Key Points
In combination with cyclophosphamide, intravenous busulfan is associated with better leukemia-free and overall survival in AML than TBI.
Abstract
Cyclophosphamide combined with total body irradiation (Cy/TBI) or busulfan (BuCy) are the most widely used myeloablative conditioning regimens for allotransplants. Recent data regarding their comparative effectiveness are lacking. We analyzed data from the Center for International Blood and Marrow Transplant Research for 1230 subjects receiving a first hematopoietic cell transplant from a human leukocyte antigen-matched sibling or from an unrelated donor during the years 2000 to 2006 for acute myeloid leukemia (AML) in first complete remission (CR) after conditioning with Cy/TBI or oral or intravenous (IV) BuCy. Multivariate analysis showed significantly less nonrelapse mortality (relative risk [RR] = 0.58; 95% confidence interval [CI]: 0.39-0.86; P = .007), and relapse after, but not before, 1 year posttransplant (RR = 0.23; 95% CI: 0.08-0.65; P = .006), and better leukemia-free survival (RR = 0.70; 95% CI: 0.55-0.88; P = .003) and survival (RR = 0.68; 95% CI: 0.52-0.88; P = .003) in persons receiving IV, but not oral, Bu compared with TBI. In combination with Cy, IV Bu is associated with superior outcomes compared with TBI in patients with AML in first CR.
Introduction
The development of the 2 most commonly used myeloablative preparative regimens for allogeneic hematopoietic cell transplantation (HCT) occurred largely in parallel. Thomas and colleagues reported cure of some patients with advanced acute leukemia using cyclophosphamide, total body irradiation (Cy/TBI), and marrow from human leukocyte antigen (HLA)-identical siblings.1 They subsequently demonstrated sustained leukemia-free survival (LFS) in more than half of patients with acute myeloid leukemia (AML) in first complete remission (CR),2 the superiority of 120 mg/kg Cy with TBI delivered in 6 fractions of 2 Gy compared with a single dose of 10 Gy,3 and equivalent survival with lower nonrelapse mortality (NRM) using 12 Gy compared with 15.75 Gy in 7 divided fractions.4
Santos and colleagues developed a preparative regimen of 16 mg/kg of orally administered busulfan (Bu) and 200 mg/kg Cy and reported an extraordinarily low rate of relapse, but a high incidence of NRM, in patients in various stages of AML.5 Tutschka reported less toxicity and NRM with a reduction in Cy dose to that used by Thomas, 120 mg/kg administered over 2 days (BuCy2).6 A multi-institutional international study of AML in first CR reported an estimated 3-year LFS of 63% following BuCy2 and transplantation from HLA-identical sibling donors,7 however, a randomized study shortly thereafter reported superior outcomes with Cy/TBI compared with BuCy2.8 Prospective and retrospective studies and metaanalyses performed during the subsequent decade raised questions regarding the superiority of Cy/TBI over BuCy,9-17 but generally suggested lower relapse rates in patients with AML following TBI.17
These comparative studies all used fixed doses of oral Bu, which are associated with substantial individual variation in plasma levels. Low steady state Bu levels are associated with relapse and rejection18,19 and high levels with hepatic venoocclusive disease (VOD) and other toxicities.18,20,21 Therapeutic monitoring of plasma Bu concentrations and individualized adjustment of the oral dose result in plasma levels within a desired range in most patients, lowered incidence of NRM, and improved survival.21-23 The development and administration of intravenous (IV) Bu has similarly decreased variability in plasma levels compared with a fixed oral dose and has been reported to reduce VOD, other toxicities and 100-day mortality.24-26 Therapeutic monitoring and dose adjustment of IV Bu further reduces the variability of its levels in plasma.27 Thus, while molecular HLA-typing and better supportive care have improved survival generally after HCT,28,29 improvements in its administration might differentially improve results using Bu.27,30,31
Unfortunately, no prospective comparisons of oral dose-adjusted or IV Bu to fixed dose oral Bu or to TBI have been performed. Moreover, there have been no large retrospective multi-institutional studies comparing outcomes among groups of patients receiving these preparative regimens following widespread implementation of these improvements in Bu administration. The Center for International Bone Marrow Transplant Research (CIBMTR) thus conducted a large retrospective multiinstitutional study to analyze outcomes in a modern cohort of patients with AML in first CR using Cy in combination with oral Bu, IV Bu, or TBI. We report here the results of this analysis.
Patients and methods
Data sources
The CIBMTR is a working group of more than 500 transplant centers worldwide that voluntarily contribute data on allogeneic and autologous transplants. Detailed demographic, disease, and transplant characteristics and outcome data are collected on a sample of registered patients including all unrelated donor (URD) transplants facilitated by the National Marrow Donor Program in the United States. Observational studies conducted by the CIBMTR are carried out with a waiver of informed consent and in compliance with HIPAA regulations as determined by the Institutional Review Board and the Privacy Officer of the Medical College of Wisconsin.
Patients
The patient population consisted of all patients reported to the CIBMTR who underwent first allogeneic HCT with an HLA-identical sibling or URD from 2000-2006 for AML in first CR who received preparation with Cy/TBI (nonfractionated dose ≥5.5 Gy, fractionated ≥9 Gy) or BuCy (≥9mg/kg) without additional chemotherapeutic agents. Patients with identical twin or cord blood donors were excluded, as were patients who underwent in vitro T cell depletion or who received posttransplant Cy. Information regarding Bu pharmacokinetics and molecular markers of risk (including FLT3 ITD and NPM1) were not collected during this period of study.
Study end points and definitions
The primary outcome studied was survival. Patients were considered to have an event at time of death from any cause; survivors were censored at last contact. Relapse was defined by hematologic criteria and NRM was considered a competing event. NRM was defined as death without evidence of leukemia recurrence; relapse was considered a competing event. LFS was defined as time to treatment failure (death or relapse). For relapse, NRM and LFS, patients alive in continuous complete remission were censored at last follow-up. Times to neutrophil and platelet recovery were calculated as the time from transplantation to achieving the first of 3 consecutive days with an absolute neutrophil count > 500/μL and platelet count > 20 000/μL, 7 days from the last platelet transfusion. Acute graft-versus-host disease (GVHD) was graded according to consensus criteria based on the pattern of severity of abnormalities in skin, gastrointestinal tract and liver.32 Chronic GVHD was diagnosed by standard criteria.33 For engraftment and GVHD, death without the event was considered a competing event. Cytogenetic abnormalities were classified according to the SWOG/ECOG cytogenetic classification system.34
Statistical methods
Probability of LFS and overall survival (OS) were calculated using the Kaplan-Meier method, with the variance estimated by Greenwood’s formula. Engraftment, GVHD, NRM, and relapse were estimated using the cumulative incidence method to account for competing risks. Comparisons of survival curves were performed using the log-rank test. Comparisons of NRM curves were performed using Gray’s method.
In multivariate analysis, a stepwise selection procedure was performed using the proportional hazards Cox model for OS, LFS, NRM, GVHD, and relapse to adjust for the following variables—patient: age, gender, and Karnofsky score at transplant; disease: interval from diagnosis to transplant, and cytogenetic risk group; and transplant-related: donor-recipient gender and Cytomegalovirus (CMV) status, graft type, graft source and degree of match, year of transplant, GVHD prophylaxis, and planned use of growth factors posttransplant. The proportional hazards assumption was assessed for each variable by testing its time dependency. If the proportional hazards assumption was violated, models were constructed breaking the posttransplant time course into 2 periods, using the maximized partial likelihood method to find the most appropriate breakpoint. Interactions were checked between each selected variable and the main effects. To avoid confounding of results by local clinical practices determined at the center level, a variable representing the transplant center35 was tested in all the models using the score test.36 If this variable was found to be an important confounder, the marginal Cox model for dependent data were used.37
The interaction between main effect categories of preparative regimen and donor type (sibling, unrelated) was tested using a cutoff of 0.01 for significance. Adjusted LFS and survival probabilities were estimated through the direct adjusted survival curves estimation method.38 SAS software, version 9.2 (SAS Institute, Cary, NC) was used in all analyses.
Results
Demographics and univariate analysis
A total of 1230 patients underwent first HCT from an HLA-matched sibling or URD from January 1, 2000 through December 31, 2006 for AML in first CR following preparation with myeloablative doses of Cy/TBI or BuCy. The median follow up of surviving patients is 5 years.
Characteristics of patients categorized according to preparative regimen are described in Table 1.39 Patients who received oral Bu were younger (median age 34 years, 37% >40 years) than patients receiving TBI (median age 37 years, 42% >40 years) who, in turn, were younger than patients who received IV Bu (median age 40 years, 49% > 40 years). More than three-fourths of patients receiving oral Bu underwent transplantation from HLA-identical siblings, while more than half of those receiving IV Bu and TBI had URD. Approximately 10% of oral Bu and TBI patients received anti-thymocyte globulin (ATG) or alemtuzumab compared with 20% of IV Bu patients. More than three-fourths of IV Bu patients, compared with roughly half of oral Bu and TBI patients, underwent transplantation from 2004-2006, the last 3 years of study. Cytogenetic classification, donor and recipient gender, and graft type were similar among the groups. The median and interquartile range (IQR) Cy dose was 118.6mg/kg (IQR: 98-120 mg/kg) for patients receiving TBI, 118.8mg/kg (IQR: 104.6-120 mg/kg) among those receiving oral Bu, and 117.1mg/kg (IQR: 100.6-120.4 mg/kg) for those receiving IV Bu. The median and IQR Bu dose was 15.7mg/kg (IQR: 13.8-16 mg/kg) for patients receiving Bu orally and 12.6 mg/kg (IQR: 10.9-12.9 mg/kg) for those receiving it intravenously.
Characteristics of patients who underwent first allogeneic transplantation with an HLA- identical sibling or unrelated donor for AML in CR1 and received preparation with Cy/TBI, oral or IV BuCy, reported to CIBMTR from 2000 to 2006
| Characteristics of patients . | Cy/TBI . | Oral BuCy . | IV BuCy . |
|---|---|---|---|
| Number of patients | 586 | 408 | 236 |
| Number of centers | 117 | 86 | 80 |
| Patient-related | |||
| Age, median (range), years | 37 (2-63) | 34 (2-62) | 40 (2-64) |
| 10 y or less | 17 (3) | 26 (6) | 27 (11) |
| 11-20 | 66 (11) | 65 (16) | 35 (15) |
| 21-30 | 110 (19) | 88 (22) | 29 (12) |
| 31-40 | 144 (25) | 80 (20) | 31 (13) |
| 41-50 | 159 (27) | 97 (24) | 65 (28) |
| Older than 50 | 90 (15) | 52 (13) | 49 (21) |
| Sex | |||
| Male | 307 (52) | 216 (53) | 122 (52) |
| Karnofsky score at transplant | |||
| Less than 90% | 128 (22) | 56 (14) | 51 (22) |
| Greater than or equal to 90% | 424 (72) | 340 (83) | 174 (74) |
| Missing | 34 (6) | 12 (3) | 11 (5) |
| Disease-related Cytogenetic classification34 | |||
| Favorable | 25 (4) | 24 (6) | 16 (7) |
| Intermediate | 244 (42) | 230 (56) | 117 (50) |
| Poor | 215 (37) | 135 (33) | 81 (34) |
| Missing | 102 (17) | 19 (5) | 22 (9) |
| Transplant-related | |||
| Time from diagnosis to transplant, median (range), months | 5 (2-44) | 5 (2-49) | 5 (2-25) |
| Donor-recipient sex match | |||
| Male - male | 198 (34) | 123 (30) | 72 (31) |
| Female - male | 109 (19) | 93 (23) | 49 (21) |
| Male - female | 152 (26) | 110 (27) | 67 (28) |
| Female - female | 126 (22) | 82 (20) | 47 (20) |
| Missing | 1 (<1) | 0 | 1 (<1) |
| Donor-recipient HLA match39 | |||
| HLA-identical sibling | 229 (39) | 309 (76) | 111 (47) |
| Well-matched URD | 268 (46) | 72 (18) | 99 (42) |
| Partially matched URD | 68 (12) | 23 (6) | 23 (10) |
| Mismatched URD | 21 (4) | 4 (<1) | 3 (1) |
| Donor-recipient CMV serology | |||
| +/+ | 160 (27) | 86 (21) | 60 (25) |
| +/− | 181 (31) | 202 (50) | 87 (37) |
| −/+ | 73 (12) | 32 (8) | 24 (10) |
| −/− | 145 (25) | 76 (19) | 58 (25) |
| Missing | 27 (5) | 12 (3) | 7 (3) |
| HLA-identical sibling donor age, median (range), years | 38 (4-66) | 30 (2-71) | 36 (3-63) |
| Unrelated donor age, median (range), years | 34 (19-58) | 35 (19-56) | 33 (19-59) |
| Graft type | |||
| Bone Marrow | 231 (39) | 165 (40) | 108 (46) |
| Peripheral blood | 355 (61) | 243 (60) | 128 (54) |
| Conditioning regimen | |||
| BuCy | 0 | 408 | 236 |
| Bu dose, median (range), mg/kg | 0 | 16 (9-27) | 13 (9-21) |
| Cy/TBI (nonfractionated 550-750) | 34 (6) | 0 | 0 |
| Cy/TBI (nonfractionated 800-1200) | 12 (2) | 0 | 0 |
| Cy/TBI (fractionated 900-1170) | 16 (3) | 0 | 0 |
| Cy/TBI (fractionated 1200-1300) | 322 (55) | 0 | 0 |
| Cy/TBI (fractionated 1320-1395) | 144 (25) | 0 | 0 |
| Cy/TBI (fractionated 1400-1500) | 58 (10) | 0 | 0 |
| ATG and Alemtuzumab usage | |||
| ATG | 56 (10) | 40 (10) | 43 (18) |
| Alemtuzumab | 2 (<1) | 1 (<1) | 5 (2) |
| Missing | 1 (<1) | 2 (<1) | 0 |
| GVHD prophylaxis | |||
| TAC + MMF ± others | 26 (4) | 4 (<1) | 5 (2) |
| TAC + MTX ± others (except MMF) | 186 (32) | 43 (11) | 79 (33) |
| TAC ± others (except MTX, MMF) | 17 (3) | 4 (<2) | 9 (4) |
| CSA + MMF ± others (except TAC) | 8 (1) | 10 (2) | 3 (1) |
| CSA + MTX ± others (except TAC, MMF) | 312 (53) | 313 (77) | 118 (50) |
| CSA ± others (except TAC, MTX, MMF) | 28 (4) | 18 (5) | 14 (6) |
| Other GVHD prophylaxis | 3 (<1) | 11 (3) | 6 (3) |
| Missing | 6 (1) | 5 (1) | 2 (<1) |
| Growth factors given posttransplant: G-CSF or GM-CSF | |||
| No | 472 (81) | 299 (73) | 166 (70) |
| Yes | 112 (19) | 109 (27) | 70 (30) |
| Missing | 2 (<1) | 0 | 0 |
| Year of transplant | |||
| 2000 | 69 (12) | 46 (11) | 8 (3) |
| 2001 | 72 (12) | 52 (13) | 5 (2) |
| 2002 | 69 (12) | 63 (15) | 16 (7) |
| 2003 | 63 (11) | 64 (16) | 23 (10) |
| 2004 | 107 (18) | 73 (18) | 54 (23) |
| 2005 | 113 (19) | 54 (13) | 74 (31) |
| 2006 | 93 (16) | 56 (14) | 56 (24) |
| Median follow-up of survivors, (range) months | 68 (3-137) | 57 (2-124) | 59 (3-120) |
| Characteristics of patients . | Cy/TBI . | Oral BuCy . | IV BuCy . |
|---|---|---|---|
| Number of patients | 586 | 408 | 236 |
| Number of centers | 117 | 86 | 80 |
| Patient-related | |||
| Age, median (range), years | 37 (2-63) | 34 (2-62) | 40 (2-64) |
| 10 y or less | 17 (3) | 26 (6) | 27 (11) |
| 11-20 | 66 (11) | 65 (16) | 35 (15) |
| 21-30 | 110 (19) | 88 (22) | 29 (12) |
| 31-40 | 144 (25) | 80 (20) | 31 (13) |
| 41-50 | 159 (27) | 97 (24) | 65 (28) |
| Older than 50 | 90 (15) | 52 (13) | 49 (21) |
| Sex | |||
| Male | 307 (52) | 216 (53) | 122 (52) |
| Karnofsky score at transplant | |||
| Less than 90% | 128 (22) | 56 (14) | 51 (22) |
| Greater than or equal to 90% | 424 (72) | 340 (83) | 174 (74) |
| Missing | 34 (6) | 12 (3) | 11 (5) |
| Disease-related Cytogenetic classification34 | |||
| Favorable | 25 (4) | 24 (6) | 16 (7) |
| Intermediate | 244 (42) | 230 (56) | 117 (50) |
| Poor | 215 (37) | 135 (33) | 81 (34) |
| Missing | 102 (17) | 19 (5) | 22 (9) |
| Transplant-related | |||
| Time from diagnosis to transplant, median (range), months | 5 (2-44) | 5 (2-49) | 5 (2-25) |
| Donor-recipient sex match | |||
| Male - male | 198 (34) | 123 (30) | 72 (31) |
| Female - male | 109 (19) | 93 (23) | 49 (21) |
| Male - female | 152 (26) | 110 (27) | 67 (28) |
| Female - female | 126 (22) | 82 (20) | 47 (20) |
| Missing | 1 (<1) | 0 | 1 (<1) |
| Donor-recipient HLA match39 | |||
| HLA-identical sibling | 229 (39) | 309 (76) | 111 (47) |
| Well-matched URD | 268 (46) | 72 (18) | 99 (42) |
| Partially matched URD | 68 (12) | 23 (6) | 23 (10) |
| Mismatched URD | 21 (4) | 4 (<1) | 3 (1) |
| Donor-recipient CMV serology | |||
| +/+ | 160 (27) | 86 (21) | 60 (25) |
| +/− | 181 (31) | 202 (50) | 87 (37) |
| −/+ | 73 (12) | 32 (8) | 24 (10) |
| −/− | 145 (25) | 76 (19) | 58 (25) |
| Missing | 27 (5) | 12 (3) | 7 (3) |
| HLA-identical sibling donor age, median (range), years | 38 (4-66) | 30 (2-71) | 36 (3-63) |
| Unrelated donor age, median (range), years | 34 (19-58) | 35 (19-56) | 33 (19-59) |
| Graft type | |||
| Bone Marrow | 231 (39) | 165 (40) | 108 (46) |
| Peripheral blood | 355 (61) | 243 (60) | 128 (54) |
| Conditioning regimen | |||
| BuCy | 0 | 408 | 236 |
| Bu dose, median (range), mg/kg | 0 | 16 (9-27) | 13 (9-21) |
| Cy/TBI (nonfractionated 550-750) | 34 (6) | 0 | 0 |
| Cy/TBI (nonfractionated 800-1200) | 12 (2) | 0 | 0 |
| Cy/TBI (fractionated 900-1170) | 16 (3) | 0 | 0 |
| Cy/TBI (fractionated 1200-1300) | 322 (55) | 0 | 0 |
| Cy/TBI (fractionated 1320-1395) | 144 (25) | 0 | 0 |
| Cy/TBI (fractionated 1400-1500) | 58 (10) | 0 | 0 |
| ATG and Alemtuzumab usage | |||
| ATG | 56 (10) | 40 (10) | 43 (18) |
| Alemtuzumab | 2 (<1) | 1 (<1) | 5 (2) |
| Missing | 1 (<1) | 2 (<1) | 0 |
| GVHD prophylaxis | |||
| TAC + MMF ± others | 26 (4) | 4 (<1) | 5 (2) |
| TAC + MTX ± others (except MMF) | 186 (32) | 43 (11) | 79 (33) |
| TAC ± others (except MTX, MMF) | 17 (3) | 4 (<2) | 9 (4) |
| CSA + MMF ± others (except TAC) | 8 (1) | 10 (2) | 3 (1) |
| CSA + MTX ± others (except TAC, MMF) | 312 (53) | 313 (77) | 118 (50) |
| CSA ± others (except TAC, MTX, MMF) | 28 (4) | 18 (5) | 14 (6) |
| Other GVHD prophylaxis | 3 (<1) | 11 (3) | 6 (3) |
| Missing | 6 (1) | 5 (1) | 2 (<1) |
| Growth factors given posttransplant: G-CSF or GM-CSF | |||
| No | 472 (81) | 299 (73) | 166 (70) |
| Yes | 112 (19) | 109 (27) | 70 (30) |
| Missing | 2 (<1) | 0 | 0 |
| Year of transplant | |||
| 2000 | 69 (12) | 46 (11) | 8 (3) |
| 2001 | 72 (12) | 52 (13) | 5 (2) |
| 2002 | 69 (12) | 63 (15) | 16 (7) |
| 2003 | 63 (11) | 64 (16) | 23 (10) |
| 2004 | 107 (18) | 73 (18) | 54 (23) |
| 2005 | 113 (19) | 54 (13) | 74 (31) |
| 2006 | 93 (16) | 56 (14) | 56 (24) |
| Median follow-up of survivors, (range) months | 68 (3-137) | 57 (2-124) | 59 (3-120) |
ATG, antithymocyte globulin; CSA, cyclosporine; G-CSF, granulocyte-colony stimulating factor; GM-CSF, granulocyte-macrophage colony-stimulating factor; MMF, mycophenolate; MTX, methotrexate; and TAC, tacrolimus
The incidence of NRM at 1 and 5 years was 21% (95% confidence interval [CI]: 17% to 24%) and 31% (95% CI: 27% to 35%) with TBI, 14% (95% CI: 10% to 17%) and 18% (95% CI: 14% to 22%) for oral Bu, and 12% (95% CI: 8-17%) and 18% (95% CI: 13% to 24%) among IV Bu patients (P < .001; Table 2; Figure 1). The estimated LFS and OS at 5 years were 41% (95% CI: 37% to 46%) and 43% (95% CI: 39% to 47%) for TBI, 54% (95% CI: 49% to 60%) and 61% (95% CI: 56% to 66%) for oral Bu and 57% (95% CI: 50-64%) and 58% (95% CI: 51% to 65%) for IV Bu (P < .001 for each). The 100-day cumulative incidence of hepatic VOD was not significantly different in patients receiving oral or IV Bu compared with TBI (9%; 95% CI: 7% to 12% vs 8%; 95% CI: 5% to 11% vs 6%; 95% CI: 4% to 8%, respectively; P = .18). The cumulative incidence of interstitial pneumonia at 100-days was 10% (95% CI: 7% to 12%) in patients receiving TBI, 5% (95% CI: 3% to 8%) in those receiving oral Bu, and 3% (95% CI: 1% to 6%) among IV Bu patients (P < .001). Neutrophil recovery at 28 days was similar among the groups (Table 2), including when the analysis was restricted to patients receiving hematopoietic cells from unrelated donors (92%; 95% CI: 88% to 94%, 93%; 95% CI: 87- 97%, 93%; 95% CI: 87% to 97%; P = .80, for TBI, oral Bu, and IV Bu respectively).
Comparison of univariate outcomes of patients who underwent first allogeneic transplant with an HLA-identical sibling donor or an unrelated donor for AML in CR1 and received preparation with Cy/TBI, oral or IV BuCy, reported to the CIBMTR between 2000 and 2006
| Outcomes . | Cy/TBI Probability (95% CI) . | Oral BuCy Probability (95% CI) . | IV BuCy Probability (95% CI) . | P values (Pointwise) . |
|---|---|---|---|---|
| Neutrophil engraftment | ||||
| NEval | 585 | 407 | 236 | |
| at 28 d | 95 (89-98) | 93 (86-97) | 95 (85-100) | .824 |
| at 100 d | 98 (90-100) | 99 (87-100) | 97 (85-100) | .964 |
| Platelet engraftment | ||||
| NEval | 582 | 390 | 234 | |
| at 28 d | 62 (58-67) | 73 (67-79) | 67 (60-74) | .012 |
| at 100 d | 88 (83-93) | 92 (85-97) | 91 (81-97) | .625 |
| Acute GVHD (grade II-IV) | ||||
| NEval | 584 | 408 | 236 | |
| at 100 d | 51 (47-55) | 39 (34-44) | 40 (33-46) | <.001 |
| Acute GVHD (grade III-IV) | ||||
| NEval | 586 | 408 | 236 | |
| at 100 d | 25 (21-28) | 14 (11-18) | 17 (13-22) | <.001 |
| Chronic GVHD | ||||
| NEval | 572 | 401 | 229 | |
| at 1 y | 44 (39-49) | 42 (36-47) | 49 (41-57) | .303 |
| at 5 y | 52 (47-57) | 48 (42-53) | 55 (46-63) | .314 |
| NRM | ||||
| NEval | 586 | 407 | 236 | |
| at 1 y | 21 (17-24) | 14 (10-17) | 12 (8-17) | .002 |
| at 5 y | 31 (27-35) | 18 (14-22) | 18 (13-24) | <.001 |
| Progression/relapse | ||||
| NEval | 586 | 407 | 236 | |
| at 1 y | 20 (17-24) | 16 (13-20) | 23 (17-28) | .155 |
| at 5 y | 28 (24-32) | 27 (23-32) | 25 (19-31) | .726 |
| LFS | ||||
| NEval | 586 | 407 | 236 | <.001* |
| at 1 y | 59 (55-63) | 70 (65-75) | 65 (59-72) | .002 |
| at 5 y | 41 (37-46) | 54 (49-60) | 57 (50-64) | <.001 |
| Overall survival | ||||
| NEval | 586 | 408 | 236 | <.001* |
| at 1 y | 65 (61-68) | 76 (71-80) | 72 (66-78) | <.001 |
| at 5 y | 43 (39-47) | 61 (56-66) | 58 (51-65) | <.001 |
| Outcomes . | Cy/TBI Probability (95% CI) . | Oral BuCy Probability (95% CI) . | IV BuCy Probability (95% CI) . | P values (Pointwise) . |
|---|---|---|---|---|
| Neutrophil engraftment | ||||
| NEval | 585 | 407 | 236 | |
| at 28 d | 95 (89-98) | 93 (86-97) | 95 (85-100) | .824 |
| at 100 d | 98 (90-100) | 99 (87-100) | 97 (85-100) | .964 |
| Platelet engraftment | ||||
| NEval | 582 | 390 | 234 | |
| at 28 d | 62 (58-67) | 73 (67-79) | 67 (60-74) | .012 |
| at 100 d | 88 (83-93) | 92 (85-97) | 91 (81-97) | .625 |
| Acute GVHD (grade II-IV) | ||||
| NEval | 584 | 408 | 236 | |
| at 100 d | 51 (47-55) | 39 (34-44) | 40 (33-46) | <.001 |
| Acute GVHD (grade III-IV) | ||||
| NEval | 586 | 408 | 236 | |
| at 100 d | 25 (21-28) | 14 (11-18) | 17 (13-22) | <.001 |
| Chronic GVHD | ||||
| NEval | 572 | 401 | 229 | |
| at 1 y | 44 (39-49) | 42 (36-47) | 49 (41-57) | .303 |
| at 5 y | 52 (47-57) | 48 (42-53) | 55 (46-63) | .314 |
| NRM | ||||
| NEval | 586 | 407 | 236 | |
| at 1 y | 21 (17-24) | 14 (10-17) | 12 (8-17) | .002 |
| at 5 y | 31 (27-35) | 18 (14-22) | 18 (13-24) | <.001 |
| Progression/relapse | ||||
| NEval | 586 | 407 | 236 | |
| at 1 y | 20 (17-24) | 16 (13-20) | 23 (17-28) | .155 |
| at 5 y | 28 (24-32) | 27 (23-32) | 25 (19-31) | .726 |
| LFS | ||||
| NEval | 586 | 407 | 236 | <.001* |
| at 1 y | 59 (55-63) | 70 (65-75) | 65 (59-72) | .002 |
| at 5 y | 41 (37-46) | 54 (49-60) | 57 (50-64) | <.001 |
| Overall survival | ||||
| NEval | 586 | 408 | 236 | <.001* |
| at 1 y | 65 (61-68) | 76 (71-80) | 72 (66-78) | <.001 |
| at 5 y | 43 (39-47) | 61 (56-66) | 58 (51-65) | <.001 |
NEval, number evaluated.
Log-rank test P value.
Univariate cumulative incidence for nonrelapse mortality according to preparative regimen.
Univariate cumulative incidence for nonrelapse mortality according to preparative regimen.
Multivariate analysis
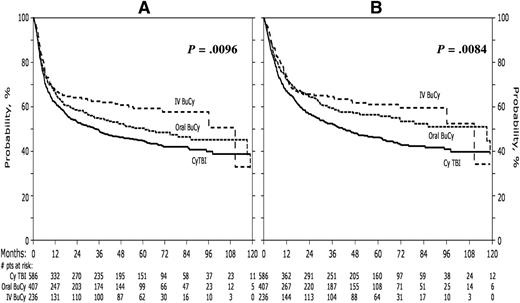
NRM occurred significantly less frequently among patients receiving IV Bu (relative risk [RR] = 0.58; 95% CI: 0.39-0.86; P = .0066; Table 3) compared with TBI. The proportional hazards assumption of the main effect in relapse was violated. Thus the Cox model for relapse was constructed breaking the posttransplant time course into 2 periods: less than or equal to 1 year and greater than 1 year. The incidence of relapse within the first year following transplant did not differ among the groups, however, relapse beyond the first year occurred significantly less frequently in those receiving IV, but not oral, Bu compared with TBI (RR = 0.23; 95% CI: 0.08-0.65; P = .006). LFS (RR = 0.70; 95% CI: 0.55-0.88; P = .0028) and OS (RR = 0.68; 95% CI: 0.52-0.88; P = .0034) were significantly higher in patients receiving IV Bu compared with TBI (Figure 2). OS was not significantly better
Multivariate analysis
| . | Oral BuCy vs Cy/TBI RR (95% CI) . | IV BuCy vs Cy/TBI RR (95% CI) . | Oral BuCy vs IV BuCy RR (95% CI) . | Overall P value . |
|---|---|---|---|---|
| aGVHD III-IV* | 0.72 (0.53-0.98) | 0.72 (0.51-1.02) | 1.00 (0.67-1.49) | .048 |
| cGVHD† | 1.01 (0.73-1.39) | 1.13 (0.87-1.47) | 0.89 (0.58-1.37) | .65 |
| NRM‡ | 0.72 (0.52-1.01) | 0.58 (0.39-0.86) | 1.24 (0.79-1.95) | .011 |
| Relapse (≤1 y)§ | 0.81 (0.59-1.11) | 1.06 (0.76-1.49) | 0.76 (0.52-1.12) | .31 |
| Relapse (>1 y)§ | 1.22 (0.78-1.90) | 0.24 (0.084-0.66) | 5.19 (1.85-14.57) | .0074 |
| LFS‖ | 0.87 (0.72-1.06) | 0.70 (0.55-0.88) | 1.25 (0.96-1.62) | .0096 |
| OS‖ | 0.78 (0.60-1.01) | 0.68 (0.52-0.88) | 1.15 (0.85-1.56) | .0084 |
| . | Oral BuCy vs Cy/TBI RR (95% CI) . | IV BuCy vs Cy/TBI RR (95% CI) . | Oral BuCy vs IV BuCy RR (95% CI) . | Overall P value . |
|---|---|---|---|---|
| aGVHD III-IV* | 0.72 (0.53-0.98) | 0.72 (0.51-1.02) | 1.00 (0.67-1.49) | .048 |
| cGVHD† | 1.01 (0.73-1.39) | 1.13 (0.87-1.47) | 0.89 (0.58-1.37) | .65 |
| NRM‡ | 0.72 (0.52-1.01) | 0.58 (0.39-0.86) | 1.24 (0.79-1.95) | .011 |
| Relapse (≤1 y)§ | 0.81 (0.59-1.11) | 1.06 (0.76-1.49) | 0.76 (0.52-1.12) | .31 |
| Relapse (>1 y)§ | 1.22 (0.78-1.90) | 0.24 (0.084-0.66) | 5.19 (1.85-14.57) | .0074 |
| LFS‖ | 0.87 (0.72-1.06) | 0.70 (0.55-0.88) | 1.25 (0.96-1.62) | .0096 |
| OS‖ | 0.78 (0.60-1.01) | 0.68 (0.52-0.88) | 1.15 (0.85-1.56) | .0084 |
Other significant factors in the multivariate model are noted by symbol. Bold indicates P value < .05. aGVHD, acute GVHD; cGVHD, chronic GVHD.
*Sex, donor type.
Donor type, recipient age, donor and recipient sex match, and graft type.
Recipient age, KPS, donor type, time from diagnosis to transplant.
KPS and Cytogenetic risk group.
Recipient age, KPS, donor type, Cytogenetic risk group.
Adjusted probabilities of LFS and OS for all patients according to preparative regimen. LFS is represented in panel A. OS is represented in panel B.
Adjusted probabilities of LFS and OS for all patients according to preparative regimen. LFS is represented in panel A. OS is represented in panel B.
in patients receiving IV Bu compared with oral Bu nor for those receiving oral Bu compared with TBI.
In order to determine whether administration of higher radiation doses might contribute to the inferior outcomes with TBI, radiation dose was categorized as standard (≤12.5 Gy) or high-dose (>12.5 Gy) in a separate multivariate analysis (Table 4). High dose TBI was associated with significantly higher rates of NRM than standard dose TBI (RR = 1.46; 95% CI: 1.03-2.07; P = .032), but LFS and OS were not significantly different. IV, but not oral Bu, was associated with significantly less NRM than standard dose TBI (RR = 0.67; 95% CI: 0.46-0.97; P = .0032). Relapse beyond 1 year after transplantation occurred significantly less frequently following IV, but not oral, Bu compared with standard (RR = 0.25; 95% CI:0.09- 0.73; P = .011) or high dose TBI (RR = 0.21; 95% CI: 0.07-0.61; P = .0045). LFS (RR = 0.74; 95% CI: 0.58-0.95; P = .016) and overall survival (RR = 0.72; 95% CI: 0.55-0.92; P = .0097) were significantly better for IV, but not oral, Bu than for standard dose TBI. No interactions between the main effect and the covariates in the final model, including donor type (P = .04 for survival and P = .11 for LFS) and age (P = .43 for survival and P = .22 for LFS) were significant.
Multivariate analysis (TBI dose divided into standard and high dose)
| . | Oral BuCy vs TBI≤1250 RR (95% CI) . | IV BuCy vs TBI≤1250 RR (95% CI) . | TBI>1250 vs TBI≤1250 RR (95% CI) . | Oral BuCy vs IV BuCy RR (95% CI) . | Oral BuCy vs TBI>1250 RR (95% CI) . | IV BuCy vs TBI>1250 RR (95% CI) . | Overall P value . |
|---|---|---|---|---|---|---|---|
| aGVHD III-IV* | 0.72 (0.52-1.00) | 0.72 (0.50-1.04) | 0.99 (0.71-1.39) | 1.00 (0.67-1.49) | 0.73 (0.49-1.07) | 0.73 (0.48-1.09) | .11 |
| cGVHD† | 0.99 (0.73-1.34) | 1.28 (0.98-1.67) | 1.40 (1.10-1.79) | 0.78 (0.54-1.11) | 0.71 (0.54-0.92) | 0.91 (0.66-1.25) | .0058 |
| NRM‡ | 0.83 (0.59-1.14) | 0.67 (0.46-0.97) | 1.46 (1.03-2.07) | 1.24 (0.79-1.93) | 0.56 (0.36-0.87) | 0.46 (0.27-0.76) | .015 |
| Relapse (≤year)§ | 0.77 (0.55-1.08) | 1.01 (0.71-1.45) | 0.85 (0.58-1.28) | 0.76 (0.52-1.12) | 0.90 (0.59-1.36) | 0.90 (0.59-1.36) | .40 |
| Relapse (>1 y)§ | 1.31 (0.80-2.18) | 0.25 (0.09-0.73) | 1.23 (0.67-2.27) | 5.19 (1.85-14.57) | 1.07 (0.60-1.91) | 0.21 (0.07-0.61) | .017 |
| LFS‖ | 0.92 (0.74-1.13) | 0.74 (0.58-0.95) | 1.16 (0.93-1.45) | 1.25 (0.96-1.62) | 0.79 (0.62-1.01) | 0.63 (0.48-0.83) | .011 |
| OS‖ | 0.82 (0.66-1.02) | 0.72 (0.55-0.92) | 1.18 (0.94-1.47) | 1.15 (0.87-1.51) | 0.69 (0.54-0.90) | 0.61 (0.46-0.80) | .0017 |
| . | Oral BuCy vs TBI≤1250 RR (95% CI) . | IV BuCy vs TBI≤1250 RR (95% CI) . | TBI>1250 vs TBI≤1250 RR (95% CI) . | Oral BuCy vs IV BuCy RR (95% CI) . | Oral BuCy vs TBI>1250 RR (95% CI) . | IV BuCy vs TBI>1250 RR (95% CI) . | Overall P value . |
|---|---|---|---|---|---|---|---|
| aGVHD III-IV* | 0.72 (0.52-1.00) | 0.72 (0.50-1.04) | 0.99 (0.71-1.39) | 1.00 (0.67-1.49) | 0.73 (0.49-1.07) | 0.73 (0.48-1.09) | .11 |
| cGVHD† | 0.99 (0.73-1.34) | 1.28 (0.98-1.67) | 1.40 (1.10-1.79) | 0.78 (0.54-1.11) | 0.71 (0.54-0.92) | 0.91 (0.66-1.25) | .0058 |
| NRM‡ | 0.83 (0.59-1.14) | 0.67 (0.46-0.97) | 1.46 (1.03-2.07) | 1.24 (0.79-1.93) | 0.56 (0.36-0.87) | 0.46 (0.27-0.76) | .015 |
| Relapse (≤year)§ | 0.77 (0.55-1.08) | 1.01 (0.71-1.45) | 0.85 (0.58-1.28) | 0.76 (0.52-1.12) | 0.90 (0.59-1.36) | 0.90 (0.59-1.36) | .40 |
| Relapse (>1 y)§ | 1.31 (0.80-2.18) | 0.25 (0.09-0.73) | 1.23 (0.67-2.27) | 5.19 (1.85-14.57) | 1.07 (0.60-1.91) | 0.21 (0.07-0.61) | .017 |
| LFS‖ | 0.92 (0.74-1.13) | 0.74 (0.58-0.95) | 1.16 (0.93-1.45) | 1.25 (0.96-1.62) | 0.79 (0.62-1.01) | 0.63 (0.48-0.83) | .011 |
| OS‖ | 0.82 (0.66-1.02) | 0.72 (0.55-0.92) | 1.18 (0.94-1.47) | 1.15 (0.87-1.51) | 0.69 (0.54-0.90) | 0.61 (0.46-0.80) | .0017 |
Other significant factors in the multivariate model are noted by symbol. Bold indicates P value < .05. Abbreviations are explained in Table 3.
Sex, donor type.
Recipient age, donor and recipient sex match, and graft type.
Recipient age, KPS, donor type, time from diagnosis to transplant.
KPS and Cytogenetic risk group.
Recipient age, KPS, donor type, Cytogenetic risk group.
Discussion
Historically, Cy/TBI has been the standard myeloablative preparative regimen for HCT. Despite extensive analysis, there had been no previous evidence that BuCy might yield better LFS or OS than Cy/TBI in patients with AML, and some suggestion that it leads to worse outcomes.8,9,14-17
It is not clear from some reports, however, that oral Bu was optimally administered: every 6 hours (as opposed to shorter and/or varied time periods between doses) and with an extended interval between the last Bu dose and the first Cy dose,40 as was recognized by the investigators of 1 such study.14 It is also unclear whether guidelines for adjustment of Bu dose in overweight patients and for administration of other drugs, which might affect Bu metabolism,28,31 were consistently followed. In addition, many of the patients receiving Bu in these studies did so early in the learning curves of participating institutions. Most importantly though, all of these published prospective studies were performed using orally administered, fixed dose Bu, following which, extensive inter-patient variation in absorption and metabolism can lead to 3-fold differences in Bu exposure.18-21,28,31,40,41 By decreasing variability in plasma levels, the use of IV and/or dose-adjusted Bu appears to improve results, although the clinical impact of these approaches has not been adequately measured.
In the present study, multivariate analysis revealed significantly better outcomes with IV Bu than with TBI. NRM and relapse beyond 1 year occurred significantly less frequently and LFS and survival were significantly better following IV, but not oral, Bu compared with TBI. In order to determine whether greater toxicity with more intensive TBI regimens might have contributed to these outcomes, the TBI group was divided into those receiving standard or high dose TBI. IV Bu maintained its association with lower incidences of NRM and relapse beyond 1 year and better LFS and OS compared with standard dose TBI. Interaction tests to analyze outcomes based on age and whether the donor was an HLA- identical sibling or matched URD were not statistically significant, indicating that the difference in outcomes among the preparative regimens does not depend on patient age or donor type. Further, hematopoietic recovery among patients with unrelated donors was similar among the groups, supporting the effectiveness of Bu in matched unrelated, as well as related donor transplantation.
A recent prospective observational cohort study of patients with AML, myelodysplastic syndrome, or chronic myeloid leukemia in various stages of disease undergoing matched related or unrelated donor transplants after myeloablative conditioning demonstrated better 2 -year survival following IV Bu (in combination with Cy or fludarabine) compared with TBI (in combination with Cy or etoposide).42 Although the patient population and preparative regimens were much more heterogeneous and follow up was comparatively brief compared with our study, the improved overall survival reinforces the results reported here.
While the substantial difference in NRM between IV Bu and TBI is the primary factor in improved LFS and OS, the data indicating a decreased incidence of relapse beyond 1 year are intriguing. Bu is effective in noncycling cells43 including primitive hematopoietic progenitors.44 Evidence from animal models,45 including a non-human primate model of human transplantation,46 indicates that Bu is a potent stem cell toxin. In the primate transplant model, quiescent primitive hematopoietic cells which survive Bu treatment require many months to contribute to hematopoiesis.47 Relapse beyond a year after transplantation may result from the recovery of injured quiescent primitive leukemic cells. By reducing the variability in drug exposure, IV administration may more effectively eradicate these primitive, quiescent, drug-resistant leukemic precursors.
Since information regarding pharmacokinetic (PK) data were not collected, it is unclear to what extent dose-adjustment of oral or IV Bu contributed to the reported outcomes. In 2008, more than 60% of patients reported to the CIBMTR who received oral Bu and 50% of those receiving IV Bu had PK data (X.Z., CIBMTR, personal communication), suggesting that many patients in the present study likely had PK data and dose adjustment. We are unable to assess their impact. This study did not address the issue of whether PK levels with dose adjustment should be performed with IV Bu, but this approach could further improve results.28,41 It appears likely that improvements in Bu administration, including IV administration and dose adjustment, are responsible for differentially improved results with Bu which surpassed those achieved with TBI.
The considerable technical requirements of TBI and its lack of universal availability have contributed to making Bu an attractive alternative, including in developing countries. Oral Bu is considerably cheaper than the IV formulation and it is possible that PK monitoring and dose adjustment of orally administered Bu might yield similarly favorable results and be more cost effective than IV Bu, but this was not addressed in the present study.
It is important to recognize the limitations of this study. First, the study is retrospective and it is not known why individual patients were designated to receive specific preparative regimens. Second, patient characteristics vary among the groups for multiple factors including age, proportion of URD and the year in which transplantation was performed. Multivariate analyses were performed to correct for these differences. Variation in dose of TBI, Bu and Cy, and the use of ATG or alemtuzumab may also have influenced results.
Despite these limitations, the results reported here are important. The rates of NRM with Bu in this study are remarkably low, particularly for a registry study with so many participating institutions. Just as 12 Gy was chosen in preference to higher dose TBI based on lower NRM in the face of similar long-term survival, it would be logical to choose Bu over TBI because of its lower NRM, even if survival were similar. Survival and LFS, however, also clearly favor the use of Bu, as do ease of administration and previous evidence of a lower incidence of some delayed complications, including cataracts,15,48 impaired growth48 and some second malignancies49 compared with TBI. In the absence of a prospective randomized comparison of Cy/ TBI and BuCy using contemporary administration, dosing, and supportive care, we suggest that IV Bu should be used in preference to TBI in patients with AML in first CR undergoing HCT from matched related or matched unrelated donors.
There is an Inside Blood commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The CIBMTR is supported by Public Health Service Grant/Cooperative Agreement U24- CA76518 from the National Cancer Institute, the National Heart, Lung and Blood Institute, and the National Institute of Allergy and Infectious Diseases, a Grant/Cooperative Agreement 5U01HL069294 from the National Heart, Lung and Blood Institute and the National Cancer Institute, a contract HHSH234200637015C with Health Resources and Services Administration (HRSA/DHHS), 2 grants N00014-06-1-0704 and N00014-08-1-0058 from the Office of Naval Research, and grants from Allos, Inc., Amgen, Inc., Angioblast, Anonymous donation to the Medical College of Wisconsin, Ariad, Be the Match Foundation, Blue Cross and Blue Shield Association, Buchanan Family Foundation, CaridianBCT, Celgene Corporation, CellGenix, GmbH, Children’s Leukemia Research Association, Fresenius-Biotech North America, Inc., Gamida Cell Teva Joint Venture Ltd., Genentech, Inc., Genzyme Corporation, GlaxoSmithKline, Kiadis Pharma, The Leukemia & Lymphoma Society, The Medical College of Wisconsin, Millennium Pharmaceuticals, Inc., Milliman USA, Inc., Miltenyi Biotec, Inc., National Marrow Donor Program, Optum Healthcare Solutions, Inc., Otsuka America Pharmaceutical, Inc., Seattle Genetics, Sigma-τ Pharmaceuticals, Soligenix, Inc., Swedish Orphan Biovitrum, THERAKOS, Inc., and Wellpoint, Inc. The views expressed in this article do not reflect the official policy or position of the National Institutes of Health, the Department of the Navy, the Department of Defense, or any other agency of the U.S. Government.
Authorship
Contribution: E.A.C., B.K.H., W.S. designed the study, analyzed results and wrote the manuscript; K.W.A. and X.Z. provided the statistical support; B.A., B.J.B., M. Aljurf, K.v.B., C.N.B., J.-Y.C., L.J.C., M.d.L., R.P.G., G.A.H., J.H., M.H., Y.I., R.T.K., M.R.L., A.W.L., D.I.M., E.O., V.R., M. Sabloff, B.N.S., M. Seftel, H.C.S., C.U., E.K.W., D.J.W., B.W., M.M.H., M. Arora, J.S., J.C., M.E.K., and R.T.M. interpreted data and critically reviewed the manuscript; and all authors approved the final manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Edward A. Copelan, Levine Cancer Institute, 1021 Morehead Medical Dr, Suite 3100, Charlotte, NC, 28204; e-mail: edward.copelan@carolinashealthcare.org.