Abstract
A graft-versus-leukemia (GVL) effect has been considered a major factor responsible for cures in patients with hematologic malignancies undergoing allogeneic bone marrow transplantation; however, associated graft-versus-host disease (GVHD) results in significant morbidity and mortality. T-cell depletion reduces the incidence and severity of GVHD but eliminates, at least partially, the GVL effect. Reinfusion of donor T lymphocytes at relapse posttransplantation can induce a potent antitumor response, but GVHD still occurs in the majority of patients. Prior transduction of T lymphocytes with the suicide gene, the viral thymidine kinase (TK), permits specific cell kill on administration of ganciclovir (GCV). Therefore, infusion of TK-transduced T lymphocytes may induce GVL effect and allow for their subsequent selective elimination in case GVHD develops. To evaluate the efficacy and feasibility of this promising approach, anti-CD3–stimulated primary human lymphocytes cultured in interleukin-2 were TK-transduced by a retroviral vector carrying both TK and neomycin-resistance genes. After selection in G418, more than 90% of the cells contained the TK gene as shown by a semiquantitative polymerase chain reaction. In addition, 1 to 5 days of GCV exposure, at clinically achievable concentrations of 20 to 50 μmol/L, induced ≥90% killing of G418-selected cells without affecting nontransduced cells. Correlation of the extent of T-cell kill and the proportion of TK-gene–transduced cells is consistent with the absence of a bystander effect. Transduced cells were CD3+ and either CD8+ or CD4+ and retained functional properties of untransduced cells. In vivo administration of GCV prevented tumor development after subcutaneous injection of TK-transduced murine myeloma cells (MOPC-11), whereas such an effect was not observed on injection of untransduced cells into the opposite flank. Our studies provide critical information that (1) adequate numbers of TK-transduced lymphocytes can be selected efficiently with ≥90% purity, (2) selected cells remain functional, (3) 24 hours of exposure to GCV at clinically achievable concentration effects ≥90% killing of selected cells, and (4) GCV is effective in vivo in killing TK-transduced cells. Based on these data, a clinical study has been initiated in patients with multiple myeloma with persistent or relapsing disease after T-cell–depleted allogeneic transplants.
ALLOGENEIC BONE MARROW transplantation is a curative treatment for selected patients with hematologic malignancies1 including multiple myeloma.2-4 Its curative potential depends in large part on an antitumor effect derived from the adoptive transfer of immunocompetent cells present in the donor graft.2,3 However, such transfer of donor lymphocytes may also lead to acute graft-versus-host disease (GVHD). GVHD is associated with severe morbidity and up to 50% mortality in cases of grade-III and -IV GVHD occurring in 14% of sibling transplants and in 35% of unrelated donor transplants.5 The incidence and severity of GVHD have been markedly decreased by T-cell depletion.6-8 However, this benefit is achieved at the expense of engraftment and graft rejection problems,9,10 a higher relapse rate,6,11-13 and delayed immune reconstitution, resulting in an increased incidence of opportunistic infection9 and the development of Epstein-Barr virus lymphoproliferative disease.14-16 The therapeutic options for patients with persistent or relapsed disease after allotransplantation are very limited. Administration of additional chemotherapy or a second bone marrow transplant is associated with high morbidity and mortality rates17 and limited disease control. A novel approach to this problem has been the administration of donor leukocytes to induce a graft-versus-leukemia (GVL) effect,18,19 producing hematologic, cytogenetic, and molecular remissions in approximately 80% of patients with chronic myelogenous leukemia relapsing after allogeneic transplantation,18-21 thus providing direct evidence of a GVL effect. Although capable of controlling and potentially eradicating the disease, this approach also carries considerable hazards; acute GVHD, pronounced cytopenias, and therapy-related deaths were observed in 80%, 54%, and 22% of patients, respectively.21 Manipulation of infused donor T cells with a “suicide gene” may overcome these problems with leukocyte infusions. The viral Herpes simplex thymidine kinase gene (TK) is a suitable candidate for such an approach because it will selectively phosphorylate ganciclovir (GCV), leading to its incorporation into DNA causing cell death. When applied to donor lymphocytes, GCV permits specific elimination of TK-transduced cells in case GVHD develops, possibly without abrogating the GVL effect. This assumption is based on observation in murine leukemia, in which the GVL effect precedes the onset of GVHD.22 In this model, the antitumor effect is accomplished in mice within 6 to 7 days after administration of T cells, whereas signs of GVHD appear only after day 14, suggesting that GVHD and GVL, while possibly mediated by the same cells, may be separate in time. Our studies show the feasibility and efficacy of selecting adequate numbers of biologically functional TK-gene–transduced human lymphocytes that are killed efficiently in vitro and in vivo with clinically achievable concentrations of GCV.
MATERIAL AND METHODS
Description of the vector.G1Tk1SvNa.7 is a retroviral vector derived from the Moloney murine leukemia virus. This vector contains a Herpes simplex type-I thymidine kinase (HSV-Tk1) gene cDNA, transcribed from the viral long terminal repeat (LTR), and a bacterial Neomycin-resistance (NeoR) gene, transcribed from an internal simian virus 40 (SV40) early promoter (LTR — HSV-Tk1 — SV — NeoR — LTR; see Fig 1) in the G1 vector backbone (Genetic Therapy Inc, Gaithersburg, MD). When an HSV-Tk1–transduced cell is exposed to GCV, the GCV acts as a substrate for phosphorylation by HSV-Tk1 resulting in a monophosphate (MP) form of the drug. Cellular phosphorylases convert this GCV-MP to GCV-triphosphate that is subsequently incorporated into DNA and inhibits DNA polymerase and chain elongation, resulting in cell death with the next cell division.23 This vector has been used in humans for the treatment of central nervous system tumors.24,25 The clinical grade viral supernatant was produced and kindly provided by Genetic Therapy Inc.
Lymphocyte culture.Peripheral blood samples from donors were subjected to gradient separation using Ficoll (lymphoprep; Nycomed, Oslo, Norway), and the mononuclear cells were isolated. These cells were suspended in serum-free AIM-V medium (GIBCO-BRL, Grand Island, NY) with anti-CD3 antibody (Ortho-Biotech, Raritan, NJ) at 1 μg/mL for 24 hours, at which time recombinant human interleukin-2 (rhIL-2; Chiron, Emeryville, CA) was added at 1,500 U/mL. The cells were cultured at 37°C in 5% CO2 for a period of 6 days, and transduction was performed when the cells were growing exponentially.
Transduction and G418 selection.The peripheral blood mononuclear cells cultured as above were transduced with the TK and NeoR genes using the retroviral vector G1Tk1SvNa.7. In brief, 2.0 × 106 mononuclear cells were resuspended in 4.0 mL media (AIM-V) containing 5 μg/mL protamine sulfate and 1,500 U/mL rhIL-2 and were incubated with the viral supernatant (G1Tk1SvNa.7) at an multiplicity of infection of 10:1. Fresh viral supernatant was added daily for 3 days. Cells were then resuspended in fresh medium and 48 hours later, cultured in G418 at an active concentration of 300 μg/mL for 3 days, and selected for a total of 7 days. Cells were then tested for transduction efficiency. A lymphoid cell line, H9, was also similarly transduced and selected.
GCV killing assay.Transduced lymphocytes (2.5 × 106) were suspended in tissue-culture flasks containing 5.0 mL of AIM-V (GIBCO-BRL) media with 1,500 U/mL rhIL-2. A total of three flasks were prepared. Two of the flasks received GCV on day 1 (at concentrations of 20 μmol/L and 50 μmol/L, respectively); the third flask was used as a control. Cells were incubated at 37°C in 5% CO2 for 5 days, after which the percentage of live cells (trypan blue negative) was determined.
Allostimulation.Transduced (G-418 selected) and untransduced lymphocytes were washed and cultured in the absence of IL-2 for 12 hours in 96-well round-bottom plates at 0.5 × 105 cells/well. ARH-77 cells, a human myeloma cell line, were irradiated (4,000 cGy) and added to the culture. After 3 days, 0.5 μL 3H-thymidine was added, and, 16 hours later, the incorporation in DNA was measured in a β-counter.
NeoR polymerase chain reaction (PCR) analysis.The presence of NeoR gene in transduced lymphocytes was detected by PCR analysis. Genomic DNA from the T lymphocytes were prepared by standard proteinase K-sodium dodecyl sulfate treatment followed by phenol/chloroform extraction. Each 100-μL reaction consisted of 1 μg genomic DNA, 1.5 mmol/L Mg2Cl, 0.2 mmol/L deoxynucleotide triphosphates (dNTPs), 50 mmol/L KCl, 10 mmol/L Tris-HCl (pH, 8.3), 1 U AmpliTaq DNA polymerase (Perkin Elmer-Cetus, Emeryville, CA), and 50 pmol synthetic primers. The NeoR specific primer pair used was 5′ GGT GGA GAG GCT ATT CGG CTA TGA 3′ and 5′ ATC CTG ATC GAC AAG ACC GGC TTC 3′. The human Actin-specific primers 5′ CAT TGT GAT GGA CTC CGG AGA CGG 3′ and 5′ CAT CTC CTG CTC GAA GTC TAG AGC 3′ were used as the internal standard to compare the amount of PCR-amplifiable DNA in each reaction. DNA was amplified using 30 cycles of incubation at 94°C (for 30 seconds), 61°C (for 30 seconds), and 72°C (for 45 seconds), followed by a 10-minute extension period at 72°C. The amplified fragments were separated by electrophoresis on 2% agarose gel, stained by ethidium bromide, Southern-transferred on nitrocellulose membrane, and probed with P32-labeled NeoR-specifc probe. Each PCR reaction was calibrated by construction of a standard curve generated by simultaneous amplifications of serial dilutions of DNA from H9 cell line that contains a single copy of integrated G1Tk1SvNa.7 provirus.
Fluorescence-activated cell sorting (FACS) analysis.Lymphocytes were centrifuged, and 1 × 106 cells were stained with 10 μL of antibody and incubated on ice for 15 minutes. Cells were washed and resuspended in 0.5 mL phosphate-buffered saline and analyzed. Phenotypic analysis was performed with a FACS Scanner, using monoclonal antibodies to CD3, CD4, CD8, CD16, CD19, and CD56 (Becton Dickinson, San Jose, CA).
In vivo GCV activity.To test the efficacy of GCV-induced killing of TK-transduced cells in vivo, a murine myeloma cell line MOPC-11 was TK-transduced with G1Tk1SvNa.7 virus and G418-selected. Transduced MOPC-11 cells (1 × 106) were injected into one flank of Balb/c mice, and an identical number of untransduced cells was injected into the other flank. Mice received daily intraperitoneal injections of GCV at a dose of 20 mg/kg starting on the day of injection of transduced and untransduced cells. The appearance and size of the tumor were observed daily.
RESULTS
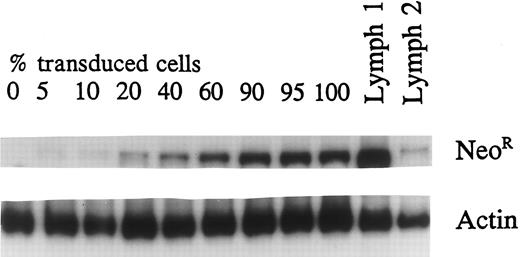
Efficacy of transduction.The optimal duration of selection in G418 after transduction of lymphocytes with NeoR and TK genes was determined by culturing transduced and untransduced cells in G418 for a variable number of days. Exposure of these cells to G418 from 1 to 5 days was equally effective in killing all nontransduced cells by day 7 of culture (data not presented). Cell samples before and after G418 selection and subsequent culture in IL-2 were subjected to semiquantitative PCR analysis using both NeoR and Actin-specific primers. TK transduction increased from a median of 37% (range, 15% to 64%) before selection to over 90% after G418 selection (Fig 2). In a typical transduction procedure, 107 peripheral blood mononuclear cells obtained from 10 mL peripheral blood, doubles on stimulation with anti-CD3 for 6 days. During the subsequent 3 days of transduction and an additional 2 to 3 days of culture in IL-2, the cell number doubles again to 4 × 107. After 3 days of G418 selection and further culture for a total of 7 days, the number of live cells remains unchanged because of the expansion of transduced cells and the death of untransduced cells. This effectively provides 4 × 107 cells, more than 90% of which contain the TK gene. Further culture in IL-2 may be undertaken to obtain the required number of cells for clinical use.
Quantititative PCR was performed using a transduced, selected lymphoid cell line. DNA from transduced cells was mixed in different proportions with untransduced-cell DNA to generate standard curve. Sample no. 1 is the TK-transduced and G418-selected primary lymphocyte population showing greater than 90% transduction. Sample no. 2 is the transduced unselected population with 16% transduced cells.
Quantititative PCR was performed using a transduced, selected lymphoid cell line. DNA from transduced cells was mixed in different proportions with untransduced-cell DNA to generate standard curve. Sample no. 1 is the TK-transduced and G418-selected primary lymphocyte population showing greater than 90% transduction. Sample no. 2 is the transduced unselected population with 16% transduced cells.
FACS analysis of the selected TK-transduced lymphocytes showed CD3 expression in 95% to 99% of the cells. The predominant phenotype was CD8+ (65% to 80%), whereas the percentage of CD4+ cells varied from 0.5% to 14%. CD56+ (0.5% to 2%) and CD19+ (<1%) cells represented a minority of the cells. TK gene transduction and G418 selection did not change the lymphocyte phenotype significantly (data not shown).
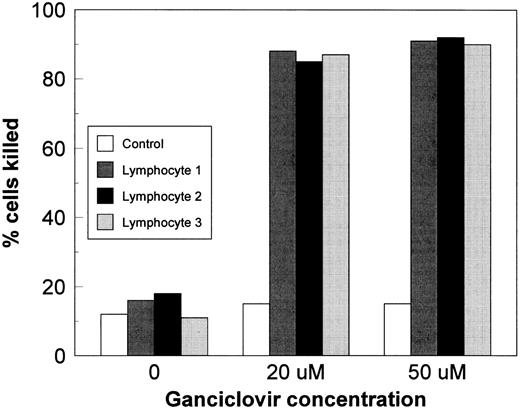
In vitro killing of transduced lymphocytes by GCV.Transduction efficiency was also assessed in seven consecutive donor samples by the extent of GCV-induced cell kill, which reflects the percentage of cells expressing adequate concentrations of TK protein. This represents a biologically more accurate test of transduction efficiency because GCV selectively kills only the transduced cells. In the setting of clinical cytomegalovirus infections, GCV serum concentrations vary between 5 and 50 μmol/L (Drug Information file).26 Therefore, GCV concentrations of 20 and 50 μmol/L were selected for our studies that did not kill untransduced cells after 5 days of culture (Fig 3). In contrast, a mean of 85% (±3.5%) of TK-transduced lymphocytes were killed with 20 μmol/L and greater than 90% (mean, 91.5% ± 3.8%) were killed with 50 μmol/L of GCV, indicating that over 90% of G418-selected cells expressed sufficient TK protein.
Transduced and untransduced lymphocytes were cultured for 5 days in GCV. Over 85% of TK-transduced cells were killed at both GCV concentrations.
Transduced and untransduced lymphocytes were cultured for 5 days in GCV. Over 85% of TK-transduced cells were killed at both GCV concentrations.
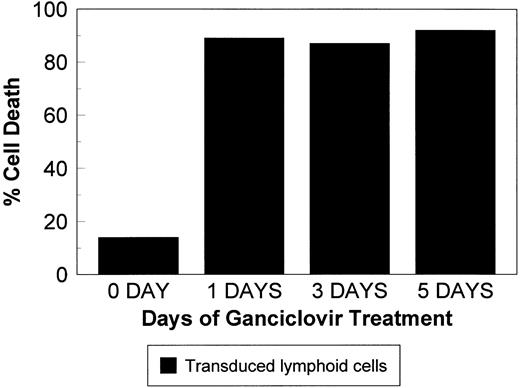
To determine the GCV exposure time required to induce maximal killing of TK-transduced cells, TK-transduced lymphoid H9 cells were selected in G418. A limiting dilution was applied to obtain clones with high sensitivity to GCV. These cells were cultured in GCV at 20 μmol/L for 1, 3, or 5 days. GCV was then removed, and the cells were resuspended in fresh medium. The percentage of live cells (Trypan-blue–negative) was determined on day 5. As shown in Fig 4, cell kill did not decrease by shortening the GCV exposure time from 5 days to 1 day.
Transduced lymphoid cells were cultured in the presence of 20 μmol/L GCV for 1, 3, or 5 days, at which time cells were resuspended in culture media, and the percentage of dead cells was assessed on day 5. Exposure to GCV for 1 day is sufficient for effective killing.
Transduced lymphoid cells were cultured in the presence of 20 μmol/L GCV for 1, 3, or 5 days, at which time cells were resuspended in culture media, and the percentage of dead cells was assessed on day 5. Exposure to GCV for 1 day is sufficient for effective killing.
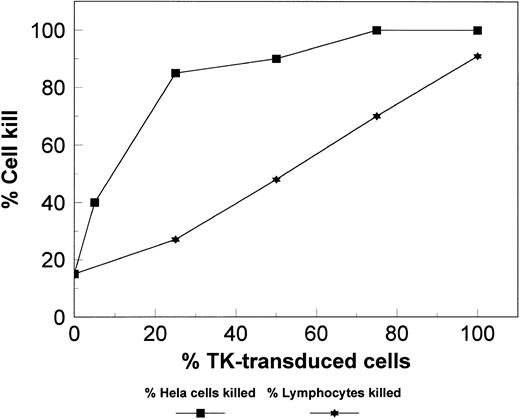
To examine the presence of a “bystander effect,” the extent of TK transduction and GCV-induced cell kill were compared. A bystander effect has been observed in TK-transduced adherent cells where the percentage of cells killed is greater than what could be predicted from the extent of TK gene transduction, possibly as a result of close interaction of transduced and untransduced cells.27 The contact between lymphocytes in suspension cultures is not close so that a bystander effect is not anticipated. Indeed, mixing experiments of transduced and untransduced cells indicated that the cell kill induced by 5-day exposure to 20 μmol/L GCV was proportional to the percentage of TK-transduced cells (Fig 5). However, a bystander effect was observed with adherent Hela cells, in which 84% cell kill was observed although only 25% were TK-transduced.
Various combinations of transduced and untransduced lymphoid cell populations were cultured together with GCV. The percentage (%) of cells killed was similar to the percentage (%) of transduced cells in the culture, suggesting no additional killing by the bystander effect. Transduced Hela cells, an adherent cell line, were used as a positive control that shows the bystander effect.
Various combinations of transduced and untransduced lymphoid cell populations were cultured together with GCV. The percentage (%) of cells killed was similar to the percentage (%) of transduced cells in the culture, suggesting no additional killing by the bystander effect. Transduced Hela cells, an adherent cell line, were used as a positive control that shows the bystander effect.
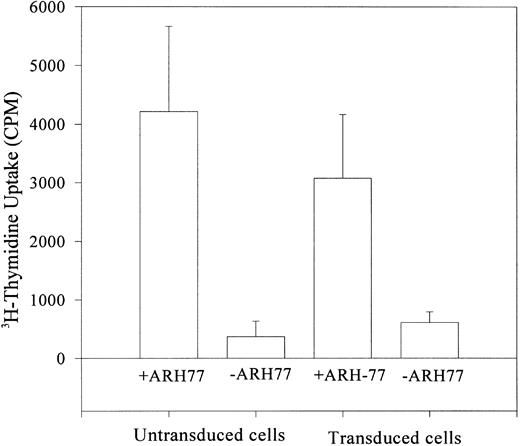
Functional activity of the transduced cells.The functional capacity of TK-transduced and G418-selected primary human lymphocytes was evaluated by their alloreactivity. Cell growth was interrupted by depriving cells of IL-2 for 12 hours. These cells were then stimulated with an allogeneic myeloma cell line (ARH-77). Nontransduced lymphocytes cultured in similar conditions served as positive controls, whereas transduced cells grown in the absence of the myeloma cell line represented the negative controls. After 72 hours of culture with ARH-77 cells, 3H-thymidine incorporation into DNA was measured. Both transduced and untransduced cells showed similar proliferative activity, whereas cells cultured in the absence of ARH-77 cells failed to show any proliferative response (Fig 6).
Incorporation of 3H-thymidine (cpm) after stimulation of IL-2–deprived lymphocytes by allogeneic cells (ARH-77 myeloma cells). Transduced and control cells showed allostimulation, suggesting functional integrity of transduced G418-selected cells.
Incorporation of 3H-thymidine (cpm) after stimulation of IL-2–deprived lymphocytes by allogeneic cells (ARH-77 myeloma cells). Transduced and control cells showed allostimulation, suggesting functional integrity of transduced G418-selected cells.
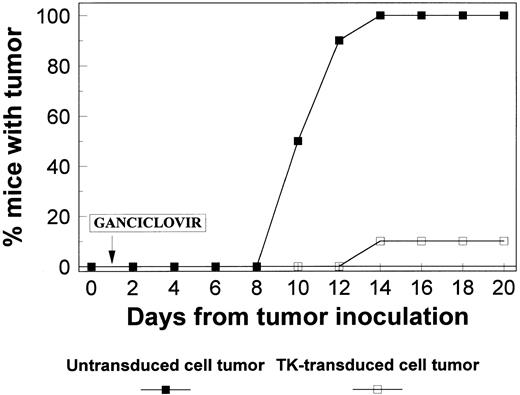
In vivo killing of transduced myeloma cells by GCV.To evaluate whether TK-expressing cells can be killed in vivo by GCV, TK-transduced and nontransduced MOPC-11 cells (a murine myeloma cell line) were injected into separate flanks of Balb/c mice. Transduced and untransduced cells showed similar growth characteristics in the animal model in the absence of GCV treatment (data not shown). GCV therapy was started at a dose of 20 mg/kg after tumor cell inoculation. Nontransduced cells gave rise to palpable tumors in all 10 mice, and the tumor growth continued until the mice were killed. In contrast, only 1 of 10 sites injected with TK-transduced cells developed a tumor that did not grow beyond 5 mm (Fig 7).
Cytotoxic effect of in vivo administration of GCV was examined in mice injected with TK-transduced cells in one flank and with untransduced cells in the other flank. GCV was injected, and the animals were observed for tumor development. All 10 flanks injected with untransduced cells developed tumors (>25 mm), whereas only 1 tumor developed in flanks injected with transduced cells and reached only 5 mm in size.
Cytotoxic effect of in vivo administration of GCV was examined in mice injected with TK-transduced cells in one flank and with untransduced cells in the other flank. GCV was injected, and the animals were observed for tumor development. All 10 flanks injected with untransduced cells developed tumors (>25 mm), whereas only 1 tumor developed in flanks injected with transduced cells and reached only 5 mm in size.
DISCUSSION
It is becoming increasingly clear that immunocompetent donor cells play an important role in tumor control after allogeneic transplantation. In chronic myelogenous leukemia, the infusion of donor cells at the time of relapse can eliminate at least 6 logs of tumor cells, as evidenced by PCR negativity for bcr-abl.19,21,28 We have observed a profound graft-versus-myeloma effect in patients relapsing after T-cell–depleted allogeneic transplantation.29 The need to control severe GVHD associated with donor leukocyte infusions has prompted this study. Although the feasibility of transduction with the viral TK gene has been shown,30 31 important clinical issues such as selection of adequate numbers of highly purified population of transduced cells, maintenance of biological function after TK transduction, and efficacy of killing with clinically achievable levels of GCV have not been addressed previously.
Our study shows efficient (>90%) TK-gene transduction of primary lymphocytes after a 3-day exposure to G418, using both a semiquantitative PCR method and a functional assay according to which 5 days of GCV treatment killed greater than 90% of G418-selected cells. This is a significant improvement over previous studies using unselected lymphocytes as a vehicle for gene transfer. 32-33 In contrast to the results of previous studies in solid tumor cells, no evidence of a “bystander effect” was observed in the case of TK-transduced lymphocytes. Maximal cell kill was obtained with clinically achievable GCV concentrations of 20 μmol/L and 50 μmol/L and was not enhanced by prolongation of exposure beyond 24 hours. Because T-cell clones were not selected for higher sensitivity to GCV, our results reflect the clinical situation of heterogeneity in GCV sensitivity of infused cells.
The presence of the NeoR gene in the transduced cell population provides an opportunity to track these infused lymphocytes in vivo and to study their phenotype in vitro. In case of the development of acute GVHD after transduced lymphocyte infusion, it is anticipated that the majority of TK-transduced cells will be inactivated by GCV administration, resulting in control of GVHD. Conventional treatment of GVHD suppresses the growth of offending lymphocytes but with reactivation on cessation of immunosuppression.34 In contrast, TK-transduced cells should be permanently removed. It will be of interest to determine if rapid elimination by GCV of the majority of T cells will result in a more prompt resolution of acute GVHD. Furthermore, by eliminating the transduced cells with GCV at a preset time, we can test the hypothesis of whether a graft-versus-tumor effect precedes GVHD, as has been suggested by animal studies.22
An intriguing question is why some cells are not killed by GCV within 5 days. Failure to kill a small proportion of the infused cells may be caused by (1) a failure of transduction; (2) a low level of expression or silencing of the TK gene; (3) the limitation of our in vitro assay to determine early TK-induced toxicity (ie, because the toxic effects induced by GCV in TK-transduced cells require observation for more than 48 hours, GCV-induced cell kill may have been underestimated); or (4) a failure of transduced cells to enter G1/S phase of cell cycle (a requirement for GCV effect). Because only dividing cells are killed with GCV, cells not undergoing division during 5 days of observation will have escaped killing. Our preliminary data show that the majority of these cells eventually die on longer observation. We also have preliminary evidence showing that, in some of these cells, although transduced, the TK gene is not functionally expressed; therefore, these cells are not susceptible to GCV killing. The in vivo characteristics of the surviving cells remain unknown and need to be clarified to maximize the efficiency of this therapy.
We have observed an excellent graft-versus-myeloma effect in three consecutive patients with the infusion of as few as 106 T lymphocytes/kg. If greater than 90% of donor lymphocytes are transduced, less than 105/kg untransduced cells will be infused. Such a low number of T cells does not result in clinically important GVHD.34 Moreover, the infusion of small numbers of donor lymphocytes (<106 CD3+cells/kg) is presently an accepted form of treatment for Epstein-Barr virus-related lymphoproliferative disease, with only a limited number of patients developing severe GVHD.35 In 81 patients with acute leukemia after T-cell–depleted transplants, Naparstek et al36 infused sequentially increasing numbers of T-cells, up to 107 cells/kg; only 8 developed moderate to severe GVHD. However, chronic GVHD is potentially a greater problem.
Finally, the current study confirms that the TK-transduced cells are biologically active. The transduced cells can be stimulated by a myeloma cell line in a fashion similar to that of nontransduced cells. In addition, the transduction and selection process does not affect the phenotype of these cells, as observed by FACS analysis showing similar patterns as nontransduced lymphocytes stimulated with anti-CD3 and IL-2.
In summary, this study provides novel and critical clinical information on successful selection of biologically active TK-transduced lymphocytes after relatively short G418 exposure. The selected cells are killed with clinically achievable concentrations of GCV. Moreover, adequate numbers of selected transduced cells for clinical use can be easily obtained. Based on our data, a clinical study has been initiated to test whether TK-transduced lymphocytes administered to patients with persistent or relapsed multiple myeloma after T-cell–depleted allogeneic transplantation can induce remissions and are susceptible to elimination by GCV in case GVHD develops.
ACKNOWLEDGMENT
The authors thank Drs K. Cornetta and A. Srivastava (Indiana University Medical Center, Indianapolis, IN) for their critical review of the manuscript.
Supported in part by a grant from USPHS National Heart Blood and Lung Institute (HL55695), by the American Cancer Society (DHP 153), and by the National Cancer Institute (Grants No. CA71092 and CA55819).
Address reprint requests to N.C. Munshi, MD, University of Arkansas for Medical Sciences, 4301 W Markham, Slot 508, Little Rock, AR 72205.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal