In this issue of Blood, Demarest et al demonstrate that maintenance of T-cell acute lymphoblastic leukemia (T-ALL) tumors requires continued expression of activated Notch1 while c-Myc expression is expendable for tumor maintenance.1
The data reported in this paper use a mouse model system where either the activated form of Notch1 (NotchIC) or c-Myc is under the control of the Tet operator and inducible by removal of doxycycline from the drinking water. Induction of either NotchIC or c-Myc is sufficient to drive the formation of T-cell leukemias that resemble human T-ALL. The authors have also used retroviral expression vectors that express either NotchIC or c-Myc. Using these tools, bone marrow stem cells from animals expressing inducible Notch (Top-Notch) are infected with a retroviral vector carrying the c-Myc gene and transferred into lethally irradiated syngenic mice and monitored for tumor formation. Alternatively, bone marrow stem cells from Tet-o-Myc mice (expression of c-Myc induced by removal of doxycycline) are infected with a retroviral vector carrying NotchIC and used for transfer into syngenic mice. This experimental system allows the investigators to either turn off c-Myc while retaining NotchIC expression or to turn off NotchIC while retaining c-Myc expression. Using this elegantly designed system, these investigators show that NotchIC but not c-Myc expression is required to maintain the tumor (see figure).
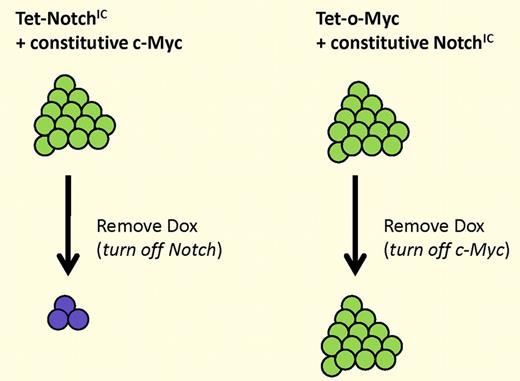
Continued expression of NotchIC, but not c-Myc, is required for tumor maintenance. (Left) Tumors generated by inducible NotchIC and constitutive c-Myc regress when Notch expression is extinguished. (Right) Tumors generated by inducible c-Myc and constitutive NotchIC do not regress when c-Myc expression is extinguished.
Continued expression of NotchIC, but not c-Myc, is required for tumor maintenance. (Left) Tumors generated by inducible NotchIC and constitutive c-Myc regress when Notch expression is extinguished. (Right) Tumors generated by inducible c-Myc and constitutive NotchIC do not regress when c-Myc expression is extinguished.
Several proto-oncogenes, including LMO1/2, TAL1/2, HOX11, LYL1, Notch1, and c-Myc, have been implicated in the development of T-ALL (reviewed in Teitell and Pandolfi2 ). Particularly relevant to the data reported by Demarest et al, activating mutations in Notch1 are found in > 60% of human T-ALL and introduction of these mutations in murine hematopoietic stem cells by retroviral infection leads to T-ALL in 100% of animals transplanted with these cells.3,4 These data suggest that activating mutations in Notch1 are a dominant event in the development of T-ALL in humans and mice and have led investigators to question how activation of Notch1 contributes to tumor development. An emerging consensus is that one major function of Notch is the regulation of cell growth. Supporting this, several groups indentified a number of downstream targets of Notch signaling in T-ALL including c-Myc5-7 the PI3K-AKT-mTOR pathway,8 cyclin D1 and D3, and CDK4 and CDK6.9,10 In many instances, when Notch1 activation is blocked by inhibition of γ-secretase, the enzyme required for cleavage and activation of membrane bound Notch, ensuing growth arrest could be rescued by introduction of a gene encoding one of these downstream targets. The observation that inhibition of γ-secretase and blockade of Notch activation leads to growth arrest and the well-known link between c-Myc and cell-cycle progression, coupled with data demonstrating overlapping gene expression profiles of Notch or c-Myc in leukemia cells, suggest that Notch and c-Myc might compensate for each other in maintaining tumors. To test this hypothesis, the authors of this study used the inducible mouse models described above.
In addition to their main finding that Notch1 (but not c-Myc) is required for tumor maintenance, Demarest and colleagues show that expression of c-Myc has no effect on tumor latency in Top-Notch mice; however, expression of NotchIC greatly enhanced time to tumor formation in Tet-o-Myc mice (110 days for Tet-o-Myc vs 42.62 days for Tet-o-Myc + NotchIC). Earlier data from this group provided compelling evidence that Notch suppresses the p53 pathway.11 Others have demonstrated that c-Myc–induced tumorigenesis requires inactivation of p53, presumably to inhibit p53-dependent apoptosis. These observations led the authors to postulate that Notch1 drives T-ALL development and tumor maintenance by sustaining cell-cycle progression while inhibiting p53-mediated apoptosis. In support of this model, in Top-Notch/c-Myc tumors, p53 expression is extinguished; however, when NotchIC is turned off by doxycyline, p53 protein levels are up-regulated suggesting tumor regression is mediated by p53-dependent apoptosis. The authors use this evidence to propose that the dominance of NotchIC over c-Myc can be explained, at least in part, by the ability of NotchIC to suppress p53-mediated apoptosis. While the data support this hypothesis, it would be useful to directly test it by precisely modulating p53 levels in Top-Notch/c-Myc tumors.
The results presented by Demarest et al advance our understanding of the role played by NotchIC in the development of T-ALL. In particular, the data indicate that inhibition of γ-secretase, which blocks Notch activation, is likely to block cell proliferation and p53 driven apoptosis. Recent data from the Ferrando laboratory suggest that many instances where T-ALL cells escape growth arrest are accompanied by mutational inactivation of the tumor suppressor PTEN.3 Inactivation of PTEN directly results in activation of the PI3K-Akt-mTOR signaling pathway, thus bypassing NotchIC-induced activation of this important cell growth pathway. Therapies that combine γ-secretase inhibitors with inhibitors of p53-mediated apoptosis as well as the PI3K-Akt-mTOR pathway are likely candidates to treat refractory T-ALL. Future research is likely to explore the relevance of the insights gained from the mouse model presented in this report to human T-ALL. In addition, an exploration of the ability to transplant tumors from animals where either Notch or c-Myc expression is extinguished may provide insights concerning the role of these genes in the maintenance of tumor initiating cells.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■
REFERENCES
National Institutes of Health

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal