLA-A, -B, -C, and -DRB1 alleles corresponded with increased incidence of aGVHD. Only HLA-A and HLA-B mismatches were associated with cGVHD and reduced OS.10,22,23 Another study suggested that the reduced survival associated with a single HLA mismatch was specific to patients with chronic phase–CML.24 Analysis of National Marrow Donor Program data demonstrated that a single mismatch at HLA-A, -B, -C, or -DRB1 (7/8 match) was associated with approximately 9% increase in mortality.11 Most studies have also demonstrated that mismatching at 2 or more loci compounds the risk.10,11,24
It should be noted, however, that most of the patients in these large registry studies received myeloablative conditioning and a T-cell replete graft. Several factors may influence the impact of HLA mismatch on outcome in the RIT versus myeloablative setting. For example, increasing age, the presence of residual host antigen-presenting cells and the inevitable period of recipient/donor chimerism post-RIT may all influence rates of GVHD. Consequently, the results of registry data in the myeloablative setting should not be extrapolated to RIT. As RIT has only become more widely practiced over the last 5 to 10 years, there is less data with regards to the impact of HLA mismatch in this setting. A study from the Dana-Farber Cancer Institute observed an increased mortality associated with an HLA-C mismatch in patients using a T-cell replete, busulphan- and fludarabine-conditioned regimen.12 The 2-year TRM in this study was 48% in HLA-C mismatched cases versus 16% in those without an HLA-C mismatch. HLA-C mismatch was also associated with an increased rate of grade 3 to 4 aGVHD. These findings were supported by another study using a total body irradiation and fludarabine-based approach in which the presence of a class I HLA mismatch was associated with a high rate of grade 2 to 4 aGVHD and a 2-year TRM of 47%.13 Although a Japanese study failed to demonstrate an adverse impact of a single HLA-mismatch on survival, rates of aGVHD and graft failure were increased and multiple HLA mismatches were associated with a markedly adverse outcome.14
The excess mortality associated with an HLA mismatch in the previous studies12-14 was attributable, at least in part, to an increased rate of GVHD. The incorporation of in vivo alemtuzumab in the conditioning regimen has been shown to be highly effective in reducing the incidence of severe aGVHD and ceGVHD.15,21 In the present cohort, only 5 cases of grade 3 aGVHD (3%) and no cases of grade 4 aGVHD occurred before DLI. Furthermore, there was no evidence of an adverse impact of the presence of an HLA mismatch on TRM, GVHD, or OS. It may be, therefore, that the adverse impact of HLA mismatch was overcome through partial T-cell depletion. Although one study of alemtuzumab-based RIT for myelodysplasia demonstrated an adverse impact of class II HLA mismatch, heterogeneity in the risk groups was a major confounding factor.25 A recent study incorporating antithymocyte globulin also demonstrated that T-cell depletion may overcome the adverse impact of HLA disparity, although the majority of patients in this study received a myeloablative transplantation.26
It is possible that this benefit of alemtuzumab in reducing GVHD is balanced by a reduction in the degree of disease control. However, the majority of patients in our cohort who relapsed without GVHD received a DLI. It is important to note, therefore, that there was no evidence of an increase in aGVHD or cGVHD between MUDs and MMUDs either before or after the inclusion of GVHD occurring after DLI. Furthermore, the rate of DLI was not different between MUDs and MMUDs, excluding this as a confounding factor.
The increased rates of late graft failure and graft rejection in the presence of an HLA mismatch are of potential interest. The presence of a serologically detectable class I HLA antigen mismatch has previously been shown to be associated with an increase in the rate of graft failure in the T-cell replete myeloablative setting.9,10 The incidences of graft failure or rejection in the MUD cohort were low (3% and 0%, respectively). Although late graft failure and rejection were both more common in the MMUD cohort, the incidences were still relatively modest (8% and 8%, respectively). Furthermore, 4/7 patients with graft failure were rescued by an additional infusion of CD34-selected stem cells without further conditioning. Drugs used for the treatment of CMV infection, most notably ganciclovir, could potentially contribute to graft failure, particularly in the T cell–depleted setting where CMV infection rates are particularly high. An imbalance in infection rates between MUDs and MMUDs could therefore influence the apparent excess of graft failure in the MMUD cohort. However, the incidence of graft failure in those receiving antiviral therapy for CMV (3/59, 5%) was not significantly different to that in those without CMV infection (4/98, 4%; P = 1.00). Furthermore, graft failure after treatment for CMV was only documented in 2 cases, and these patients only received a total of 2 to 4 weeks of foscarnet. Thus antiviral usage per se does not appear responsible for the difference seen in late graft failures.
There are several important limitations of this study, most notably, the sample size is relatively small in comparison to the large registry studies described here and, consequently, the study is insufficiently powered to confidently exclude a small impact of HLA mismatch on OS. This is particularly relevant for the exploratory analysis of 1- versus 2-loci HLA-mismatched transplantation outcomes, where larger numbers of mismatched patients will be required to confirm the apparent lack of adverse impact of mismatch at 2 loci. The results are important, however, in that they support further exploration of the use of such donors in this setting. The small number of different types of mismatch also precludes a meaningful analysis of the impact of mismatch at individual loci. Nevertheless, as patients included in the study represent a cohort treated consecutively in a single transplant program using identical conditioning, these data are of significant practical clinical relevance. The strikingly similar OS and TRM of the MUDs and MMUDs exclude a major impact of HLA mismatch on survival using this FMC approach.
We conclude that 8 to 9/10 MMUD RIT is a viable option using T-cell depletion with 100 mg alemtuzumab in vivo, without a significant adverse impact on TRM or OS compared with 10/10 MUD. Relatively few cases were mismatched at more than 1 locus at the antigenic level, and extrapolation of our results to this degree of mismatch is currently premature. The long-term OS of 49% after MMUD RIT is encouraging given the inclusion mainly of patients with multiply relapsed/refractory hematologic malignancy. Given the adverse outcome associated with MMUD allogeneic transplantation using non-T cell–depleted regimens, an FMC-based RIT is worthy of consideration when a fully HLA-matched donor is not available.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by funding from Leukaemia and Lymphoma Research (London; A.J.M., E.C.M., R.C., and K.S.P.). The study was undertaken at University College London Hospitals/University College London, which received a proportion of funding from the Department of Health, National Institute for Health Research Biomedical Research Centres Funding Scheme, and the Royal Free Hospital, London. The funding sources had no involvement in study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
Authorship
Contribution: A.J.M. and K.S.P. designed the study, collected and analyzed data, and wrote the paper. Further data collection was performed by K.J.T., S. Mohamedbhai, S.D., and G.O. K.J.T., E.C.M., A.K.F., P.D.K., R.H., R.C., D.C.L., and S. Mackinnon contributed to patient care; and K.J.T., R.C., and S. Mackinnon also contributed to writing the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Karl S. Peggs, Department of Haematology, UCL Cancer Institute, Paul O'Gorman Bldg, 72 Huntley St, London WC1E 6BT, United Kingdom; e-mail: k.peggs@cancer.ucl.ac.uk.


![Figure 3. Outcome according to presence of antigenic mismatch. (A) CI of ceGVHD including cases occurring post-DLI. (B) CI of TRM (28% [21%-38%] vs 23% [13%-41%] at 1 year). (C) Kaplan-Meier curves of OS.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/115/25/10.1182_blood-2010-01-265413/4/m_zh89991054070003.jpeg?Expires=1769080061&Signature=moKkcPX-2C6~iBLE5BVIY4MlR9a3d08LpgTjuKZ2C1ASFRV2pPduyUcAv0G85SAldIk6reatt2b3dkjueADRDFg8knqr~U8ST4q0wkdu-RuF7T3zCNeH2h~xBNZtj-Rt-oc0b6grExcdfSXSdAciRwCLwK6XUSLU~5H43UYd-Y52HecFOy2ioMwOULrmW1otEwxw-o9dCGmIwtWGj~VBNlqkIxEtT5W0Y8nCSox-J-mldNuKLbL1iuDJpPeD7MGeeFXYY~kyhaqSwdDXhAF9FH~VFcaocKtKeLq21M7Hd6UfLSUmqKbGgYFvJf-isIwoKQ3WojbFwoQafzn-Kib6Ww__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal