In this issue of Blood, 2 studies identify somatic KRAS mutations in pediatric patients who presented with features of autoimmune lymphoproliferative syndrome (ALPS), hyper γ globulinemia, and autoimmune cytopenias.1,2 With a previous report of an NRAS mutation in an adult with lifelong lymphoproliferation,3 these cases define a new and unexpected role of mutant RAS in hematologic disorders, furthering our understanding of lymphoid growth control and raising the possibility of therapeutic intervention.
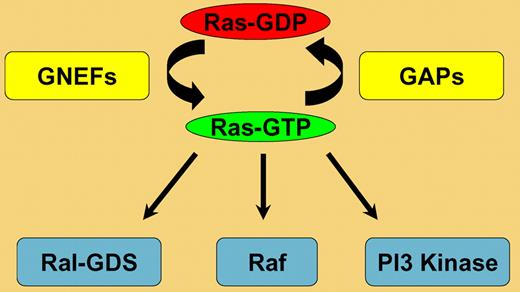
Ras proteins regulate cell fates by cycling between active guanosine triphosphate–bound and inactive guanosine diphosphate–bound conformations (Ras-GTP and Ras-GDP; see figure). Ras activation is mediated by guanine nucleotide exchange factors (GNEFs). These proteins induce guanine nucleotide dissociation in response to extracellular stimuli, which allows Ras to associate with GTP. Upon GTP binding, Ras undergoes a conformational change and can interact productively with Raf1, phosphoinositide-3-OH (PI3) kinase, Ral-GDS, and other effectors. Ras-GTP is hydrolyzed to Ras-GDP through an intrinsic GTPase activity. This reaction is greatly augmented by GTPase-activating proteins (GAPs), which greatly accelerate GTP hydrolysis by stabilizing a transition state between Ras-GTP and Ras-GDP (see figure). Thus, the competing activities of GNEFs and GAPs control Ras-GTP levels in vivo.
Somatic RAS mutations, which introduce amino acid substitutions at codons 12, 13, and 61, are found in 30% of human cancers.4 These mutant Ras proteins accumulate in the GTP-bound conformation due to defective intrinsic GTPase activity and resistance to GAPs. NRAS and KRAS mutations are common in myeloid malignancies, particularly chronic and juvenile myelomonocytic leukemia (CMML and JMML).5 Molecular genetic analysis of JMML has provided compelling evidence that aberrant activation of Ras signaling initiates this aggressive myeloproliferative disorder (MPD). By contrast, NRAS and KRAS mutations are likely acquired as secondary events during the development of acute myeloid leukemia. Although NRAS and KRAS mutations have been reported in ∼ 10% of lymphoid malignancies, the mechanisms by which oncogenic Ras contributes to lymphoid disease are largely unknown.
Studies in mice have provided insights regarding the consequences of expressing Kras and Nras oncogenes at endogenous levels. Thymic lymphomas are observed in ∼ 30% of mice harboring a latent oncogenic KrasG12D allele that is expressed after spontaneous recombination.6 Using the interferon-regulated Mx1-Cre transgene to activate a conditional mutant KrasLSL-G12D allele in hematopoietic cells causes an aggressive MPD that resembles JMML and CMML.7 Interestingly, however, adoptive transfer experiments showed that Kras mutant hematopoietic stem cells (HSCs) efficiently initiate T-lineage acute lymphoblastic leukemia (T-ALL) in irradiated mice, and that these leukemias frequently acquire somatic Notch1 mutations.8 The Mx1-Cre system has been used to induce a latent NrasLSL-G12D “knock-in” allele in mice resulting in both MPD and a polyclonal lymphoproliferative disorder (LPD).9 Together, these studies—showing that endogenous K-RasG12D and N-RasG12D protein expression perturbs myeloid and lymphoid growth control—are consistent with the discovery of NRAS and KRAS mutations in patients with LPD.
Most cases of ALPS are caused by loss-of-function mutations in components of the CD95 (Fas) apoptotic pathway.10 Affected individuals show increased numbers of “double-negative” T (DNT) cells, which lack CD4 and CD8 expression and show defective Fas-mediated apoptosis. By contrast, the DNT population is not increased in patients with KRAS or NRAS mutations, and Fas pathway activation provokes a normal apoptotic response. However, the T cells of these individuals fail to undergo apoptosis in response to interleukin-2 (IL-2) withdrawal. These new reports offer other complimentary insights into aspects of this disease. Takagi and colleagues demonstrated the KRAS mutation in multiple populations of hematopoietic cells, suggesting that the initial mutation occurred in the HSC or early multipotent progenitor compartment.2 Consistent with this idea, the myeloid progenitors of one of their cases showed hypersensitivity to granulocyte macrophage–colony stimulating factor, which is a cellular hallmark of JMML. Niemela et al found that patient T cells that were deprived of IL-2 failed to induce the proapoptotic protein BIM,1 which was also true in the previous case with an NRAS mutation.10 Together, the clinical and biologic features of LPD in individuals with KRAS and NRAS mutations establish this disorder as a distinct entity of Ras-associated lymphoproliferative disease (RALD).10 The pathogenesis of RALD likely involves the dominant clonal outgrowth of progeny of a primitive hematopoietic cell that sustains a RAS gene mutation.
If somatic RAS mutations cause either MPD or LPD, the factors that determine which disease emerges are unclear. In mice, genetic background strongly modulates the disease phenotype with LPD more prominent in the inbred C57Bl/6 strain.8,9 Modifier loci could also exist in humans. Differences in the cell type in which the inciting mutations occur and/or in the nature of any cooperating mutations could determine whether a patient develops RALD, JMML, or another hematologic disease. Finally, the nature of the somatic RAS mutation might impact clinical behavior. The most common cancer-associated RAS mutations encode codon 12 substitutions, which markedly reduce GTPase activity. By contrast, the RAS mutations identified in RALD alter codon 13 and are less deleterious biochemically. Interestingly, spontaneous improvement was reported in a few children who were diagnosed with JMML and had RAS codon 13 mutations.11
These new reports have implications for the diagnosis and management of patients with LPD, hyper γ globulinemia, and autoimmune cytopenias (Evan syndrome). In human developmental disorders, the discoveries of germline NF1 and PTPN11 mutations in affected persons implicated hyperactive Ras as an underlying molecular mechanism and led to the identification of HRAS, KRAS, NRAS, SOS, BRAF, CRAF, and MEK1 as causative genes.12 Similarly, mutations in genes encoding other proteins involved in Ras signaling may cause LPD in other clinical contexts. Finally, potent small molecule inhibitors of kinase effectors of Ras-GTP have been developed as anticancer agents (see figure). In vitro studies performed by Oliveira and colleagues10 provide intriguing evidence that drugs that target MEK, a downstream component of the Raf/MEK/ERK pathway, may reverse the cellular and biochemical defects seen in RALD. Similarly, inhibitors of PI3 kinase signaling, which plays a central role in lymphocyte survival and is frequently altered by somatic mutations in T-ALL,13 are appealing as potential therapeutic agents. Defining the nature and spectrum of Ras pathway mutations in RALD and related lymphoid disorders therefore has important biologic and translational implications.
Conflict-of-interest disclosure: The authors declare no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal