Key Points
The tumor suppressor PP2A is repressed in Jak2V617F-driven myleoproliferative neoplasms by a Jak2/PI3K/PKC/SET signaling pathway.
PP2A-activating (eg, FTY720, OSU-2S) but not sphingosine-1-phosphate agonistic (eg, FTY720-P) drugs selectively kill Jak2V617F+ cells.
Abstract
FTY720 (Fingolimod, Gilenya) is a sphingosine analog used as an immunosuppressant in multiple sclerosis patients. FTY720 is also a potent protein phosphatase 2A (PP2A)–activating drug (PAD). PP2A is a tumor suppressor found inactivated in different types of cancer. We show here that PP2A is inactive in polycythemia vera (PV) and other myeloproliferative neoplasms characterized by the expression of the transforming Jak2V617F oncogene. PP2A inactivation occurs in a Jak2V617F dose/kinase-dependent manner through the PI-3Kγ-PKC–induced phosphorylation of the PP2A inhibitor SET. Genetic or PAD-mediated PP2A reactivation induces Jak2V617F inactivation/downregulation and impairs clonogenic potential of Jak2V617F cell lines and PV but not normal CD34+ progenitors. Likewise, FTY720 decreases leukemic allelic burden, reduces splenomegaly, and significantly increases survival of Jak2V617F leukemic mice without adverse effects. Mechanistically, we show that in Jak2V617F cells, FTY720 antileukemic activity requires neither FTY720 phosphorylation (FTY720-P) nor SET dimerization or ceramide induction but depends on interaction with SET K209. Moreover, we show that Jak2V617F also utilizes an alternative sphingosine kinase-1–mediated pathway to inhibit PP2A and that FTY720-P, acting as a sphingosine-1-phosphate-receptor-1 agonist, elicits signals leading to the Jak2-PI-3Kγ-PKC-SET–mediated PP2A inhibition. Thus, PADs (eg, FTY720) represent suitable therapeutic alternatives for Jak2V617F MPNs.
Introduction
FTY720 is an oral sphingosine analog used in relapsing multiple sclerosis patients for its immunosuppressive activity, which depends on lymphocyte sequestration to the lymph nodes. FTY720 undergoes phosphorylation (FTY720-P) by sphingosine kinase 2 (SPHK2) to act as an immunosuppressant, and binds/internalizes the sphingosine-1-phosphate receptor (S1PR1).1 FTY720 also selectively induces apoptosis of neoplastic but not normal cells2 ; this anticancer activity does not require phosphorylation but mostly depends on its ability to activate protein phosphatase 2A (PP2A).2 In Philadelphia-positive (Ph+) leukemias, PP2A-activating drugs (PADs; eg, FTY720) promote breakpoint cluster region (BCR)–ABL1 inactivation/degradation, inhibition of survival factors, and therefore, apoptosis of Ph+ blasts.3,4 In vivo, FTY720 treatment translates into toxicity-free long-term survival of leukemic animals.3
Ph− myeloproliferative neoplasms (MPNs), including almost all polycythemia vera (PV) and ∼60% essential thrombocythemia (ET) and primary myelofibrosis (PMF), express a constitutively active Jak2 kinase (Jak2V617F), which induces a PV-like syndrome in mice and, perhaps, also contributes to ET and PMF pathogenesis. Jak2V617F transforms bone marrow (BM) stem/progenitor cells5-12 by aberrantly activating pathways (eg, signal transducer and activator of transcription, extracellular signal-regulated kinase–1/2, PI-3K/Akt), transducing mitogenic/survival signals leading to cytokine (eg, erythropoietin)–independent growth of erythroid progenitors.6,11,13-17 Inhibition of Jak2 with tyrosine kinase inhibitors (TKIs) is effective in PV animal models and reduces splenomegaly in patients but does not decrease leukemic allele burden or BM fibrosis, and because of the nonselectivity for mutated Jak2, TKI treatment is often accompanied by anemia and thrombocytopenia.18-20 Moreover, increasing TKI dosage does not improve outcome, suggesting that MPN-initiating clone(s) are insensitive to Jak2 inhibition and that Jak2-independent genetic and epigenetic processes may cooperate with Jak2V617F for MPN induction and maintenance.21,22 Thus, better understanding of the biology of Jak2V617F+ MPNs is essential for the development of more successful therapies.
Here we show that PP2A tumor suppressor activity is inhibited in MPNs by the Jak2V617F/PI-3Kγ/PKC-induced SET phosphorylation. Reactivation of PP2A by PADs (FTY720 and its non-immunosuppressive derivatives) exerts strong antileukemic activity in primary CD34+ PV progenitors, Jak2V617F+ cell lines, and Jak2V617F+ leukemic animals without toxicity toward normal cells/organs. FTY720’s anticancer activity, which relies on inactivation/downregulation of PP2A targets (eg, Jak2V617F), depends on interaction/sequestration of the PP2A inhibitor SET but does not require conversion into FTY720-P that, unexpectedly, seems to favor oncogenic Jak2 signaling by inhibiting PP2A upon acting as a S1PR1 agonist.
Methods
Cells and clonogenic assays
Nonidentifiable Jak2V617F MPN (BM) and peripheral blood (PB) patient samples were obtained from The Ohio State University (OSU) Comprehensive Cancer Center (Columbus, OH), MD Anderson Cancer Center (Houston, TX), Hammersmith Hospital (London, UK), and Memorial Sloan-Kettering Cancer Center (New York, NY) leukemia tissue banks. Frozen samples of healthy donor CD34+ BM cells (NBM) were purchased from Cincinnati Children’s Hospital (Cincinnati, OH). Primary cells, murine pro-B Ba/F3, the human erythroleukemia TF-1 and HEL cell lines, and their derivatives were cultured, retro/lentivirally transduced, and selected as described in the supplemental Data, found on the Blood Web site. All studies with human specimens were conducted in accordance with the Declaration of Helsinki and were performed with The OSU Institutional Review Board approval. Colony-forming cell (CFC) assays were carried out by plating 103 cells from Jak2V617F cell lines or 104 CD34+ PV cells in 0.9% methylcellulose (MethoCult M3234 or H4435; Stem Cell Technologies, Inc., Vancouver, BC, Canada). Colonies (>125 µm) were scored 7 and 15 days later, respectively.
Chemical and biological reagents
Cells were treated with the kinase, phosphatase, or sphingolipid pathway inhibitors or activators (see details in the supplemental Data) used at concentrations, times, and schedules indicated in the Results section. The origin and subcloning strategies for the plasmids MSCV-puro-Jak2(V617F), MigR1-Jak2(wild-type [WT] and V617F), MigRI-HA-PP2Ac, pLL3.7-shSET, pCDH-FLAG-SET, and SET point-mutants are described in detail in the supplemental Data.
In vivo studies
Four- to 6-week-old immunocompromised imprinting control region–severe combined immunodeficiency (SCID) mice (n = 50) were intravenously (IV) injected with 105 Ba/F3-Jak2V617F cells (n = 30) or used as controls (n = 20). After engraftment (1 week), 15 mice were intraperitoneally FTY720-treated daily, and 15 mice were vehicle (saline solution) treated. As controls, 10 age-matched SCID mice received neither cells nor treatment, and the other 10 received FTY720 at the same regimen. At 5-week posttransplant, 3 mice/group (censored), respectively, were sacrificed, and organs (BM, spleen, liver, kidney, and heart) were subjected to macroscopic and microscopic (hematoxylin and eosin [H&E] staining) evaluation of leukemic-cell infiltration. At 8 weeks posttransplant, organs from the last 4 (2 just died and 2 moribund were sacrificed) untreated and 4 FTY720-treated (censored) leukemic mice were subject to histopathologic analysis. PB was collected by retro-orbital survival bleeding for SNaPshot analysis of Jak2 alleles (see supplemental Data). Following 10 weeks of treatment, untreated (age matched) and FTY720-treated (10 weeks; 10 mg/kg/d) control mice (n = 3 per group) were used to assess the effect of long-term FTY720 treatment on cardiac function (see supplemental Data). Kaplan-Meier survival analysis was performed on untreated (n = 10) and FTY720-treated (n = 8) cell-injected and FTY720-treated age-matched (n = 7) animals, because 5 untreated and 7 FTY720-treated leukemic animals were censored, as described above. All the animal protocols/procedures were approved by OSU Institutional Animal Care and Use Committee.
Immunoblots and protein-based assays
Cell lysates (see supplemental Data for details) in radioimmunoprecipitation assay buffer were used in immunoblotting with the monoclonal antibodies: anti-SET/I2PP2A (clone H-120; Santa Cruz Biotechnology, Santa Cruz, CA), -Jak2 (Cell Signaling Technology, Beverly, MA), -pJak2Y1007/1008 (Cell Signaling), -pJak2Y972 (Millipore, Billerica, MA), -PP2Ac (Upstate, Billerica, MA), - pPP2AcY307 (Epitomics, Burlingame, CA), and -Grb2 (BD Biosciences, Franklin Lakes, NJ). For immunoprecipitation and to detect the SET-FTY720 interaction, cell lysates (1 mg) in radioimmunoprecipitation assay buffer were incubated for 1 hour at 4°C with either the primary antibody or FTY720-phenoxy-biotin (Cayman Chemical, Ann Arbor, MI) followed by the anti-aminonitrobenzoxadiazoles (NBD) antibody (see supplemental Data for details). For SET dimerization assays, lysates of Ba/F3 expressing FLAG-SET and green fluorescent protein (GFP)-SET cells were coincubated and used in anti-FLAG immunoprecipitations and anti-GFP immunoblotting (see supplemental Data). PP2A, sphingosine kinase activities, DAG kinase-mediated ceramide quantitation, and mass spectrometry of ceramide subspecies are described in the supplemental Data.
Statistical analysis
For in vitro studies, data were statistically compared with the 2-tailed Student t test. For animal studies, the estimated probabilities for survival were calculated with the Kaplan-Meier method, and the log-rank test was used to evaluate the differences among survival distributions. A P value of less than .01 (mouse model P < .05) was considered statistically significant.
Results
PP2A tumor suppressor activity is inhibited in MPNs in a Jak2V617F/SET-dependent manner
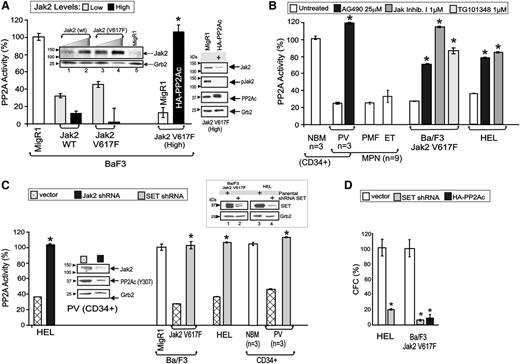
To evaluate the role of PP2A in Jak2V617F-expressing cells, interleukin -3–dependent Ba/F3 progenitors were transduced with WT or V617F Jak2 and sorted for high and low GFP expression to obtain cells with graded Jak2 expression levels (Figure 1A, inset). PP2A assays showed that Jak2 overexpression significantly decreased PP2A activity with decrease of phosphatase activity (∼55% vs ∼99% inhibition) (Figure 1A). Notably, PP2A inhibition was similar in Jak2 (WT)– and Jak2V617F-expressing cells; however, only the Ba/F3-Jak2V617F (high) cells acquired cytokine independence. In these cells, restoration of PP2A activity by ectopic HA-PP2Ac expression resulted in decreased Jak2V617F activity/expression (Figure 1A) and, consequently, suppression of cytokine-independent survival, as indicated by the ∼90% reduction of their clonogenic potential (Figure 1D). PP2A assays with CD34+ progenitors or mononuclear cells (MNCs) from the BM or PB cells from healthy individuals (n = 3) and PV, MF, and ET patients (n = 9) revealed that marked reduction (∼80% inhibition) of PP2A activity is common in MPNs carrying a Jak2V617F allele (Figure 1B). Likewise, inhibition of Jak2 by treatment of the Ba/F3-Jak2V617F and the Jak2V617F homozygous human embryo lung (HEL) cells—and primary PV CD34+ progenitors (n = 3) with the Jak inhibitors AG490 (25 µM), Jak inhibitor I (1 µM), and TG101348 (1 μM)—restored PP2A activity to levels similar to those detected in CD34+ BM progenitors from healthy individuals and nontransformed Ba/F3 cells (Figure 1A-B). Accordingly, shRNA-mediated Jak2V617F (Jak2–short hairpin RNA [shRNA]) downmodulation (∼75% decrease) in both HEL cells and CD34+ PV progenitors rescued PP2A activity, as demonstrated by the PP2A assay (∼65% increase) and decreased levels (∼73% inhibition) of Y307-phosphorylated PP2Ac (inactive2 ), respectively (Figure 1C). To elucidate the mechanism whereby Jak2V617F suppresses PP2A activity, we expressed shRNAs in CD34+ PV progenitors, Ba/F3-Jak2V617F, and HEL cells to negatively modulate expression of the direct PP2A inhibitor SET.4,23,24 Lentiviral-mediated SET knock-down (Figure 1C, inset) fully restored PP2A activity in all cell types (Figure 1C). Consistent with the proapoptotic role of active PP2A, Ba/F3-Jak2V617F+ and HEL cells expressing SET shRNA had a 91% and 80% reduction in CFCs, respectively (Figure 1D). Thus, Jak2V617F uses SET to inactivate PP2A, thereby maintaining survival of leukemic cells.
Jak2V617F suppresses PP2A activity in a SET-dependent manner. (A) Graph shows PP2A activity in Ba/F3 cells transduced with the MigR1 vector (open bars), Ba/F3 cells expressing low (light gray bars) and high (solid bars) levels of WT and V617F Jak2 kinase, and in HA-PP2A–expressing Ba/F3-Jak2V617F cells. Inset shows Jak2 levels in Ba/F3 cells transduced with WT or V617F Jak2, and sorted for high and low GFP expression. Western blots (right) show Jak2 expression and activity and PP2Ac expression in vector- and HA-PP2A–transduced Ba/F3-Jak2V617F cells. (B) PP2A activity in untreated and Jak inhibitor (25 µM AG490; 1 µM Jak inhibitor I; 1 µM TG101348)–treated primary CD34+ NBM, PV, PMF, and ET patient samples, Ba/F3-Jak2V617F and HEL cells. (C) PP2A activity in Jak2 shRNA-expressing HEL cells and SET shRNA-expressing Ba/F3-Jak2V617F, HEL and primary CD34+ PV cells. Inset shows levels of inactive PP2Ac (pY307) in Jak2 shRNA-expressing PV CD34+ progenitors. SET downmodulation in SET shRNA-expressing Ba/F3-Jak2V617F and HEL cells (top right). (D) Percentage of CFC in HEL and Ba/F3-Jak2V617F cells transduced with empty vector (open bars) or with the SET shRNA (light gray bars), and in HA-PP2Ac–transduced Ba/F3-Jak2V617F cells (solid bars). *P ≤ .01.
Jak2V617F suppresses PP2A activity in a SET-dependent manner. (A) Graph shows PP2A activity in Ba/F3 cells transduced with the MigR1 vector (open bars), Ba/F3 cells expressing low (light gray bars) and high (solid bars) levels of WT and V617F Jak2 kinase, and in HA-PP2A–expressing Ba/F3-Jak2V617F cells. Inset shows Jak2 levels in Ba/F3 cells transduced with WT or V617F Jak2, and sorted for high and low GFP expression. Western blots (right) show Jak2 expression and activity and PP2Ac expression in vector- and HA-PP2A–transduced Ba/F3-Jak2V617F cells. (B) PP2A activity in untreated and Jak inhibitor (25 µM AG490; 1 µM Jak inhibitor I; 1 µM TG101348)–treated primary CD34+ NBM, PV, PMF, and ET patient samples, Ba/F3-Jak2V617F and HEL cells. (C) PP2A activity in Jak2 shRNA-expressing HEL cells and SET shRNA-expressing Ba/F3-Jak2V617F, HEL and primary CD34+ PV cells. Inset shows levels of inactive PP2Ac (pY307) in Jak2 shRNA-expressing PV CD34+ progenitors. SET downmodulation in SET shRNA-expressing Ba/F3-Jak2V617F and HEL cells (top right). (D) Percentage of CFC in HEL and Ba/F3-Jak2V617F cells transduced with empty vector (open bars) or with the SET shRNA (light gray bars), and in HA-PP2Ac–transduced Ba/F3-Jak2V617F cells (solid bars). *P ≤ .01.
Jak2V617F-dependent and PI-3Kγ/PKC–mediated SET phosphorylation shuts down PP2A
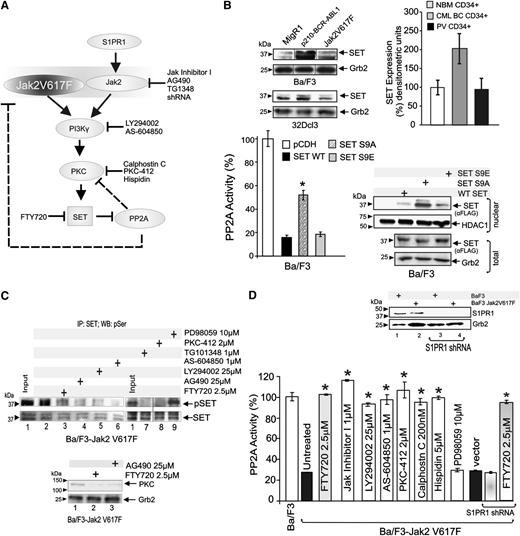
In chronic myelogenous leukemia (CML), PP2A inhibition depends on the BCR-ABL1-induced overexpression of SET4,24 (Figure 2B, top); however, Jak2V617F expression in 32Dcl3 (Figure 2B, top), Ba/F3 (Figure 2B, top), and TF-1 (not shown) cells did not increase SET levels. Accordingly, SET expression was similar in CD34+ progenitors form PV patients (n = 3) and healthy individuals (NBM), whereas, as expected,4,24 it was increased in CD34+ progenitors from CML-blast crisis (CML-BC) (n = 3) patients (Figure 2B, top), suggesting that Jak2V617F-induced posttranslational modification(s) control(s) SET ability to inhibit PP2A in MPNs. Reportedly, SET undergoes PI-3Kγ–mediated serine 9 and 24 phosphorylation by PKC that controls SET nuclear export and, therefore, its ability to act as a inhibitor of PP2A.23,25,26 Thus, Jak2V617F-generated signals may suppress PP2A activity through the PI-3Kγ-PKC–mediated SET phosphorylation on these serine residues (Figure 2A). In Ba/F3 cells, ectopic expression of WT and phosphomimetic mutation of SET serine 9 into glutamic acid (S9E) resulted in an ∼80% inhibition of PP2A activity (Figure 2B, bottom left). Conversely, only a ∼40% decrease was observed upon ectopic expression of the nonphosphorylatable SET S9A mutant (Figure 2B, bottom left) that, as expected, was mostly nuclear localized (Figure 2B, bottom right). Because PP2A activity was not significantly affected upon expression of SET serine 24 mutants (not shown), it is highly plausible that serine 9 phosphorylation accounts for most of the SET inhibitory function on PP2A. Accordingly, Ba/F3-Jak2V617F treatment with the pan-PI-3K (LY294002; 25 µM, 6 hours), the selective PI-3Kγ (AS-604850; 1 µM, 6 hours), and 3 inhibitors of conventional PKCs (PKC-412, 2 µM, 6 hours; Hispidin, 5 μM, 6 hours; Calphostin C, 200 nM, 6 hours) markedly reduced SET serine phosphorylation (Figure 2C) and restored normal PP2A activity (Figure 2D). A similar effect was also observed upon treatment of Ba/F3-Jak2V617F cells with TG101348 (1 µM; 6 hours), AG490 (25 µM; 6 hours), and Jak inhibitor I (1 µM; 6 hours) (Figure 2C-D). Further supporting the existence of a Jak2V617F-PI-3Kγ/PKC-SET axis, both Jak2 inhibition and FTY720 impaired expression of conventional PKCs (Figure 2C, bottom), and FTY720 also reduced SET phosphorylation (Figure 2C, top). As expected, inhibition of a nonrelated pathway (eg, mitogen-activated protein kinase) by the mitogen-activated protein kinase inhibitor PD98059 (10 µM; 6 hours) did not alter SET phosphorylation or PP2A activity (Figure 2C-D). Reportedly, activation of the S1PR1 activates Jak2, which recruits PI-3K into a PP2A-containing complex, thereby resulting in PP2A inhibition.27-29 Although S1PR1 levels were not altered by Jak2V617F expression (Figure 2D, inset), to determine whether S1PR1 contributes to PP2A inactivation through enhancement of Jak2(WT) signaling, S1PR1 shRNAs were expressed in Ba/F3-Jak2V617F cells (Figure 2D, inset). Inhibition of S1PR1 expression neither rescued PP2A activity nor halted FTY720 PP2A-activating function (Figure 2D), suggesting that the main mechanism used by Jak2V617F to suppress PP2A does not require S1PR1 and that the FTY720 PP2A-activating function is S1PR1-independent in Jak2V617F cells.
Jak2V617F-driven PI-3Kγ/PKC–mediated signals induce inhibition of PP2A through SET phosphorylation. (A) Molecular pathway model of Jak2/Jak2V617F-mediated PP2A inhibition. Chemical inhibitors are indicated in black. (B) SET and Grb2 protein levels in MigR1-, Jak2V617F-, and BCR-ABL1-expressing Ba/F3 and 32Dcl3 cells (upper left); SET protein levels in CD34+ BM progenitors from healthy individuals (NBM) and PV and CML-BC patients (n = 3 each) (upper right). PP2A activity in Ba/F3 cells transduced with empty vector (pCDH), WT, nonphosphorylatable S9A, and phosphomimetic S9E mutant SET FLAG-tagged constructs (bottom panel). Inset shows ectopic WT and mutant SET proteins (nuclear fraction). (C) Levels of serine phosphorylated SET (pSET) in SET immunoprecipitates (SET) from lysates of untreated, kinase (Jak2, PI-3K, PI-3Kγ, PKC, and MEK1) inhibitors and FTY720-treated Ba/F3-Jak2V617F cells. (D) PP2A activity in untreated and drug-treated Ba/F3-Jak2V617F cells and in Ba/F3-Jak2V617F cells transduced with empty vector or expressing a S1PR1 shRNA and treated with 2.5 µM FTY720. Inset shows S1PR1 levels in parental and S1PR1 shRNA-expressing Ba/F3-Jak2V617F cells. *P ≤ .01.
Jak2V617F-driven PI-3Kγ/PKC–mediated signals induce inhibition of PP2A through SET phosphorylation. (A) Molecular pathway model of Jak2/Jak2V617F-mediated PP2A inhibition. Chemical inhibitors are indicated in black. (B) SET and Grb2 protein levels in MigR1-, Jak2V617F-, and BCR-ABL1-expressing Ba/F3 and 32Dcl3 cells (upper left); SET protein levels in CD34+ BM progenitors from healthy individuals (NBM) and PV and CML-BC patients (n = 3 each) (upper right). PP2A activity in Ba/F3 cells transduced with empty vector (pCDH), WT, nonphosphorylatable S9A, and phosphomimetic S9E mutant SET FLAG-tagged constructs (bottom panel). Inset shows ectopic WT and mutant SET proteins (nuclear fraction). (C) Levels of serine phosphorylated SET (pSET) in SET immunoprecipitates (SET) from lysates of untreated, kinase (Jak2, PI-3K, PI-3Kγ, PKC, and MEK1) inhibitors and FTY720-treated Ba/F3-Jak2V617F cells. (D) PP2A activity in untreated and drug-treated Ba/F3-Jak2V617F cells and in Ba/F3-Jak2V617F cells transduced with empty vector or expressing a S1PR1 shRNA and treated with 2.5 µM FTY720. Inset shows S1PR1 levels in parental and S1PR1 shRNA-expressing Ba/F3-Jak2V617F cells. *P ≤ .01.
FTY720 long-term treatment increases survival and decreases leukemia allelic burden without cardiologic toxicity
Reportedly, PADs have strong antileukemic activity and safety profile in animal models of acute and chronic leukemias.2,3 To evaluate the potential of using PADs and, specifically, FTY720 in Jak2V617F MPN patients, we used an aggressive mouse model of Jak2V617F leukemia in which each transplanted cytokine-independent Ba/F3-Jak2V617F cell represents a potential leukemia-initiating cell.30 SCID mice (n = 30) were IV injected with Ba/F3-Jak2V617F cells (5 × 105 cells/mouse). After 7 days, all mice were engrafted (presence in PB of GFP+ Jak2V617F cells) and FTY720 was intraperitoneally administered (10 mg/kg/d; LD50 = 300 mg/kg) to 15 mice. As controls, 10 age-matched mice only received daily FTY720 treatment, whereas 15 cell-injected mice were left untreated. After 4 weeks of FTY720 treatment (5 weeks posttransplant), Jak2V617F burden was assessed by SNaPshot assay on PB samples of untreated and FTY720-treated leukemic mice (n = 3 per group). A significant reduction of the V617F burden (red curves) and the almost sole expression of WT Jak2 allele (blue curves) was observed in FTY720-treated animals (Figure 3A, red box). As expected, the Jak2 allelic status was nearly 100% mutated in cells from PB of untreated leukemic animals (Figure 3A, gray box), and completely WT in PB isolated from an age-matched healthy control (Figure 3A, white box). Mice were thereafter sacrificed (censored in the Kaplan-Meier) and organs evaluated by visual inspection and light microscopy. Untreated Jak2V617F mice (gray) showed massive splenomegaly (n = 3 per group; P < .05), whereas spleens from FTY720-treated Ba/F3-Jak2V617F cell–injected mice (red) resembled those of control age-matched (white) or FTY720-only treated (green) mice (Figure 3B). H&E-stained sections of spleen, BM, and liver of Ba/F3-Jak2V617F mice showed extensive infiltration of blast cells with a low degree of maturation typical of an overt leukemia-like process (Figure 3C). In contrast, spleen, BM, liver, and kidney from the FTY720-treated leukemic mice were similar to those of the age-matched and FTY720-injected control groups (Figure 3C). Notably, cardiac and renal tissue did not appear infiltrated by Ba/F3-Jak2V617F cells (Figure 3C). Near the end of the 7th week of treatment (8 posttransplant), 2 untreated animals just died, and the last 2 were moribund (censored) and, therefore, have been sacrificed together with 4 FTY720-treated mice (censored) for histopathology evaluation. Marked splenomegaly and immature cell infiltration were observed in the untreated animals, and spleens of FTY720-treated Jak2V617F animals were similar to those of age-matched controls (not shown). Accordingly, survival of FTY720-treated leukemic mice was significantly increased with a median survival time of 10.4 weeks (P < .0001). In fact, all FTY720-treated mice were alive at 8 weeks after cell transplant, whereas untreated leukemic mice (median survival of 6.7 weeks) all succumbed (Figure 3D). As expected, all control mice were still alive after 22 weeks of daily FTY720 (10 mg/kg) treatment. Furthermore, no significant changes in body weight (not shown) and no signs of toxicity were observed in control or Ba/F3-Jak2V617F mice that received 10 mg/kg FTY720 for 22 weeks.
In vivo antileukemic effects of FTY720 and lack of toxicity in long-term treated animals. (A) Jak2V617F leukemic burden at 6 weeks after Ba/F3-Jak2V617F cell injection measured by SNapShot assay in untreated (gray square) and FTY720 -treated (red square) (5 weeks; 10 mg/kg/d) mice. Age-matched mice (white square) were used as a control. Levels of WT and V617F Jak2 alleles are shown by the blue and red curves, respectively. (B) Average spleen weights of untreated or FTY720-treated age-matched and leukemic mice. (C) H&E-stained sections of spleen, BM, liver, heart, and kidney of untreated (white square) and FTY720-treated (green square) age-matched control mice and, untreated (gray square) and FTY720-treated (red square) leukemic mice. (D) Kaplan-Meier curve shows survival of untreated leukemic (gray line), FTY720-treated leukemic (red line), and age-matched control (green line) mice. (E) Electrocardiographic and cardiomyocyte contractile performance in untreated or FTY720-treated (10 weeks) control mice. *P ≤ .05.
In vivo antileukemic effects of FTY720 and lack of toxicity in long-term treated animals. (A) Jak2V617F leukemic burden at 6 weeks after Ba/F3-Jak2V617F cell injection measured by SNapShot assay in untreated (gray square) and FTY720 -treated (red square) (5 weeks; 10 mg/kg/d) mice. Age-matched mice (white square) were used as a control. Levels of WT and V617F Jak2 alleles are shown by the blue and red curves, respectively. (B) Average spleen weights of untreated or FTY720-treated age-matched and leukemic mice. (C) H&E-stained sections of spleen, BM, liver, heart, and kidney of untreated (white square) and FTY720-treated (green square) age-matched control mice and, untreated (gray square) and FTY720-treated (red square) leukemic mice. (D) Kaplan-Meier curve shows survival of untreated leukemic (gray line), FTY720-treated leukemic (red line), and age-matched control (green line) mice. (E) Electrocardiographic and cardiomyocyte contractile performance in untreated or FTY720-treated (10 weeks) control mice. *P ≤ .05.
Because the U.S. Food and Drug Administration–approved FTY720 may in some cases mildly affect cardiac performance,31 contractile cardiomyocytes performance and electrocardiography in nondiseased animals treated long term (10 weeks) with FTY720 (10 mg/kg/d) were compared with those of untreated control mice. Representative traces (Figure 3E, left) showed that myocyte contraction (cell shortening and calcium transient amplitude) was unaffected by FTY720 treatment (Figure 3E, middle). In addition, isoproterenol stimulation (1 µM) produced the expected increase in amplitude, an effect that was unchanged by FTY720 (Figure 3E, middle). Likewise, FTY720 did not alter basal or isoproterenol-stimulated relaxation (cell relengthening and calcium transient time to 50% relaxation, RT50) (Figure 3E, right). Finally, the heart rate of treated mice remained identical to that of untreated mice. Bradycardia, which is one of the adverse effects given by the SPHK2-dependent conversion of FTY720 into the phosphorylated immunosuppressive FTY720-P32 (Figure 4A), was completely absent (Figure 3E, bottom).
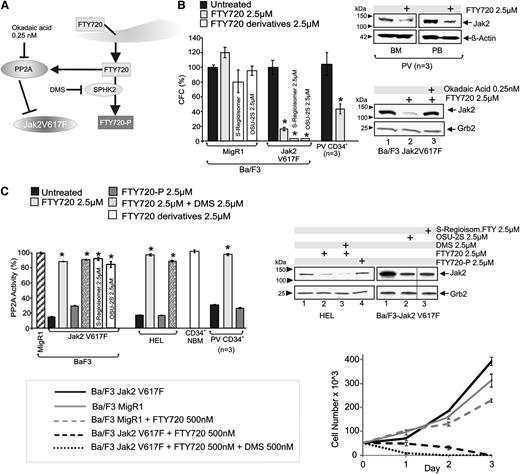
FTY720 phosphorylation is dispensable for its antileukemic activity on Jak2V617F cells. (A) Schematic representation of FTY720 conversion into FTY720-P and FTY720/PP2A interplay; inhibitors are indicated in black. (B) Effect of FTY720 and its non-immunosuppressive derivatives (OSU-2S and FTY720-S-regioisomer) on the clonogenic potential, expressed as percentage of CFCs, of Ba/F3, Ba/F3-Jak2V617F, and primary CD34+ PV cells (left). Jak2 expression in vehicle- and FTY720-treated CD34+ progenitors derived from the BM or PB of PV patients (top right). Jak2 expression in Ba/F3-Jak2V617F cells treated with the PP2A activator FTY720 alone or in combination with okadaic acid used at concentration (0.25 nM) that inhibits PP2A activity only (bottom right). (C) PP2A activity in Ba/F3-Jak2V617F, HEL, primary CD34+ NBM, and PV cells untreated or treated with FTY720 (2.5 µM) alone or in combination with the sphingosine kinase inhibitor DMS (2.5 µM), the immunosuppressive and S1PR1 agonist FTY720-P (2.5 µM), or with the nonimmunosuppressive FTY720 derivatives (FTY720-S-regioisomer and OSU-2S) (left). Jak2 protein levels in HEL cells untreated and treated with FTY720 (2.5 µM) alone or in combination with DMS (2.5 µM) or treated with FTY720-P (2.5 µM) (top right). Proliferation of Ba/F3-Jak2V617F cells (or Ba/F3-MigR1 used as a control) untreated or treated with FTY720 (500 nM) alone or in combination with DMS (500 nM) (bottom right).
FTY720 phosphorylation is dispensable for its antileukemic activity on Jak2V617F cells. (A) Schematic representation of FTY720 conversion into FTY720-P and FTY720/PP2A interplay; inhibitors are indicated in black. (B) Effect of FTY720 and its non-immunosuppressive derivatives (OSU-2S and FTY720-S-regioisomer) on the clonogenic potential, expressed as percentage of CFCs, of Ba/F3, Ba/F3-Jak2V617F, and primary CD34+ PV cells (left). Jak2 expression in vehicle- and FTY720-treated CD34+ progenitors derived from the BM or PB of PV patients (top right). Jak2 expression in Ba/F3-Jak2V617F cells treated with the PP2A activator FTY720 alone or in combination with okadaic acid used at concentration (0.25 nM) that inhibits PP2A activity only (bottom right). (C) PP2A activity in Ba/F3-Jak2V617F, HEL, primary CD34+ NBM, and PV cells untreated or treated with FTY720 (2.5 µM) alone or in combination with the sphingosine kinase inhibitor DMS (2.5 µM), the immunosuppressive and S1PR1 agonist FTY720-P (2.5 µM), or with the nonimmunosuppressive FTY720 derivatives (FTY720-S-regioisomer and OSU-2S) (left). Jak2 protein levels in HEL cells untreated and treated with FTY720 (2.5 µM) alone or in combination with DMS (2.5 µM) or treated with FTY720-P (2.5 µM) (top right). Proliferation of Ba/F3-Jak2V617F cells (or Ba/F3-MigR1 used as a control) untreated or treated with FTY720 (500 nM) alone or in combination with DMS (500 nM) (bottom right).
Conversion into FTY720-P is dispensable for FTY720 antileukemic activity in Jak2V617F cells
FTY720 efficiently restores PP2A activity in leukemic blasts (Figure 4A).2,3 Treatment of CD34+ PV progenitors (n = 3) and Ba/F3-Jak2V617F cells with FTY720 (2.5 µM) reduced the clonogenic potential by 62% and 84%, respectively (Figure 4B), without impairing that of Ba/F3 (Figure 4B) and CD34+ NBM3 cells. Consistent with the effect of ectopic HA-PP2Ac, SET shRNA and Jak2 shRNA expression (Figure 1), FTY720-induced decreased survival of Jak2V617F primary cells, and cell lines corresponded with restoration of physiological PP2A activity to levels similar to those in CD34+ NBM progenitors and parental Ba/F3 cells (Figure 4C), and Jak2V617F downregulation in primary PV CD34+ (BM and PB) progenitors and Ba/F3-Jak2V617F cells (Figure 4B, right). Notably, dependence of Jak2V617F downregulation on PP2A reactivation was confirmed by cotreatment with okadaic acid used at a concentration (0.25 nM) that inhibits PP2A activity only33 (Figure 4B). To assess whether PP2A activation requires intracellular reconversion of phosphorylated (immunosuppressive) FTY720 into its nonphosphorylated native form, we treated primary CD34+ PV progenitors (n = 3) and HEL and Ba/F3-Jak2V617F cells with phosphorylated FTY720 (FTY720-P; 2.5 µM, 6 hours). FTY720-P neither restored PP2A activity (Figure 4C, dark gray) nor triggered Jak2V617F downregulation (Figure 4C, right, lane 4). Accordingly, the sphingosine kinase inhibitor N,N-dimethylsphingosine (DMS) (2.5 µM), which prevents the de novo conversion of FTY720 to FTY720-P, did not impede FTY720-induced PP2A activation in Ba/F3-Jak2V617F and HEL cells (Figure 4C, left) but potentiated the FTY720-induced loss of Jak2V617F protein (Figure 4C, right, lane 3) and greatly enhanced the FTY720 (500 nM)–induced killing of Ba/F3-Jak2V617F cells (Figure 4C, bottom right), likely by effectively increasing the amount of active FTY720. As expected,3 MigR1-transduced Ba/F3 cells continued to proliferate when exposed to 500 nM FTY720 (Figure 4C, bottom right). To further demonstrate that conversion to FTY720-P is unnecessary for the FTY720 antitumor activity, we treated Ba/F3-Jak2V617F cells with 2 FTY720 derivatives (S-FTY720-regioisomer34 and OSU-2S35 ) that neither interact with S1PR1 nor undergo SPHK2-dependent phosphorylation and do not induce lymphopenia.36 Both nonphosphorylatable FTY720 derivatives restored PP2A activity, decreased Jak2V617F levels, and impaired clonogenic potential of Ba/F3-Jak2V617F but not of parental Ba/F3 cells (Figure 4B-C).
FTY720-P (immunosuppressive) augments Jak2 and suppresses PP2A activities
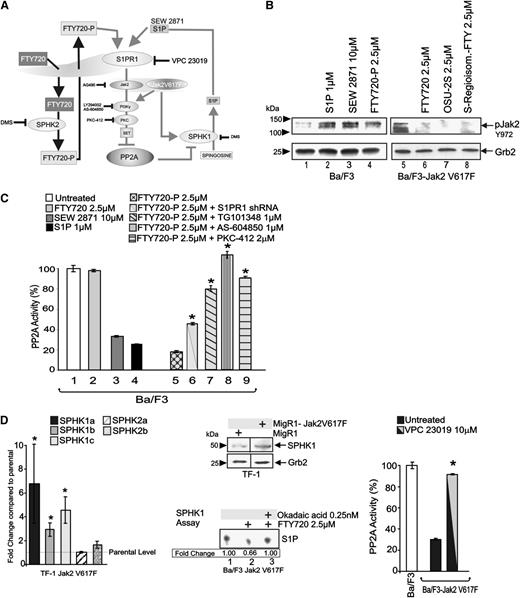
Although FTY720-P does not rescue PP2A, and downregulation of S1PR1 (a receptor mediating FTY720-P immunosuppressive activity on B and T cells1,37 ) does not prevent PP2A activation by FTY720 in Jak2V617F-transformed cells (Figure 2), it is unclear whether the FTY720-P/S1PR1 interaction elicits signals modulating FTY720 anticancer activity. Unexpectedly, we found that FTY720-P suppresses PP2A through a mechanism that closely parallels that of Jak2V617F in Ba/F3 progenitors (Figure 5A). In fact, treatment of Ba/F3 cells with FTY720-P (2.5 μM; 6 hours) strongly increased levels of Y972 phosphorylated (active) Jak2 (Figure 5B, lane 4) and suppressed PP2A activity (∼80% reduction) (Figure 5C, lane 5). An identical effect was also obtained upon exposure to the natural S1PR1-5 ligand S1P (1 µM; 6 hours) and to the S1PR1-specific agonist SEW 2871 (10 µM, 6 hours). Furthermore, shRNA-mediated S1PR1 downregulation dampened the FTY720-P inhibitory effect on PP2A (Figure 5C, lane 6), whereas treatment with OSU-2S and (S)-FTY720-regioisomer (2.5 μM; 6 hours) did not enhance but markedly inhibited active Jak2 levels in Ba/F3-Jak2V617F cells (Figure 5B, lanes 5-8). Interestingly, PP2A inhibition induced in Ba/F3 cells by FTY720-P was antagonized by cotreatment with TG101348 (1 µM), AS-604850 (1 µM), or PKC-412 (2 µM) (Figure 5C, lanes 7-9), suggesting that S1PR1 agonists (eg, FTY720-P) induce signals suppressing PP2A through activation of the Jak2(WT)/PI-3Kγ/PKC pathway.
FTY720-P promotes Jak2 and suppresses PP2A activities. (A) Schematic representation of the FTY720-P–induced and SIPR1/Jak2 (WT and V617F)–mediated PP2A inhibition. (B) Western blots show levels of active Jak2 (pY972) and Grb2 in parental Ba/F3 and Ba/F3-Jak2V617F cells untreated and treated with the S1PR1 agonists FTY720-P (2.5 µM), SEW 2871 (10 µM), and S1P (1 µM), and with the PP2A activators FTY720 (2.5 µM), OSU-2S (2.5 µM), and (S)-FTY720-regioisomer (2.5 µM), respectively. (C) PP2A activity in Ba/F3 cells untreated and treated with FTY720 (2.5 µM), SEW 2871 (10 µM), S1P (1 µM), FTY720-P (2.5 µM), and FTY720-P in combination with S1PR1 shRNA, TG101348 (1 µM), AS-604850 (1 µM), or PKC-412 (2 µM). (D) Effect of Jak2V617F expression on the messenger RNA levels (quantitative reverse-transcription polymerase chain reaction) of specific SPHK1/2 isoforms in TF-1 cells. Fold change are relative to levels of SPHK1 and SPHK2 isoforms in parental TF-1 cells (left). Effect of Jak2V617F expression on sphingosine kinase 1 (SPHK1) expression in TF-1 cells (middle top). SPHK1 kinase activity measured as fold change in the amount of sphingosine converted to sphingosine-1-phosphate (S1P) in Ba/F3-Jak2V617F cells untreated and treated with FTY720 (2.5 µM) alone or in combination with okadaic acid (0.25 nM) (middle bottom). PP2A activity in Ba/F3 cells and in parental and S1PR1 shRNA-expressing Ba/F3-Jak2V617F cells left untreated or treated with the transduced with the S1PR1 antagonist VPC 23019 (right).
FTY720-P promotes Jak2 and suppresses PP2A activities. (A) Schematic representation of the FTY720-P–induced and SIPR1/Jak2 (WT and V617F)–mediated PP2A inhibition. (B) Western blots show levels of active Jak2 (pY972) and Grb2 in parental Ba/F3 and Ba/F3-Jak2V617F cells untreated and treated with the S1PR1 agonists FTY720-P (2.5 µM), SEW 2871 (10 µM), and S1P (1 µM), and with the PP2A activators FTY720 (2.5 µM), OSU-2S (2.5 µM), and (S)-FTY720-regioisomer (2.5 µM), respectively. (C) PP2A activity in Ba/F3 cells untreated and treated with FTY720 (2.5 µM), SEW 2871 (10 µM), S1P (1 µM), FTY720-P (2.5 µM), and FTY720-P in combination with S1PR1 shRNA, TG101348 (1 µM), AS-604850 (1 µM), or PKC-412 (2 µM). (D) Effect of Jak2V617F expression on the messenger RNA levels (quantitative reverse-transcription polymerase chain reaction) of specific SPHK1/2 isoforms in TF-1 cells. Fold change are relative to levels of SPHK1 and SPHK2 isoforms in parental TF-1 cells (left). Effect of Jak2V617F expression on sphingosine kinase 1 (SPHK1) expression in TF-1 cells (middle top). SPHK1 kinase activity measured as fold change in the amount of sphingosine converted to sphingosine-1-phosphate (S1P) in Ba/F3-Jak2V617F cells untreated and treated with FTY720 (2.5 µM) alone or in combination with okadaic acid (0.25 nM) (middle bottom). PP2A activity in Ba/F3 cells and in parental and S1PR1 shRNA-expressing Ba/F3-Jak2V617F cells left untreated or treated with the transduced with the S1PR1 antagonist VPC 23019 (right).
Consistent with the existence of a S1PR1/Jak2-mediated PP2A inhibitory pathway in addition to that initiated by Jak2V617F, SPHK1 isoforms (1A, 1B, and 1C), but not SPHK2 messenger RNA or protein levels, were markedly enhanced by Jak2V617F expression in cytokine-dependent TF-1 erythroleukemia cells (Figure 5D, left and middle panels). Interestingly, a SPHK1 assay revealed that FTY720 (2.5 µM) treatment of Ba/F3-Jak2V617F cells impaired SPHK1 activity in a PP2A-dependent manner as cotreatment with 0.25 nM okadaic acid antagonized the effect of FTY720 (Figure 5D, middle lower). Accordingly, S1PR1 inhibition, achieved by exposing Ba/F3-Jak2V617F cells to the S1PR1/3 inverse agonist VPC 23019 (10 µM), efficiently rescued PP2A activity (Figure 5D, right). Thus, restoration of PP2A activity by FTY720 also prevents SPHK1-dependent S1P production, thereby decreasing the negative effect of S1PR1 signaling on PP2A.
FTY720 sequesters SET and activates PP2A in the absence of SET dimerization or induction of ceramide
The proapoptotic sphingolipid ceramide activates PP2A38 upon binding to SET lysine 209 (K209);39,40 thus, we evaluated whether FTY720 activates PP2A by directly interacting with SET in hematopoietic cells. Phosphatase assays show that PP2A activity was similarly suppressed in Ba/F3 cells ectopically expressing WT or K209D SET (Figure 6A). However, it was efficiently restored by FTY720 (2.5 μM) in cells expressing WT but not K209D SET (Figure 6A), suggesting that FTY720, like ceramide, requires interaction with SET K209 for activating PP2A. To further confirm that FTY720-SET interaction is occurring in hematopoietic cells, we performed anti-NBD immunoprecipitations and monomeric avidin-based chromatography using Ba/F3-Jak2V617F cells treated with FTY720-phenoxy-NBD and FTY720-phenoxy-biotin, respectively (Figure 6B, left). Notably, both FTY720 conjugates restored PP2A activity in Ba/F3-Jak2V617F cells (Figure 6B, right). Anti-SET western blots clearly showed association of SET with both FTY720 conjugates (Figure 6B, lane 3). As expected, SET was detected neither in anti-IgG immunoprecipitates nor in chromatography with nonconjugated FTY720-treated cells (Figure 6B, lane 2).
FTY720-dependent PP2A activation in myeloid cells depends on the SET K209–FTY720 interaction but not on SET dimerization or increased ceramide levels. (A) PP2A activity in untreated and FTY720-treated Ba/F3 cells expressing WT or K209D SET proteins. (B) Pull-down assays demonstrate FTY720-SET interaction in NBD immunoprecipitates of Ba/F3-Jak2V617F cell lysates treated with NBD-conjugated FTY720 (top left) and in avidin-mediated affinity chromatography with lysates of Ba/F3-Jak2V617F cells treated with biotin-tagged FTY720 (bottom left). PP2A activity in Ba/F3-Jak2V617F cells treated with FTY720 (2.5 µM), FTY720-NBD (2.5 µM), and FTY720-biotin (2.5 µM) (right). (C) Diacylglycerol kinase assay shows gross ceramide levels in untreated and FTY720-treated Ba/F3-Jak2V617F cells (top). LC-MS/MS measurement of levels of specific ceramides in FTY720-treated Ba/F3-Jak2V617F cells expressed as percentage of those in untreated cells (bottom). The ceramide C18 able to induce PP2A activation is depicted as a solid bar. (D) PP2A activity in parental and WT or nondimerizing (TAF-1β PME mutant) SET-expressing Ba/F3 cells (left). Western blot show levels of GFP-SET in FLAG immunoprecipitates from lysates of Ba/F3-FLAG-SET coincubated with equal amount of Ba/F3-GFP-SET cell lysates in the presence of DMSO or FTY720 (right). DMSO, dimethylsulfoxide.
FTY720-dependent PP2A activation in myeloid cells depends on the SET K209–FTY720 interaction but not on SET dimerization or increased ceramide levels. (A) PP2A activity in untreated and FTY720-treated Ba/F3 cells expressing WT or K209D SET proteins. (B) Pull-down assays demonstrate FTY720-SET interaction in NBD immunoprecipitates of Ba/F3-Jak2V617F cell lysates treated with NBD-conjugated FTY720 (top left) and in avidin-mediated affinity chromatography with lysates of Ba/F3-Jak2V617F cells treated with biotin-tagged FTY720 (bottom left). PP2A activity in Ba/F3-Jak2V617F cells treated with FTY720 (2.5 µM), FTY720-NBD (2.5 µM), and FTY720-biotin (2.5 µM) (right). (C) Diacylglycerol kinase assay shows gross ceramide levels in untreated and FTY720-treated Ba/F3-Jak2V617F cells (top). LC-MS/MS measurement of levels of specific ceramides in FTY720-treated Ba/F3-Jak2V617F cells expressed as percentage of those in untreated cells (bottom). The ceramide C18 able to induce PP2A activation is depicted as a solid bar. (D) PP2A activity in parental and WT or nondimerizing (TAF-1β PME mutant) SET-expressing Ba/F3 cells (left). Western blot show levels of GFP-SET in FLAG immunoprecipitates from lysates of Ba/F3-FLAG-SET coincubated with equal amount of Ba/F3-GFP-SET cell lysates in the presence of DMSO or FTY720 (right). DMSO, dimethylsulfoxide.
Because FTY720 increases ceramide levels in specific culture conditions,40 we investigated whether FTY720 altered gross ceramide levels and, specifically, that of the PP2A-activating C18 ceramide40 (Figure 6C, solid bar). diacylglycerol kinase/thin layer chromatography and liquid chromatography (LC)/mass spectrometry (MS) assays showed that FTY720 did not alter gross and specific ceramide levels in Ba/F3-Jak2V617F cells (Figure 6C), further suggesting that ceramide is dispensable for PP2A induction in FTY720-treated Ba/F3-Jak2V617F cells. Finally, anti-GFP immunoblot on anti-FLAG immunoprecipitates from vehicle- and FTY720-treated Ba/F3-FLAG-SET lysates coincubated with Ba/F3-GFP-SET lysates (1:1) showed that FTY720 decreased SET dimerization (Figure 6D, right). Moreover, expression of the dimerization-deficient SET mutant (TAF-1β PME) inhibited PP2A activity as well as SET(WT) (Figure 6D, left), indicating that SET dimerization is not required for both PP2A inhibition and FTY720 interaction in hematopoietic progenitors.
Discussion
PP2A tumor suppressor activity plays a pivotal role in cancer emergence, development, and progression.2 Similar to BCR-ABL1+ leukemias4,24 and consistent with the notion that high levels of Jak2V617F circumvents the requirement for erythropoietin receptor in transformation of myeloid progenitors,41 we showed that PP2A activity is inactivated in Jak2V617F MPN progenitors, and this occurs in an oncogene dose- and kinase-dependent manner and is mediated by the PP2A inhibitor SET. Furthermore, though expression of Jak2V617F is per se sufficient to inhibit PP2A in MPN progenitor cells, expression of endogenous WT Jak2 in normal BM progenitors is not sufficient to suppress PP2A activity because the WT Jak2 signaling pathway leading to inhibition of PP2A requires the constitutive engagement of the S1PR1 receptor (Figure 2A). The possibility that Jak2V617F can directly inhibit PP2A through PP2AcY307 phosphorylation is supported by the fact that interleukin-3–stimulation induces Jak2-PP2A association and phosphorylation-mediated PP2A inhibition in myeloid precursors,42 and by our data indicating that Jak2V617F-driven PI-3Kγ/PKC activation is responsible for PKC-dependent SET activation. This is not unexpected, considering that SET was already suggested to be a PKC substrate23 and that Jak2 associates and activates PII1PP2A-3K that, in turn, recruits PP2A and induces canonical PKC activation via PIP3 production.27,43 Thus, SET might work as a scaffold protein recruiting the Jak2V617F -containing complex to PP2A. Accordingly, BCR-ABL1 uses Jak2 to induce SET-dependent PP2A inactivation.24 To ensure that SET mediates PP2A inhibition in Jak2V617F cells, we also targeted the related PP2A inhibitor I1PP2A (inhibitor 1 of PP2A) by administering DMS and sphingosine that, reportedly,44 target I1PP2A and restore PP2A activity when it is repressed by I1PP2A. These compounds failed to induce PP2A activity in Jak2V617F cells (not shown), indicating that I1PP2A unlikely plays a role in Jak2V617F-induced PP2A inhibition.
Inhibition of PP2A in MPNs appears essential for leukemogenesis; in fact, genetic (HA-PP2Ac or SET-shRNA expression) and pharmacologic (FTY720, S-FTY720-regioisomer, and OSU-2S) reactivation of PP2A results in Jak2V617F inactivation/downregulation, reduced clonogenic potential, and impaired in vivo Jak2V617F+ leukemogenesis. Accordingly, PADs (eg, FTY720) also selectively suppress, in a PP2A-mediated manner, the BCR-ABL1 kinase-independent and Jak2-regulated survival/self-renewal of TKI-resistant CML stem cells both in vitro and in BM transplantation assays.36 Moreover, as reported,2,3 long-term FTY720 administration had neither adverse effects on normal hematopoiesis nor toxicity in nonhematopoietic organs. In this regard, FTY720-treated animals had normal cardiac cell function, consistent with the notion that a clinically manageable bradycardia and atrioventricular conduction block are only observed in multiple sclerosis patients at the time of FTY720 therapy initiation.45
The in vitro and in vivo antileukemic activity of FTY720 against Jak2V617F MPN cells is independent of FTY720 phosphorylation; analogous with the effects of SPHK2 modulation and FTY720-P treatment in CML and KitD816V AML cells,2 the S1PR1 agonist and immunomodulator FTY720-P neither induced PP2A activity nor antagonized Jak2V617F or Jak2V617F-driven cell proliferation. Conversely, we showed that FTY720 nonimmunosuppressive derivatives (OSU-2S and S-FTY720-regioisomer), which do not interact with S1PR1,2 efficiently downregulated Jak2V617F activity. This is highly important, because exposure of hematopoietic precursors to S1PR1 agonists, including S1P and FTY720-P, led to PP2A inhibition. Additionally, Jak2 activation was increased in Ba/F3 cells treated with S1PR1 agonists, including FTY720-P, despite the notion that S1P receptors are G- protein–coupled receptors that typically do not signal through receptor-bound kinases such as Jak2,46 although Jak2 Y972 was also found phosphorylated in response to Jak2 activation by another G protein–coupled receptor.47 Thus, it is not surprising that an inverse SPHK1 modulation was observed in response to FTY720 treatment and Jak2V617F expression. Indeed, in accordance with a previous study,48 not only was SPHK1 activity decreased following PP2A activation by FTY720 and increased by Jak2V617F but the antiapoptotic S1P,49 product of SPHK1 activity, also inhibited PP2A upon triggering the S1PR1-Jak2-π-3Kγ/PKC-SET pathway in nontransformed and Jak2V617F hematopoietic precursors. This seems not to be the case for the FTY720 conversion enzyme, SPHK2, which remained unaltered by Jak2V617F expression. Conversely, FTY720 in its phosphorylated form seems to act primarily as a S1PR1 agonist only in mature lymphocytes to induce immunosuppression.1 Here we showed that S1PR1 agonists may suppress PP2A also in hematopoietic progenitors; however, LC/electrospray ionization (ESI)/MS/MS analysis indicated that most of intracellular FTY720 given to myeloid precursors is nonphosphorylated.36 Thus, the limiting factor for a wide use of FTY720 as an antileukemia agent depends on the levels and activity of the FTY720-converting enzyme SPHK2 that, if aberrantly elevated, can antagonize FTY720 anticancer activity. However, there is no evidence indicating that SPHK2 activity is increased in hematologic malignancies.
Mechanistically, we showed that the activity of FTY720 as a PAD in Jak2V617F MPNs depends on the sequestration of SET through interaction with SET K209 but not on SET dimerization or induction of ceramide. SET dimerization has been shown to be critical for SET-enhanced DNA replication49 but not for PP2A inhibition in a cell-free system.50
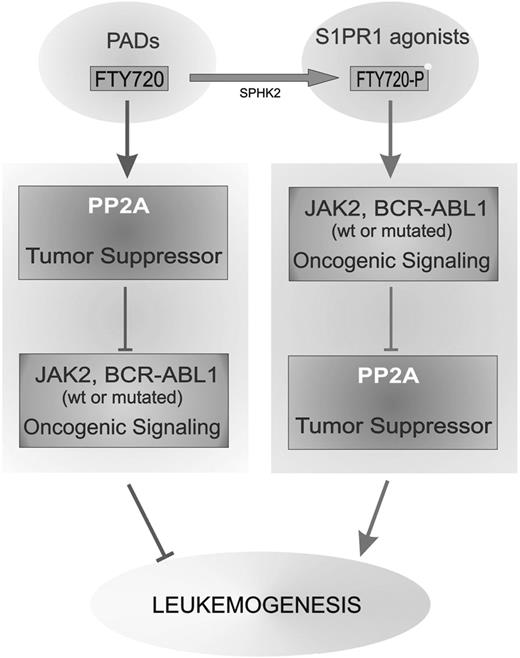
Thus it is likely that the PP2A inhibitory effects of SET are dependent on its subcellular localization and that the importance of SET serine phosphorylation is limited to allow cytoplasmic localization and ability to interact/inhibit cytoplasmic PP2A that, otherwise, would suppress Jak2V617F leukemogenic activity. Because PP2A has a pleiotropic anticancer activity,2 our data suggest that the use of FTY720 and, better, other PADs lacking S1PR1 agonist activity (Figure 7) might be used together with TKIs for treating Jak2V617F+ MPNs and other malignancies (eg, Ph+ leukemias) characterized by inactivation of the PP2A tumor suppressor.
Antagonizing effect of FTY720 and FTY720-P on leukemogenesis. Schematic representation on the effect of PP2A-activating drugs (PADs, eg, FTY720) and sphingosine-1-phosphate-receptor-1 agonists (eg, FTY720-P) on leukemogenesis through the opposite effect on the interplay between oncogenic kinase (eg, Jak2V617F and BCR-ABL1) signaling and tumor suppressor (ie, PP2A) activity.
Antagonizing effect of FTY720 and FTY720-P on leukemogenesis. Schematic representation on the effect of PP2A-activating drugs (PADs, eg, FTY720) and sphingosine-1-phosphate-receptor-1 agonists (eg, FTY720-P) on leukemogenesis through the opposite effect on the interplay between oncogenic kinase (eg, Jak2V617F and BCR-ABL1) signaling and tumor suppressor (ie, PP2A) activity.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked ‘‘advertisement’’ in accordance with 18 USC section 1734.
Acknowledgments
The authors thank J. Van Brocklyn for providing reagents, J. Perrin for assistance in procuring MPN specimens, and S. Lee for editorial assistance.
This work was supported in part by National Institutes of Health—NCI grants CA095512 and CA163800 (to D.P.), CA16058 (to The OSU Comprehensive Cancer Center CCC), CA88932 and DE016572 (to B.O.), CA49639 (to R.B.A.), the Leukemia and Lymphoma Society Scholarship Program (to D.P.), and the American-Italian Cancer Foundation (to P.N.). O.J.J. is a graduate student of the OSU Integrated Biomedical Science Graduate Program (IBGP).
Authorship
Contribution: J.J.O., R.S., C.J.W., S.R., J.G.H., G.F., A.-K.E., S.A.S., and P.N. performed experiments; J.R.V.B., R. Briesewitz, K.N., R. Bittman, M.A.C., O.A.-W., R.L., R.B.A., A.Q.-C., J.M.G., J.A., A.R., D.M., M.T.Z., G.M., and B.O. provided essential reagents, including patient specimens, and discussed results; J.J.O., P.N., and D.P. analyzed results and made the figures; and J.J.O. and D.P. designed the research and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Danilo Perrotti, The Ohio State University Comprehensive Cancer Center, 892 Biomedical Research Tower, 460 West 12th Ave, Columbus, OH 43210-2207; e-mail: danilo.perrotti@osumc.edu.