Abstract
Programmed cell death (apoptosis) of activated lymphocytes is critical to immune homeostasis. The cell surface protein Fas (CD95) and its ligand play a pivotal role in regulating lymphocyte apoptosis, and defective expression of either Fas or Fas ligand results in marked over accumulation of mature lymphocytes and autoimmune disease in mice. The results of recent studies suggest that defective lymphocyte apoptosis caused by mutations of the Fas gene can result in a severe autoimmune lymphoproliferative syndrome (ALPS) in humans. To define the clinical, genetic, and immunologic spectrum of ALPS, 9 patients and their families were extensively evaluated with routine clinical studies, lymphocyte phenotyping, genotyping, and in vitro assays for lymphocyte apoptosis. Individual patients were followed up for 3 months to 6 years. ALPS was identified in 9 unrelated children as manifested by moderate to massive splenomegaly and lymphadenopathy, hypergammaglobulinemia, autoimmunity, B-cell lymphocytosis, and the expansion of an unusual population of CD4−CD8− T cells that express the α/β T-cell receptor (TCR). All patients showed defective lymphocyte apoptosis in vitro. Heterozygous mutations of the Fas gene were detected in 8 patients. One ALPS patient lacked a Fas gene mutation. Healthy relatives with Fas mutations were identified in 7 of 8 ALPS kindreds. These relatives also showed in vitro abnormalities of Fas-mediated lymphocyte apoptosis, but clinical features of ALPS were not present in the vast majority of these individuals. ALPS is a unique clinical syndrome in which in vitro abnormalities of lymphocyte apoptosis are associated with abnormal lymphoproliferation and autoimmunity. These findings provide evidence that apoptosis of activated lymphocytes is an important mechanism for maintaining immunologic homeostasis and self-tolerance in humans. Fas gene mutations account for impaired lymphocyte apoptosis in only a subset of patients with ALPS.
MATURE LYMPHOCYTES UNDERGO a life cycle of activation and effector response followed by apoptosis or programmed cell death. Apoptosis maintains immune homeostasis by limiting lymphocyte accumulation and minimizing reactions against self-antigens.1,2 Antigenic restimulation induces proliferating T cells to express a surface receptor Fas (CD95/Apo1) and its ligand whose interaction triggers a dedicated molecular pathway leading to cell death.3,4 The in vivo importance of this pathway in maintaining lymphocyte homeostasis is shown by the lymphoproliferative and autoimmune disease that develops in lpr and gld strains of mice that are genetically deficient in Fas and Fas ligand expression, respectively.5,6 The relevance of the Fas apoptotic pathway to human disease was postulated in 1992, with our description of two children with massive, nonmalignant lymphoid hyperplasia and autoimmune disease.7 In 1995 Rieux-Laucat et al and ourselves documented that this novel disorder, termed autoimmune lymphoproliferative syndrome (ALPS), was associated with inherited heterozygous mutations in the Fas gene.8 9 We now present a comprehensive picture of the clinical, genetic, and immunologic spectrum of ALPS through an analysis of nine kindreds.
MATERIALS AND METHODS
Study Population
Between 1990 and 1995, 20 patients were referred to our service at the Warren Magnuson Clinical Center of the National Institutes of Health (NIH; Bethesda, MD) for evaluation of unexplained lymphadenopathy, splenomegaly, or both. Nine of these patients had features of ALPS, and their clinical and demographic features are summarized in Table 1. The other 11 patients lacked one or more key features of ALPS, which include elevated numbers of T-cell receptor (TCR) α/β CD4−CD8−T cells, in vitro apoptosis defects, and autoimmune disease. Participating patients and their families consented to evaluation and follow-up examination under approved clinical protocols. Individual patients were followed up for 3 months to 6 years. Patients and their family members were evaluated at the NIH Clinical Center with a complete history and physical examination, routine laboratory studies, peripheral blood lymphocyte (PBL) phenotyping, genotyping, and in vitro lymphocyte apoptosis assays.
Clinical and Epidemiologic Features of Nine Patients With ALPS
| Patient No. . | Age at Presentation . | Race/Sex . | Presenting Features . | Adenopathy* . | Splenomegaly/Hepatomegaly . | Neutropenia . | Autoimmune Disease . |
|---|---|---|---|---|---|---|---|
| 1 | 4 mo | W/M | Adenopathy | 3+ | +/+ | − | ITP |
| Hepatosplenomegaly | |||||||
| 2 | 18 mo | W/F | Adenopathy | 4+ | +/+ | − | Glomerulonephritis, positive ANA† |
| Hepatosplenomegaly | Hemolytic anemia‡ | ||||||
| 3 | 5 yr | W/M | Adenopathy | 2+ | +/− | + | Hemolytic anemia, ITP |
| Splenomegaly | |||||||
| 4 | 2 yr | W/M | Adenopathy | 3+ | +/+ | + | Urticarial rash |
| Hepatosplenomegaly | |||||||
| 5 | 2 mo | W/M | Splenomegaly | 4+ | +/− | + | Hemolytic anemia, ITP |
| 6 | 4 mo | B/F | Adenopathy | 4+ | +/+ | − | Hemolytic anemia |
| Splenomegaly | |||||||
| 14 | 9 mo | W/F | Splenomegaly | 4+ | +/+ | − | Hemolytic anemia |
| Hemolytic anemia | |||||||
| 17 | 4 mo | W/F | Adenopathy | 4+ | +/+ | + | Hemolytic anemia |
| Hepatosplenomegaly | |||||||
| 20 | 3 yr | W/F | Adenopathy | 2+ | +/+ | − | Guillain-Barrè syndrome |
| Hepatosplenomegaly |
| Patient No. . | Age at Presentation . | Race/Sex . | Presenting Features . | Adenopathy* . | Splenomegaly/Hepatomegaly . | Neutropenia . | Autoimmune Disease . |
|---|---|---|---|---|---|---|---|
| 1 | 4 mo | W/M | Adenopathy | 3+ | +/+ | − | ITP |
| Hepatosplenomegaly | |||||||
| 2 | 18 mo | W/F | Adenopathy | 4+ | +/+ | − | Glomerulonephritis, positive ANA† |
| Hepatosplenomegaly | Hemolytic anemia‡ | ||||||
| 3 | 5 yr | W/M | Adenopathy | 2+ | +/− | + | Hemolytic anemia, ITP |
| Splenomegaly | |||||||
| 4 | 2 yr | W/M | Adenopathy | 3+ | +/+ | + | Urticarial rash |
| Hepatosplenomegaly | |||||||
| 5 | 2 mo | W/M | Splenomegaly | 4+ | +/− | + | Hemolytic anemia, ITP |
| 6 | 4 mo | B/F | Adenopathy | 4+ | +/+ | − | Hemolytic anemia |
| Splenomegaly | |||||||
| 14 | 9 mo | W/F | Splenomegaly | 4+ | +/+ | − | Hemolytic anemia |
| Hemolytic anemia | |||||||
| 17 | 4 mo | W/F | Adenopathy | 4+ | +/+ | + | Hemolytic anemia |
| Hepatosplenomegaly | |||||||
| 20 | 3 yr | W/F | Adenopathy | 2+ | +/+ | − | Guillain-Barrè syndrome |
| Hepatosplenomegaly |
Abbreviations: W, white; B, black; ITP, idiopathic thrombocytopenic purpura.
Multiple chains of 1- to 2-cm nodes indicated by 2+, multiple chains of nodes visible without palpation by 3+, and massive adenopathy distorting normal anatomical landmarks by 4+.
Positive immunofluorescent assay for antinuclear antibody, 1:320 nucleolar pattern.
Direct antiglobulin (Coomb's) test was positive for both IgG and C3d in all patients with hemolytic anemia.
Immunologic Studies
Cell culture and induction of apoptosis.PBLs were isolated over Ficoll-Hypaque gradients (Pharmacia Biotech, Piscataway, NJ) and in some instances cryopreserved. PBLs were activated with 10 μg/mL of phytohemagglutinin (PHA) and then maintained in recombinant human interleukin-2 (IL-2) as previously described.8 For some studies, Epstein-Barr virus (EBV)-transformed B-cell lines were established using standard techniques. Apoptosis was induced by incubating cells with or without an apoptotic stimulus, followed by marking of nonviable cells with propidium iodide and quantitation by flow cytometry. Activated PBLs were exposed to antihuman CD3ε monoclonal antibody (MoAb; 64.1)-coated microtiter plates as previously described.8 The percentage of cell loss was calculated as follows: 1 − (Cell Number Recovered From Wells With anti-CD3ε/ (Cell Number Recovered From Wells Without anti-CD3ε) × 100. Apoptosis by the Fas pathway was detected by exposure of test cells to an agonist anti-Fas MoAb (CH11; Kamiya Biomedical Corp, Thousand Oaks, CA) as previously described.8 Quantitation of viable cells and calculation of percentage of cell loss were performed as above.
Flow cytometry.Anticoagulated PB specimens were stained for flow cytometry using a whole-blood lysis method and analyzed with a FACScan (Becton Dickinson, San Jose, CA) using Cell Quest software (Becton Dickinson). The directly conjugated MoAbs used included anti-CD3, anti-CD4, and anti-CD8 (Becton Dickinson); anti-TCR α/β ( pan TCR α/β) and γ/δ (pan TCR γ/δ; T Cell Diagnostics, Woburn, MA). Irrelevant MoAbs of the IgG1, IgG2a, and IgG2b subclasses (directly fluorochrome conjugated) were used to ascertain background staining. To calculate the absolute numbers of each lymphocyte subgroup, the percentage of cells staining positive was multiplied times the absolute count of PBLs obtained using a Coulter counter (Coulter, Hialeah, FL) on the same blood sample. Analysis of Fas surface expression was performed with antihuman Fas MoAbs UB2 or ZB4 (Kamiya Biomedical Corp) as described.8 Analysis of Fas ligand surface expression was performed using a Fas-Fc fusion protein and biotin-antihuman-IgG1 as described.10
Portions of lymph nodes and splenic tissue were submitted for routine histopathology, immunohistochemistry on both paraffin and frozen sections, and immunophenotypic analyses on cells in suspension by flow cytometry, as described.7
Detection of Fas Mutations
DNA and RNA samples were prepared from activated lymphocytes using standard methods. Reverse transcriptase-polymerase chain reaction (RT-PCR), cDNA analysis, and single-strand conformation polymorphism (SSCP) analysis were performed as previously described.8
RESULTS
Clinical Features
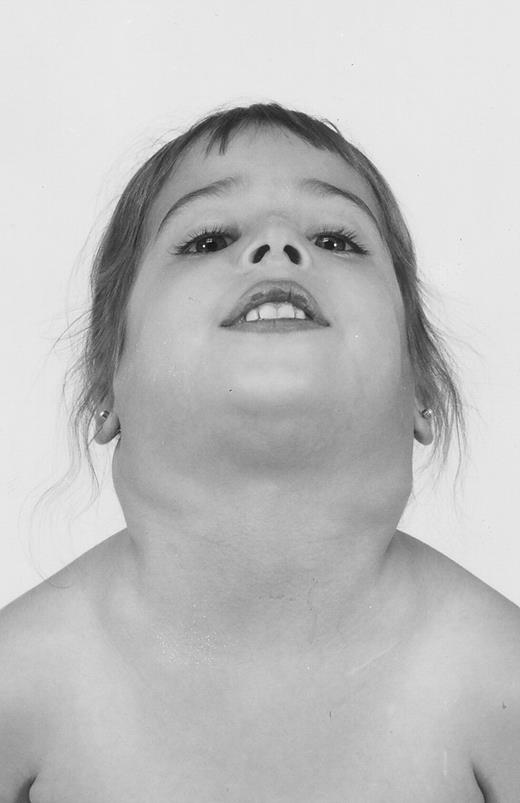
The 9 patients with ALPS presented at a median age of 9 months (range, 2 to 60 months) with persistent splenomegaly, peripheral lymphadenopathy, or both. Lymphadenopathy and splenomegaly were frequently of massive proportions, with distortion of normal anatomical landmarks (Table 1 and Fig 1). Lymphadenopathy persisted for 2 or more years in all patients and patient no. 17 has had documented lymphadenopathy for 23 years. The degree of lymphadenopathy fluctuated with time but remained moderate to extreme in all patients during the study period.
The above lymphoproliferative disease was associated with clinically significant autoimmune disease in 8 of 9 patients; patient no. 4 only showed a recurrent urticarial rash (Table 1). Glomerulonephritis associated with antinuclear antibodies was present in a single patient. Antinuclear antibodies were not detected in the other 7 patients. Five patients developed hemolytic anemia associated with IgG autoantibodies to red blood cells. Episodes of hemolysis in these patients were frequently severe, with 4 patients experiencing at least one episode in which hemoglobin levels decreased to less than 7 mg/dL. Three patients developed idiopathic thrombocytopenic purpura (ITP), with platelet counts of <10,000/μL on one or more occasions; 2 of these 3 patients also manifested hemolytic anemia (Table 1). Patient no. 20 developed a severe inflammatory polyneuropathy consistent with Guillian-Barrè syndrome at 6 years of age.
Neutropenia (absolute count <1,000 cells/mm3 ) developed in 4 patients. In patients no. 3, 5, and 17, neutropenia appeared to result from autoimmune mechanisms, as it developed after splenectomy and in the setting of normal myeloid cellularity on bone marrow (BM) aspiration. Neutrophil counts normalized in patients no. 3 and 5 after corticosteroid therapy. In patient no. 4, neutropenia was associated with normal myeloid cellularity on BM aspiration and the presence of an enlarged spleen.
Splenectomy was performed in all but patients no. 4 and 6. Indications for splenectomy were hypersplenism (2 patients), severe hemolytic anemia (2 patients), refractory thrombocytopenia (1 patient), and suspicion of lymphoma (1 patient). No patient developed opportunistic infection or other clinical evidence of immunodeficiency, and all patients tested negative for antibodies to the human immunodeficiency virus (HIV).
Based on studies in lpr mice with an ALPS-like phenotype in which these therapies were effective,11 12 patient no. 2 received sequential therapeutic trials of prednisone, interferon-α, IL-2, and cyclosporin A for treatment of her lymphoproliferative disease. Therapy with prednisone resulted in a transient decrease in the degree of lymphadenopathy, but the lymph nodes returned to their original size after tapering of the prednisone dose. None of the other treatments resulted in a change in the degree of her lymphadenopathy or her clinical status.
In view of the substantial toxicities and lack of efficacy of the various agents used to treat patient no. 2 and the fact that the lymphoproliferative disease was not threatening any vital organ function, no attempt was made to specifically treat the lymphoproliferative disease in the other 8 patients. However, in 5 patients, severe episodes of autoimmune hemolytic anemia, ITP, or both required systemic corticosteroid therapy. In these patients, the size of individual lymph nodes transiently decreased (but remained enlarged) during treatment with ≥1 mg/kg/d of prednisone, but, in all 5 patients, lymph nodes returned to their original size after tapering of the prednisone dose. In patients no. 1 and 3, multiple courses of high-dose corticosteroid and intravenous Ig therapy were required for control of the ITP.
Pathological Features
Histopathological analyses of 14 lymph nodes from 8 affected patients showed pathological changes similar to those described in our first 2 patients.7 There was architectural preservation with florid reactive follicular hyperplasia and marked paracortical expansion with immunoblasts and plasma cells (data not shown). The features resemble those of viral lymphadenitis except for the conspicuous absence of histiocytes normally observed to contain apoptotic debris. The paracortical expansion may be extensive enough to consider a differential diagnosis of immunoblastic lymphoma with many cells expressing the Ki-67 antigen indicative of active proliferation.13 Analyses of splenic tissue from 1 patient showed lymphoid hyperplasia of the white pulp with histologic features similar to those of the lymph nodes.
Flow-Cytometric and Immunologic Studies
The lymphocyte phenotyping data from the 9 patients with ALPS are shown in Table 2. All patients showed an increase on multiple determinations of both the percent and absolute number of CD3+T lymphocytes that expressed the α/β TCR, but did not express either CD4 or CD8 (double-negative T cells). The majority of double-negative T cells expressed HLA-DR, but not CD25. Increased numbers of CD3+T cells that were CD4−CD8− were also detected in the paracortical region of lymph node tissue and in splenic tissue (Table 2). Of the 9 patients, 8 had an increase on multiple determinations in the absolute number and percentage of B lymphocytes in the peripheral blood, as well as elevated serum levels of one or more Ig isotypes (Table 2).
Immunologic Features of Nine Patients With ALPS
| . | Patient No. . | Normal Range . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| . | 1 . | 2 . | 3 . | 4 . | 5 . | 6 . | 14 . | 17 . | 20 . | . |
| Lymphocyte phenotype (cells/μL [% of lymphocytes]) | ||||||||||
| PBL | 13,344 | 9,176 | 13,370 | 2,074 | 2,470 | 12,328 | 5,152 | 3,286 | 975 | 473-4,800 |
| T cells | ||||||||||
| CD3+ | 9,434 (71) | 6,830 (65) | 9,586 (72) | 1,429 (69) | 1,756 (71) | 8,297 (67) | 2,880 (56) | 2,160 (59) | 857 (88) | 832-2,028 (61-84) |
| CD4+ | 2,936 (22) | 1,471 (14) | 3,610 (27) | 519 (25) | 553 (22) | 3,464 (28) | 927 (18) | 961 (26) | 390 (40) | 480-1,339 (33-59) |
| CD8+ | 2,509 (19) | 1,923 (18) | 4,158 (31) | 697 (34) | 778 (31) | 3,292 (27) | 876 (17) | 745 (20) | 343 (35) | 351-911 (18-47) |
| CD3+DR+ | 5,885 (44) | 3,499 (33) | ND | 243 (12) | 447 (18) | 4,056 (33) | 1,159 (23) | 1,104 (30) | 239 (25) | <15* |
| CD3+CD4−8− TCR α/β+ | 3,746 (28) | 2,144 (20) | 2,005 (15) | 79 (4) | 452 (18) | 1,566 (13) | 1,031 (20) | 395 (11) | 146 (15) | <1%* |
| CD3+CD4−8− TCR γ/δ+ | 227 (2) | 725 (7) | 13 (0.1) | 114 (6) | 41 (7) | 999 (8) | 155 (3) | 29 (1) | 25 (3) | <10%* |
| NK cells | ||||||||||
| CD16+/56+ | 400 (3) | 494 (5) | 521 (4) | 97 (5) | 101 (4) | 2,120 (17) | 402 (8) | 303 (8) | 47 (5) | 120-490 (6.5-30) |
| B cells | ||||||||||
| CD19+ | 3,216 (24) | 2,911 (28) | 3,155 (23) | 539 (27) | 652 (26) | 1,701 (14) | 2,524 (49) | 1,155 (32) | 107 (11) | 94-378 (6-16) |
| Lymphocyte phenotype (percent of CD3+ cells) | ||||||||||
| Lymphoid tissue† | ||||||||||
| CD4− CD8− | ND | 50 | 23 | 25 | 50 | 46 | 62 | ND | ND | |
| Serum Igs (mg/dL) | ||||||||||
| IgM | 77 | 400 | 40 | 41 | 330 | 240 | 126 | 132 | 30 | 37-200 |
| IgG | 1,480 | 4,020 | 1,700 | 2,040 | 1,700 | 2,300 | 600 | 1,650 | 1,070 | 523-1,482 |
| IgA | 700 | 1,670 | 310 | 1,800 | <10 | 500 | 870 | 360 | 159 | 51-375 |
| . | Patient No. . | Normal Range . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| . | 1 . | 2 . | 3 . | 4 . | 5 . | 6 . | 14 . | 17 . | 20 . | . |
| Lymphocyte phenotype (cells/μL [% of lymphocytes]) | ||||||||||
| PBL | 13,344 | 9,176 | 13,370 | 2,074 | 2,470 | 12,328 | 5,152 | 3,286 | 975 | 473-4,800 |
| T cells | ||||||||||
| CD3+ | 9,434 (71) | 6,830 (65) | 9,586 (72) | 1,429 (69) | 1,756 (71) | 8,297 (67) | 2,880 (56) | 2,160 (59) | 857 (88) | 832-2,028 (61-84) |
| CD4+ | 2,936 (22) | 1,471 (14) | 3,610 (27) | 519 (25) | 553 (22) | 3,464 (28) | 927 (18) | 961 (26) | 390 (40) | 480-1,339 (33-59) |
| CD8+ | 2,509 (19) | 1,923 (18) | 4,158 (31) | 697 (34) | 778 (31) | 3,292 (27) | 876 (17) | 745 (20) | 343 (35) | 351-911 (18-47) |
| CD3+DR+ | 5,885 (44) | 3,499 (33) | ND | 243 (12) | 447 (18) | 4,056 (33) | 1,159 (23) | 1,104 (30) | 239 (25) | <15* |
| CD3+CD4−8− TCR α/β+ | 3,746 (28) | 2,144 (20) | 2,005 (15) | 79 (4) | 452 (18) | 1,566 (13) | 1,031 (20) | 395 (11) | 146 (15) | <1%* |
| CD3+CD4−8− TCR γ/δ+ | 227 (2) | 725 (7) | 13 (0.1) | 114 (6) | 41 (7) | 999 (8) | 155 (3) | 29 (1) | 25 (3) | <10%* |
| NK cells | ||||||||||
| CD16+/56+ | 400 (3) | 494 (5) | 521 (4) | 97 (5) | 101 (4) | 2,120 (17) | 402 (8) | 303 (8) | 47 (5) | 120-490 (6.5-30) |
| B cells | ||||||||||
| CD19+ | 3,216 (24) | 2,911 (28) | 3,155 (23) | 539 (27) | 652 (26) | 1,701 (14) | 2,524 (49) | 1,155 (32) | 107 (11) | 94-378 (6-16) |
| Lymphocyte phenotype (percent of CD3+ cells) | ||||||||||
| Lymphoid tissue† | ||||||||||
| CD4− CD8− | ND | 50 | 23 | 25 | 50 | 46 | 62 | ND | ND | |
| Serum Igs (mg/dL) | ||||||||||
| IgM | 77 | 400 | 40 | 41 | 330 | 240 | 126 | 132 | 30 | 37-200 |
| IgG | 1,480 | 4,020 | 1,700 | 2,040 | 1,700 | 2,300 | 600 | 1,650 | 1,070 | 523-1,482 |
| IgA | 700 | 1,670 | 310 | 1,800 | <10 | 500 | 870 | 360 | 159 | 51-375 |
Abbreviation: ND, not done.
Percentage of cells in the lymphocyte gate.
Percentage of T cells that are CD4− CD8− as determined by flow cytometry of peripheral lymph node (patients no. 1 through 4, 6 and 14) or spleen (patient no. 5).
FAS Gene Mutations
Because of clinical and immunologic similarities between ALPS and lpr/gld mice, we have sought functionally significant mutations in the Fas and Fas ligand genes in these patients and their families. As described previously, we have found Fas gene mutations in patients no. 1 through 5,8 and we now report 3 additional patients with distinct Fas gene mutations. Patient no. 6 has a C-to-A substitution at cDNA nucleotide 966, which produces a nonconservative substitution of aspartic acid for alanine. Patient no. 17 has a T-to-G substitution at cDNA nucleotide 1123 producing a substitution of serine for isoleucine-294 in the intracytoplasmic death domain. Patient no. 20 has a C-to-A substitution at cDNA nucleotide 413 producing a premature stop codon. Because stable mRNA bearing this mutation is expressed (data not shown), this patient is predicted to produce a soluble, secreted Fas protein of 57 amino acids truncated in the first cystein-rich domain. None of these mutations were detected on analysis of over 100 copies of the Fas gene from normal unrelated individuals.
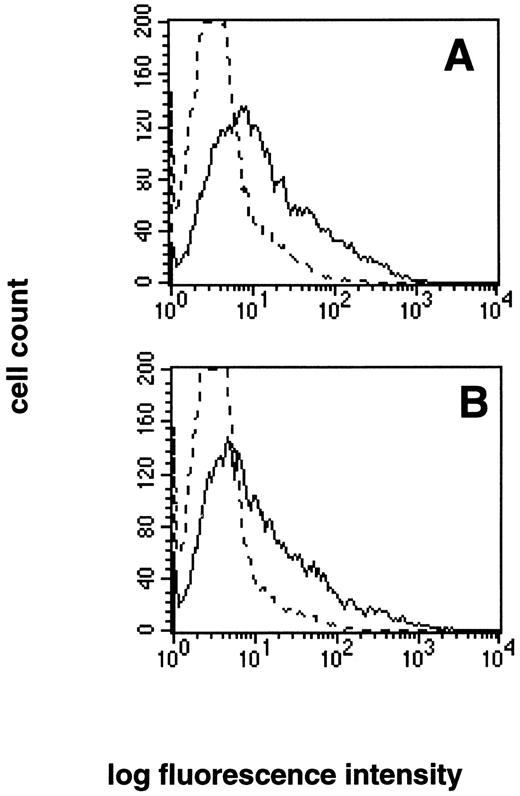
In contrast, no Fas or Fas ligand mutations were found in patient no. 14 despite complete nucleotide sequencing of the cDNA and genomic coding regions for both genes. Lymphocytes from patient no. 14 expressed normal levels of both Fas (data not shown) and Fas ligand (Fig 2).
Normal expression of Fas ligand by patient no. 14. PBLs from (A) normal control and (B) patient no. 14 were analyzed for surface expression of Fas ligand using a Fas-Fc fusion protein and biotin-antihuman IgG1. The dashed line represents staining with biotin-antihuman IgG1 alone.
Normal expression of Fas ligand by patient no. 14. PBLs from (A) normal control and (B) patient no. 14 were analyzed for surface expression of Fas ligand using a Fas-Fc fusion protein and biotin-antihuman IgG1. The dashed line represents staining with biotin-antihuman IgG1 alone.
Impaired Apoptosis
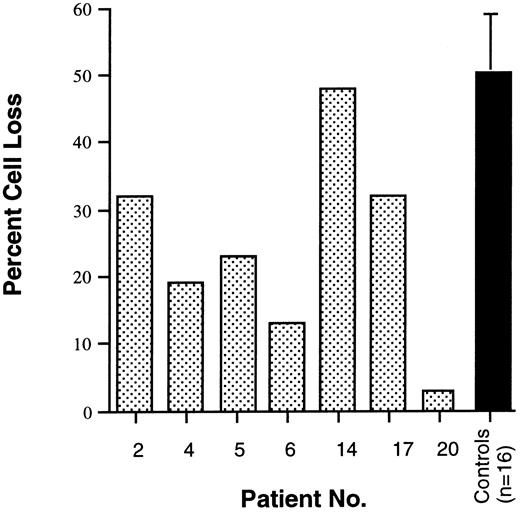
As described above, B-cell lymphocytosis, hypergammaglobulinemia, and autoantibody production are prominent features of ALPS. These abnormalities of humoral immunity led us to suspect that B cells from ALPS patients may be defective in their ability to undergo Fas-mediated apoptosis. To investigate this possibility, EBV-transformed cell lines were established from 7 of the 9 patients and analyzed for apoptosis following exposure to anti-Fas monoclonal antibody. Patients with Fas gene mutations showed defective apoptosis of EBV-transformed B-cell lines in this assay (Fig 3). However, patient no. 14, who has no Fas mutation, showed normal Fas-mediated killing of EBV-transformed B cells in vitro (Fig 3).
Fas-mediated killing of EBV-transformed B-cell lines derived from 7 ALPS patients and 16 normal controls. The percentage of cell loss was calculated from cultures of EBV-transformed B-cell lines exposed to the anti-Fas MoAb CH11. Patient no. 14 lacked a Fas mutation. Mean and standard deviation are shown for controls.
Fas-mediated killing of EBV-transformed B-cell lines derived from 7 ALPS patients and 16 normal controls. The percentage of cell loss was calculated from cultures of EBV-transformed B-cell lines exposed to the anti-Fas MoAb CH11. Patient no. 14 lacked a Fas mutation. Mean and standard deviation are shown for controls.
As reported previously, phenotypically normal T cells from patients 1 through 5 showed defective apoptosis in both a TCR restimulation assay and after cross-linking Fas with the anti-Fas MoAb CH11.8 Patients no. 6, 17, and 20 also showed defective apoptosis in both assays (Table 3). Patient no. 14, who lacked a Fas mutation, showed defective apoptosis after anti-CD3 treatment but normal or near normal apoptosis after exposure to anti-Fas (Table 3). These abnormalities in apoptosis were consistently observed for all patients on multiple determinations. Although the exact percentage of cell death varied from assay to assay, the percentage of cell loss was always significantly lower in ALPS cases when compared with concomitantly run controls. The magnitude and pattern of B- and T-cell apoptosis defects observed in vitro did not correlate with the severity of disease expression.
Summary of Lymphocyte Apoptosis Defects in Nine Patients With ALPS
| Patient No. . | Apoptosis Defect (% cell loss)3-150 . | |
|---|---|---|
| . | Anti-CD3 . | Anti-Fas . |
| 1 | 13 | 12 |
| 2 | 26 | 35 |
| 3 | 14 | 8 |
| 4 | 37 | 17 |
| 5 | 33 | 25 |
| 6 | 8 | 21 |
| 17 | 9 | 18 |
| 20 | ND | 21 |
| 143-151 | 27 | 60 |
| Normal controls (n = 3) | 51 ± 1 | 87 ± 0.5 |
| Patient No. . | Apoptosis Defect (% cell loss)3-150 . | |
|---|---|---|
| . | Anti-CD3 . | Anti-Fas . |
| 1 | 13 | 12 |
| 2 | 26 | 35 |
| 3 | 14 | 8 |
| 4 | 37 | 17 |
| 5 | 33 | 25 |
| 6 | 8 | 21 |
| 17 | 9 | 18 |
| 20 | ND | 21 |
| 143-151 | 27 | 60 |
| Normal controls (n = 3) | 51 ± 1 | 87 ± 0.5 |
Abbreviation: ND, not done.
Percentage of lymphocytes undergoing apoptosis after exposure to anti-CD3 (64.1) or anti-Fas (CH11) MoAb.
All patients have Fas mutations, except patient no. 14.
Family Studies
Detailed analyses were conducted in 65 members of 7 of the 8 kindreds with Fas mutations. The members of family 6 have yet to be fully analyzed. Mutations in the Fas gene were detected in 15 additional family members of these 7 patients. In all cases, nucleotide sequence analysis proved the mutations in the family members to be identical to those of the index cases (Fisher et al8 and data not shown), indicating that the Fas mutations were genetically transmitted through the germline and do not represent new mutations.
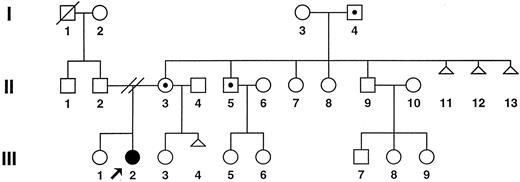
Figure 4 shows the results of genetic and immunologic studies of 1 well-studied family. The mother, maternal grandfather, and maternal uncle of patient no. 2 all carry the same Fas mutation and have no history of lymphadenopathy, autoimmune disease, or other clinical features of ALPS. Analysis of the relative's PBLs by flow cytometry showed normal lymphocyte phenotypes, with no B-cell lymphocytosis or increase in TCR α/β+ double-negative T cells (Table 4). However, when stimulated PBLs from the patient and relatives were treated with anti-Fas antibody, it was evident that relatives with the Fas gene mutation showed defective apoptosis comparable in magnitude with that of the patient. In contrast, the relatives who did not carry the Fas mutation showed normal Fas-mediated apoptosis in this assay (Table 4).
Pedigree of family no. 2: ALPS patient no. 2, (•); unaffected individuals are represented by open symbols; open symbols with dots indicate clinically normal carriers of the Fas mutation; and open triangles indicate spontaneous abortion.
Pedigree of family no. 2: ALPS patient no. 2, (•); unaffected individuals are represented by open symbols; open symbols with dots indicate clinically normal carriers of the Fas mutation; and open triangles indicate spontaneous abortion.
Immunologic Features of Kindred No. 2
| . | Fas Mutation . | % CD4−CD8− . | CD19+ cells/ . | Fas-Mediated . |
|---|---|---|---|---|
| . | . | TCRα/β+ . | mm3 (%) . | Apoptosis . |
| . | . | . | . | % Cell Loss4-150 . |
| Patient no. 2 | + | 25 | 3485 (41) | 35 |
| Mother | + | 0.6 | 135 (6) | 25 |
| Maternal grandfather | + | 0.9 | 148 (7) | 17 |
| Maternal uncle no. 1 | + | 0.7 | 80 (4) | 37 |
| Father | − | 0.2 | 234 (10) | 90 |
| Sister | − | 0.8 | 51 (3) | ND |
| Maternal uncle no. 2 | − | 0.7 | 80 (4) | 62 |
| Maternal grandmother | − | 0.1 | 89 (6) | 63 |
| . | Fas Mutation . | % CD4−CD8− . | CD19+ cells/ . | Fas-Mediated . |
|---|---|---|---|---|
| . | . | TCRα/β+ . | mm3 (%) . | Apoptosis . |
| . | . | . | . | % Cell Loss4-150 . |
| Patient no. 2 | + | 25 | 3485 (41) | 35 |
| Mother | + | 0.6 | 135 (6) | 25 |
| Maternal grandfather | + | 0.9 | 148 (7) | 17 |
| Maternal uncle no. 1 | + | 0.7 | 80 (4) | 37 |
| Father | − | 0.2 | 234 (10) | 90 |
| Sister | − | 0.8 | 51 (3) | ND |
| Maternal uncle no. 2 | − | 0.7 | 80 (4) | 62 |
| Maternal grandmother | − | 0.1 | 89 (6) | 63 |
Abbreviation: ND, not done.
Percentage of lymphocytes undergoing apoptosis after exposure to the anti-Fas MoAb CH11.
Healthy members of the other 6 kindreds in which Fas mutations could be documented were also found to show in vitro abnormalities of Fas-mediated lymphocyte apoptosis (data not shown). Clinical features of ALPS were not present in the vast majority of these individuals; however, the father and paternal uncle of patient no. 3 both developed lymphoma (T-cell–rich B-cell lymphoma and nodular lymphocyte predominance Hodgkin's disease, respectively). The father died of his lymphoma, and no tissue samples are available for analysis; however, the paternal uncle is living and is heterozygous for the same Fas mutation as patient no. 3.8 The brother of patient no. 3 is also heterozygous for this Fas mutation and has had three transient episodes of peripheral lymphadenopathy not associated with splenomegaly. A biopsy specimen of one of these enlarged lymph nodes showed follicular hyperplasia but did not show paracortical expansion or increased numbers of double-negative T cells. The full details of this kindred are the subject of a separate manuscript (A. Lin et al, manuscript submitted). Outside of family no. 3, the only other relative who showed any features of ALPS was the maternal grandfather of patient no. 5. This individual also carries a Fas mutation and was found to have 2.8% TCR α/β+ double-negative T cells in his PB. He has normal B-cell numbers and no other laboratory or clinical features of ALPS.
DISCUSSION
In this report, we describe the clinical, immunologic, and genetic features of 9 patients with a novel disorder we have termed ALPS. The defining features of this syndrome are chronic lymphadenopathy and splenomegaly, autoimmune disease including autoantibody-mediated hemolytic anemia and thrombocytopenia, expansion of an unusual population of double-negative T cells that express the α/β TCR, and defective lymphocyte apoptosis.
In all our patients, lymphadenopathy and splenomegaly developed early in childhood and were often massive. Several diagnoses, including malignant lymphoma, were entertained in these patients before the recognition of ALPS. However, the unique clinical and immunological features of ALPS allowed it to be distinguished from malignant lymphoma or other autoimmune diseases, thus avoiding unnecessary immunosuppressive or cytotoxic chemotherapy.
The clinical and immunologic features of ALPS bear a striking resemblance to a lymphoproliferative disease observed in strains of mice that carry either the lpr or gld mutations. Mice homozygous for either of these mutations develop hypergammaglobulinemia, autoantibody production, glomerulonephritis, massive lymphoid hyperplasia, and expansion of TCR α/β+ double-negative T cells.14 Genetic studies have shown that the lpr phenotype results from a recessive mutation in the Fas gene,5 whereas the gld phenotype is caused by a mutation in the gene encoding Fas ligand.6 These mutations render mature T cells resistant to activation-induced apoptosis15 that, in turn, results in the progressive accumulation of phenotypically normal and TCR α/β+ double-negative T cells in peripheral lymphoid tissue. Similar to lpr and gld mice, patients with ALPS show defective T-cell apoptosis and abnormal accumulation of TCR α/β+ double-negative T cells in the blood and peripheral lymphoid tissue. TCR α/β+ double-negative T cells are present in normal humans, where they constitute a minor (<1%) subpopulation of thymus and PB cells.16,17 In Fas-deficient lpr mice, the TCR α/β+ double-negative T cells that accumulate appear to be derived from chronically activated CD8+T cells that fail to undergo apoptosis and downregulate CD8 expression.18 A similar mechanism may account for the expansion of TCR α/β+ double-negative T cells observed in ALPS.
Another important similarity between ALPS and lpr/gld mice is the occurrence of antibody-mediated autoimmune disease. Recent studies indicate that in addition to regulating T-cell responses, Fas-mediated apoptosis may also be an important mechanism for regulating humoral immune responses and eliminating autoreactive B cells. In vitro, activated B cells express Fas and become sensitive to killing by Fas ligand-expressing T cells.19,20 Furthermore, in vivo studies in mice expressing a transgene-encoded autoantigen indicate that autoreactive B cells can be eliminated as a result of cognate T-cell–B-cell interactions that engage the Fas/Fas ligand system.21 Hypergammaglobulinemia, B-cell lymphocytosis, and pathological autoantibody production are prominent features of ALPS that point to a severe dysregulation of humoral immunity. Our demonstration in this report that EBV-transformed B-cell lines derived from patients with ALPS show defective Fas-mediated apoptosis provides the first direct evidence that the Fas/Fas ligand system may be important in eliminating autoreactive B cells and limiting the size of humoral immune responses in humans.
The clinical and immunopathological similarities between our patients and lpr/gld mice led us to investigate whether functionally significant mutations of the Fas gene were present in all patients with ALPS. Of the 9 patients, 8 were each found to possess unique heterozygous Fas gene mutations. Direct analysis of the mutant alleles from patients no. 2 through 5 by cell transfection studies showed they were unable to transmit an apoptosis signal and had dominant interfering effects on apoptosis mediated by wild-type Fas.8 The mutations in patients no. 6, 17, and 20 were not tested in the transfection system. However, none of these Fas mutations were detected in over 100 copies of chromosome 10 obtained from healthy unrelated individuals (Fisher et al8 and unpublished data). This fact, combined with the observed defects in Fas-triggered apoptosis, strongly argues that the mutations detected in these ALPS patients are functionally abnormal and do not represent normal polymorphisms of the Fas gene.
One ALPS patient lacked a Fas mutation yet still showed defective T-cell apoptosis after TCR restimulation, but not after exposure to anti-Fas antibody (Table 3). These findings are similar to those observed in mice with the gld mutation; however, patient no. 14 did not have a mutation in the gene encoding Fas ligand and expressed normal levels of the protein (Fig 2). This patient may have a defect in one or more proteins involved in triggering Fas-independent apoptosis.
In 7 patients with Fas mutations, family members who carried the same mutation were identified. Family members with these mutations frequently showed abnormal lymphocyte apoptosis in vitro; however, they show few if any clinical or immunophenotypical features of ALPS. Thus, the presence of a Fas mutation alone is not sufficient for the development of ALPS. It is likely that environmental factors or the presence of other immunoregulatory abnormalities are required for full expression of the ALPS phenotype. One hypothesis is that the gene or genes responsible for converting an asymptomatic Fas mutation into ALPS are inherited from the parent who does not carry the Fas mutation. Recent elucidation of molecules and regulatory pathways involved in lymphocyte apoptosis suggest several candidate genes worthy of study, including bcl-2, IL-1β converting enzyme (ICE), tumor necrosis factor receptor I, galectin-1, Fas-associated protein with a death domain (FADD), TNF receptor-associated protein with a death domain (TRADD), and receptor interacting protein (RIP).4 22-28 Alternatively, the healthy carriers of these Fas mutations may also carry protective genes or immunoregulatory mechanisms that counteract the deleterious effects of the Fas mutation.
Fas gene mutations are present in most currently recognized cases of ALPS (Fisher et al,8 Rieux-Laucat et al,9 and present study), and we propose considering such individuals as having ALPS type I. Individuals in whom ALPS is not associated with a Fas mutation (such as patient no. 14) should be considered to have ALPS type II. Eventually, the genetic abnormalities of patients in this latter category of ALPS may be shown, thus allowing for their more precise classification.
ACKNOWLEDGMENT
We gratefully acknowledge Drs A. Lin, D. Melnick, V. Bonagura, and J. Taylor for referring patients and relatives to the NIH for study; Galen Fisher for his contribution to the apoptosis studies of patient no. 6; and Margaret Brown for performing the flow cytometry studies on these patients.
Address reprint requests to Michael C. Sneller, MD, Bldg 10, Rm 11B-13, National Institutes of Health, Bethesda, MD 20892.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal