Key Points
TLR-stimulated macrophages synthesize, release, and hydrolyze ATP via CD39 to regulate their own activation state.
The loss of macrophage CD39 prevents regulatory macrophage development and leads to lethal inflammatory responses and septic shock in mice.
Abstract
Sepsis is a highly fatal disease caused by an initial hyperinflammatory response followed by a state of profound immunosuppression. Although it is well appreciated that the initial production of proinflammatory cytokines by macrophages accompanies the onset of sepsis, it remains unclear what causes the transition to an immunosuppressive state. In this study, we reveal that macrophages themselves are key regulators of this transition and that the surface enzyme CD39 plays a critical role in self-limiting the activation process. We demonstrate that Toll-like receptor (TLR)-stimulated macrophages modulate their activation state by increasing the synthesis and secretion of adenosine triphosphate (ATP). This endogenous ATP is paradoxically immunosuppressive due to its rapid catabolism into adenosine by CD39. Macrophages lacking CD39 are unable to transition to a regulatory state and consequently continue to produce inflammatory cytokines. The importance of this transition is demonstrated in a mouse model of sepsis, where small numbers of CD39-deficient macrophages were sufficient to induce lethal endotoxic shock. Thus, these data implicate CD39 as a key “molecular switch” that allows macrophages to self-limit their activation state. We propose that therapeutics targeting the release and hydrolysis of ATP by macrophages may represent new ways to treat inflammatory diseases.
Introduction
Failure to control inflammatory macrophage activation responses can lead to pathological diseases, best exemplified by sepsis. Despite our growing understanding of its pathogenesis, sepsis continues to affect more than 200 000 people annually in the United States, with a mortality rate as high as 50%.1,2 The severe pathology associated with sepsis occurs in response to the hyperproduction of macrophage-derived inflammatory cytokines, which can lead to vascular and tissue destruction, multiple organ failure, shock, and death.3 Intriguingly, macrophages isolated from late-stage septic individuals exhibit the phenotype of immunosuppressive, regulatory macrophages, expressing high levels of the anti-inflammatory cytokine interleukin-10 (IL-10) and low levels of tumor necrosis factor α (TNF-α) and IL-12.4-6 These observations suggest the transition from inflammatory to immunosuppressive macrophages may be critical to control initial inflammatory responses and prevent lethal septic shock.4,7,8 However, the molecular mechanism by which this transition is achieved remains poorly understood.
In the present work, we examine the role that endogenous CD39 and adenosine triphosphate (ATP) play in regulating the macrophage inflammatory response. Recently, extracellular ATP (eATP) has been characterized as a “danger signal” that can promote inflammation through P2X7-dependent activation of the NLRP3 inflammasome, leading to IL-1β production.9-12 Importantly, however, such results were shown to be dependent on the addition of millimolar levels of exogenous ATP in vitro. These high levels of eATP have yet to be discovered in vivo, and the concentration of eATP is carefully controlled by the ectonucleoside triphosphate diphosphohydralse (ENTPDase1/CD39).13,14 It was recently shown that macrophages express CD39 and that it played a role in modulating the P2X7-dependent production of IL-1β15 in the presence of exogenously added ATP. Several issues remain incompletely understood regarding how ATP and CD39 contribute to the regulation of macrophage activation. First, the potential for macrophages to be an endogenous source of ATP has not been fully addressed. Unlike most cells, macrophages primarily generate ATP through glycolysis.16,17 Moreover, macrophages increase the rate of glycolysis in response to Toll-like receptor (TLR) stimulation.18 Previous attempts to measure ATP release from macrophages have yielded inconsistent results19-21 that were likely complicated by endogenous CD39 activity. Second, if macrophages are indeed a source of ATP, the mechanism by which they release ATP into the extracellular milieu has not been previously defined. Third, there is currently a paucity of studies investigating how ATP and CD39 influence inflammasome-independent responses, such as TNF-α production.4,8,22 Finally, the specific contribution of CD39 on macrophages in directing the course of inflammatory disease remains unknown.
In the present study, we demonstrate that in response to a variety of TLR ligands, macrophages synthesize, secrete, and hydrolyze ATP to control their own activation status. Surprisingly, the ATP that is generated does not stimulate IL-1β release, but rather suppresses the production of proinflammatory cytokines like TNF-α and IL-12. Mechanistically, we show that macrophages hydrolyze self-released ATP via CD39 to generate immunosuppressive adenosine. CD39-mediated adenosine generation results in a dramatic reprogramming of the macrophage activation response to enhance not only IL-10 production but also many newly identified regulatory macrophage-specific transcripts.23-26 Finally, we demonstrate that macrophage-specific expression of CD39 is critical in preventing lethal hyperinflammatory responses to lipopolysaccharides (LPS) in vivo. Taken together, our data indicate that CD39 acts as a “molecular switch” that controls the balance between inflammatory and regulatory macrophage differentiation.
Materials and methods
Mice and macrophage isolation
BALB/c and C57BL/6 mice were purchased from the Charles River Laboratories. These studies were reviewed and approved by the University of Maryland Institutional Animal Care and Use Committee. Bone-marrow–derived macrophages were prepared from 6- to 8-week-old female Balb/c, C57BL/6, Cd39−/−,27 and A2br−/−28 mice, as previously described,29 and differentiated in 20% L929 (LC14) conditioned media unless otherwise noted. Human monocytes were obtained from healthy volunteers with their informed consent in accordance with the Declaration of Helsinki and institutional review board approval. Monocytes were purified for immediate use or differentiated in the presence of 50 ng/mL macrophage colony-stimulating factor (M-CSF) (CSF-1) for 7 days as previously described.30 Murine peritoneal macrophages were isolated as previously described.31 RAW264.7 cells (TIB-71) were obtained from ATCC (Manassas, VA).
ATP release assay
eATP release was detected using the ATPlite Luminescence ATP Detection Assay System (PerkinElmer, Waltham, MA) according to the manufacturer’s protocol but omitting the cell-lysis step.
Inorganic phosphate release assay
Adenine nucleotide hydrolysis was detected using the BIOMOL Green Reagent (Enzo Life Sciences, Farmingdale, NY) according to the manufacturer’s protocol.
Flow cytometry
CD39 expression on peritoneal macrophages was determined after gating on F4/80+CD11b+ cells. CD39 expression on human monocytes/macrophages was determined after gating on CD14+ cells.
For detailed experimental protocols and a list of reagents, primer pairs, and antibody clones used in this study, please refer to supplemental Methods found on the Blood Web site.
Results
TLR stimulation results in ATP release from macrophages
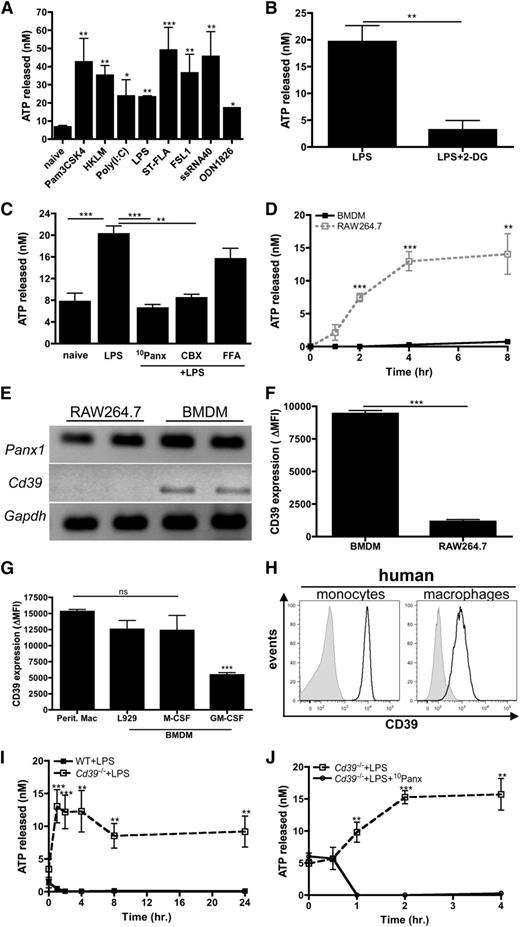
The potential for macrophages to be a source of eATP remains controversial. To address this, macrophages were stimulated with TLR agonists and the release of ATP was measured. All TLR agonists tested were capable of inducing significant ATP release from the macrophage-like cell line RAW264.7 (Figure 1A). TLR-induced ATP release was glycolysis dependent, because treatment with 2-deoxyglucose, a competitive inhibitor of glycolysis,32 inhibited ATP release (Figure 1B). Pannexin-1 is a plasma membrane channel that was recently shown to be the major mediator of ATP release from red blood cells33 and activated T cells.34 To investigate whether pannexin-1 channels were important for ATP release from macrophages, RAW264.7 cells were stimulated with LPS in the presence of 10 panx, a pannexin-1 specific inhibitor,10 and the release of ATP was measured. The results revealed that ATP secreted by macrophages was strongly dependent on pannexin-1 (Figure 1C). The blockade of pannexin-1–mediated ATP release was also recapitulated when macrophages were stimulated with LPS in the presence of carbenoxolone, but not in the presence of flufenamic acid (FFA), which preferentially inhibit pannexin and related connexin channels, respectively (Figure 1C).35 Thus, these results suggest that pannexin-1 channels are indeed the major conduits from which ATP is released from TLR-stimulated macrophages.
TLR stimulation results in ATP release from macrophages. (A) RAW264.7 cells were stimulated with the indicated TLR stimuli or left untreated. After 3 hours, ATP release was measured. The means ± standard error of the mean (SEM) of 3 independent experiments are shown and statistical analysis is relative to naïve samples. (B) RAW264.7 cells were stimulated with LPS in absence or presence of 200 μg/mL 2-deoxyglucose and levels of ATP released were measured 3 hours later. The means ± standard deviation (SD) of triplicate determinations are shown and are representative of 2 independent experiments. (C) RAW264.7 cells left untreated or stimulated with LPS alone or in the presence of 50 μM 10Panx, 10 μM carbenoxolone, or 10 μM FFA. After 3 hours, ATP release was measured. The means of 3 independent experiments ± SEM are shown. (D) Levels of ATP released from LPS-stimulated RAW264.7 cells (open squares; dashed line) or BMDMs (closed squares; solid line) were measured over time. The means ± SEM of 3 independent experiments are shown. (E) The mRNA expression of Panx1 and Cd39 in RAW264.7 cells and BMDMs is shown. Data are representative of 3 independent experiments. (F) The surface expression of CD39 on RAW264.7 cells and BMDMs was determined by FACS and expressed as ΔMFI, compared with the isotype control for each group. The means ± SD of triplicate determinations are shown and are representative of 3 independent experiments. (G) CD39 expressed on peritoneal macrophages and BMDMs differentiated in the presence of L929 conditioned media, M-CSF, or GM-CSF for 7 days was determined by flow cytometry and expressed as ΔMFI, compared with isotype control for each group. The mean ± SD of triplicate determinations is shown and is representative of 2 independent experiments. (H) Flow cytometry analysis of CD39 surface expression (black histograms) on human monocytes (left) and macrophages (right) compared with isotype controls (gray histograms). Data are representative of 3 independent experiments. (I) Levels of ATP released from LPS-stimulated Cd39−/− (open squares; dashed line) and wild-type (closed squares; solid line) BMDMs were measured over time. The means ± SEM of 3 independent experiments are shown. (J) The levels of ATP produced by Cd39−/− BMDMs stimulated with LPS in the absence (open squares; dashed line) or presence (closed circles; solid line) of 50 μM 10Panx was measured over time. The means of 3 independent experiments ± SEM are shown. MFI, mean fluorescence intensity; WT, wild-type.
TLR stimulation results in ATP release from macrophages. (A) RAW264.7 cells were stimulated with the indicated TLR stimuli or left untreated. After 3 hours, ATP release was measured. The means ± standard error of the mean (SEM) of 3 independent experiments are shown and statistical analysis is relative to naïve samples. (B) RAW264.7 cells were stimulated with LPS in absence or presence of 200 μg/mL 2-deoxyglucose and levels of ATP released were measured 3 hours later. The means ± standard deviation (SD) of triplicate determinations are shown and are representative of 2 independent experiments. (C) RAW264.7 cells left untreated or stimulated with LPS alone or in the presence of 50 μM 10Panx, 10 μM carbenoxolone, or 10 μM FFA. After 3 hours, ATP release was measured. The means of 3 independent experiments ± SEM are shown. (D) Levels of ATP released from LPS-stimulated RAW264.7 cells (open squares; dashed line) or BMDMs (closed squares; solid line) were measured over time. The means ± SEM of 3 independent experiments are shown. (E) The mRNA expression of Panx1 and Cd39 in RAW264.7 cells and BMDMs is shown. Data are representative of 3 independent experiments. (F) The surface expression of CD39 on RAW264.7 cells and BMDMs was determined by FACS and expressed as ΔMFI, compared with the isotype control for each group. The means ± SD of triplicate determinations are shown and are representative of 3 independent experiments. (G) CD39 expressed on peritoneal macrophages and BMDMs differentiated in the presence of L929 conditioned media, M-CSF, or GM-CSF for 7 days was determined by flow cytometry and expressed as ΔMFI, compared with isotype control for each group. The mean ± SD of triplicate determinations is shown and is representative of 2 independent experiments. (H) Flow cytometry analysis of CD39 surface expression (black histograms) on human monocytes (left) and macrophages (right) compared with isotype controls (gray histograms). Data are representative of 3 independent experiments. (I) Levels of ATP released from LPS-stimulated Cd39−/− (open squares; dashed line) and wild-type (closed squares; solid line) BMDMs were measured over time. The means ± SEM of 3 independent experiments are shown. (J) The levels of ATP produced by Cd39−/− BMDMs stimulated with LPS in the absence (open squares; dashed line) or presence (closed circles; solid line) of 50 μM 10Panx was measured over time. The means of 3 independent experiments ± SEM are shown. MFI, mean fluorescence intensity; WT, wild-type.
Surprisingly, very little eATP was detected from LPS-stimulated bone-marrow derived murine macrophages (BMDMs) relative to LPS-stimulated RAW264.7 cells (Figure 1D), despite similar expression levels of pannexin-1 both at the messenger RNA (mRNA) level and the protein level (Figure 1E and data not shown). We hypothesized that BMDMs may control the bioavailability of self-released eATP and express higher levels of CD39, thereby decreasing eATP detection. Indeed, Cd39 transcript levels were below detection in RAW264.7 cells but present in BMDMs (Figure 1E). CD39 expression on the surface of RAW264.7 cells was 10-fold less than on BMDMs (Figure 1F). We further evaluated whether culture conditions used to develop BMDMs influenced CD39 expression and observed that CD39 levels were significantly higher on L929- and M-CSF–differentiated BMDMs compared with granulocyte macrophage colony-stimulating factor (GM-CSF)–induced BMDMs (Figure 1G). Furthermore, L929/M-CSF-differentiated BMDMs most closely resembled peritoneal-resident macrophages with regard to CD39 expression. These observations are consistent with previous work showing that in vitro cultivation of BMDMs in the presence of M-CSF is a more representative model of tissue resident macrophages than GM-CSF–derived BMDMs.36 Human monocytes and macrophages also expressed high levels of CD39 (Figure 1H). To address whether CD39 was directly responsible for regulating levels of macrophage-derived eATP, we examined the levels of ATP released from LPS-stimulated Cd39−/− macrophages. In contrast to wild-type macrophages, eATP was detectable following LPS stimulation and was sustained for up to 24 hours postactivation in Cd39−/− macrophages (Figure 1I). These data suggest that TLR-stimulated wild-type macrophages secrete ATP but rapidly hydrolyze it, therefore preventing eATP detection. Furthermore, similar to CD39lo RAW264.7 cells, ATP secretion by Cd39−/− macrophages was critically dependent on pannexin-1 (Figure 1J). Taken together, these data demonstrate that TLR stimulation induces intrinsic ATP release from macrophages via pannexin-1 channels and that under normal conditions macrophages use CD39 to control eATP bioavailability.
eATP is rapidly hydrolyzed by macrophage ectoenzymes
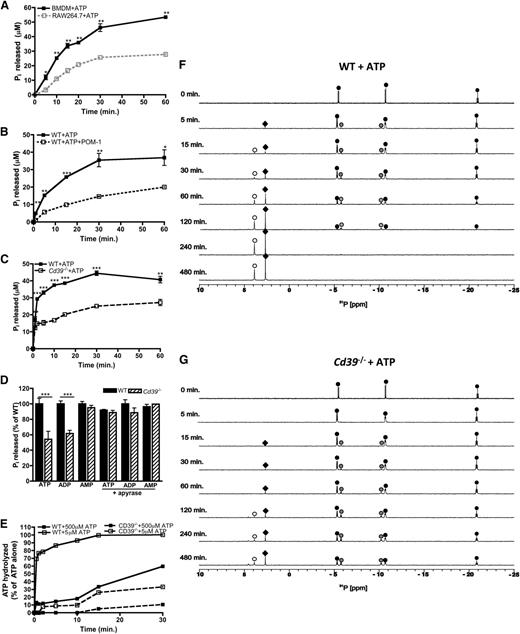
Having demonstrated that CD39 is important for regulating the levels of macrophage-derived eATP, we next characterized the CD39-specific e-NTPDase activity in macrophages. ATP hydrolysis yields the production of adenine metabolites and inorganic phosphates (Pi), so we first examined levels of Pi released over time by RAW264.7 cells and BMDMs in the presence of eATP. Consistent with the CD39 expression data, CD39lo RAW264.7 cells exhibited substantially impaired eATP hydrolysis compared with CD39+/+ BMDMs (Figure 2A). eATP hydrolysis by macrophages was also dependent on CD39, because treatment with the CD39 inhibitor POM-137 as well as CD39-deficient macrophages exhibited substantially impaired ATP hydrolysis (Figure 2B-C). The defects in purine nucleotide hydrolysis were specific to ATP and adenosine 5′-diphosphate (ADP), but not adenosine 5′-monophosphate (AMP) (Figure 2D).13 We next investigated whether genetic ablation of Cd39 could be rescued by the presence of apyrase, a soluble e-NTPDase.38 Indeed, apyrase fully complemented the loss of endogenous CD39, achieving hydrolysis rates similar to Cd39+/+ macrophages (Figure 2D). The low level of ATP hydrolysis by CD39-deficient macrophages may be due to the presence of low-affinity nonspecific phosphatases. To address this, we compared hydrolysis over time from macrophages exposed to lower levels of ATP and demonstrate that wild-type macrophages completely hydrolyze 5 μM ATP within 15 minutes, compared with 30% of 500 μM ATP hydrolyzed within the same time (Figure 2E). Importantly, macrophages lacking CD39 exhibited substantially reduced hydrolysis rates regardless of the amount of eATP present.
eATP is hydrolyzed by the macrophage ectoenzyme CD39. (A-C) The hydrolysis of 500 μM ATP by wild-type BMDMs (closed squares; solid black line) over time compared with RAW264.7 cells (open squares; gray, dashed line) (A), in the presence of 10μM POM-1 (open squares; black, dashed line) (B), or Cd39−/− (open squares; black, dashed line) BMDMs (C) and was measured by the Pi release assay. (D) The percent of adenine nucleotides hydrolyzed within 30 minures as measured by Pi released by wild-type BMDMs (solid bars) and Cd39−/− (striped bars) BMDMs in the absence (left) or presence (right) of apyrase. (A-D) The means ± SD of triplicate determinations are shown and are representative of 3 independent experiments. (E) The percent of 5 μM (open squares) or 500 μM (closed squares) ATP hydrolyzed over time by wild-type BMDMs (solid lines) and Cd39−/− (dashed lines) BMDMs. Percent of hydrolysis was compared with levels of ATP detected in the absence of cells and determined by the ATPlite assay. The data are representative of 3 independent experiments. (F-G) The 31P NMR spectra of 500 μM ATP by wild-type (F) or Cd39−/− BMDMs (G) are shown over time. The location of the α,β,γ phosphorous peaks of ATP (black circles), α,β phosphorous peaks of ADP (gray circles), phosphorous peaks of AMP (white circles), and Pi (black diamonds) was based on reference 31P NMR spectra. NMR spectra are representative of at least 3 independent experiments. WT, wild-type.
eATP is hydrolyzed by the macrophage ectoenzyme CD39. (A-C) The hydrolysis of 500 μM ATP by wild-type BMDMs (closed squares; solid black line) over time compared with RAW264.7 cells (open squares; gray, dashed line) (A), in the presence of 10μM POM-1 (open squares; black, dashed line) (B), or Cd39−/− (open squares; black, dashed line) BMDMs (C) and was measured by the Pi release assay. (D) The percent of adenine nucleotides hydrolyzed within 30 minures as measured by Pi released by wild-type BMDMs (solid bars) and Cd39−/− (striped bars) BMDMs in the absence (left) or presence (right) of apyrase. (A-D) The means ± SD of triplicate determinations are shown and are representative of 3 independent experiments. (E) The percent of 5 μM (open squares) or 500 μM (closed squares) ATP hydrolyzed over time by wild-type BMDMs (solid lines) and Cd39−/− (dashed lines) BMDMs. Percent of hydrolysis was compared with levels of ATP detected in the absence of cells and determined by the ATPlite assay. The data are representative of 3 independent experiments. (F-G) The 31P NMR spectra of 500 μM ATP by wild-type (F) or Cd39−/− BMDMs (G) are shown over time. The location of the α,β,γ phosphorous peaks of ATP (black circles), α,β phosphorous peaks of ADP (gray circles), phosphorous peaks of AMP (white circles), and Pi (black diamonds) was based on reference 31P NMR spectra. NMR spectra are representative of at least 3 independent experiments. WT, wild-type.
To further elucidate the CD39-specific e-NTPDase activity in macrophages, we used 31P nuclear magnetic resonance (NMR) spectroscopy, which enables the identification of each phosphorus-containing intermediate metabolite generated via eATP hydrolysis. NMR revealed that ATP remained intact and stable in macrophage-free culture medium for several hours (data not shown); however, eATP stability was drastically reduced in the presence of CD39+/+ macrophages as evidenced by the generation of ADP and AMP from ATP. By 4 hours, ATP was completely hydrolyzed (Figure 2F). In contrast, a substantial fraction of ATP remained intact, even after 8 hours of exposure to Cd39−/− macrophages (Figure 2G). While some ATP/ADP was hydrolyzed by CD39-deficient macrophages, which is likely due to the compensatory nonspecific phosphatases, the rate of conversion of eATP to ADP and then AMP was dramatically reduced. Collectively, these data suggest that CD39 is the major high-affinity ecto-ATPase used by macrophages to hydrolyze eATP.
ATP exposure promotes regulatory macrophage development following TLR stimulation
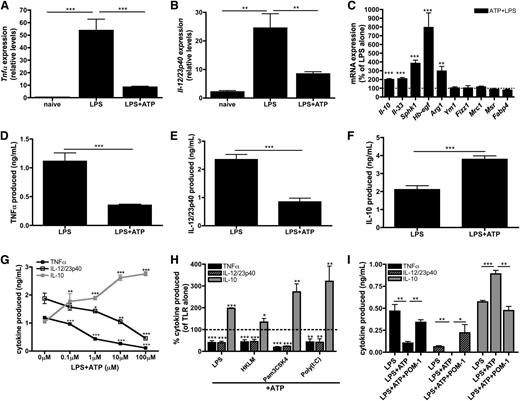
Although the inflammatory effect of millimolar levels of eATP is well documented,9-11,39 the role of submillimolar levels of eATP in influencing macrophage physiology remains unresolved. To address this, macrophages were stimulated with LPS in the presence or absence of 100 μM ATP and genetic biomarkers associated with inflammatory and regulatory macrophage activation states were assessed. Macrophages stimulated with LPS alone upregulated the expression of mRNA encoding the inflammatory cytokines TNF-α (Figure 3A) and IL-12/23p40 (Figure 3B), as expected. Macrophages stimulated in the presence of low levels of ATP suppressed Tnfα and Il-12/23p40 transcripts by ∼80% and 60%, respectively (Figure 3A-B). Additionally, a significant upregulation of many transcripts expressed by regulatory macrophages was observed including Il-10, Il-33, Hb-egf, and Sphk1.23-26 Although eATP enhanced LPS-induced Arg1 levels, it did not induce expression of Fizz1, Ym1, Mrc1, Msr1, or Fabp4, which are biomarkers of alternatively activated (IL-4/IL-13 treated) macrophages (AAMs) (Figure 3C). 40-42 Thus, eATP did not merely inhibit inflammatory macrophage activation, but rather actively promoted the development of a regulatory macrophage phenotype.
ATP promotes regulatory macrophage development. (A-B) Tnfα (A) and Il-12/23p40 (B) mRNA expression in naïve wild-type BMDMs or BMDMs stimulated with LPS in the absence or presence of 100 μM ATP was determined after 4 hours of stimulation. The means of 3 independent experiments ± SEM are shown. (C) The percent increase of regulatory macrophage-associated transcripts in wild-type BMDMs in the presence of LPS and 100 μM ATP are shown at 4 hours poststimulation by qRT-PCR. The dashed line represents the expression of regulatory transcripts induced by LPS alone. The means of at least 3 independent experiments ± SEM are shown. (D-F) Secreted levels of TNF-α (D), IL-12/23p40 (E), and IL-10 (F) by wild-type BMDMs stimulated with LPS in the absence or presence of 100μM ATP at 8 hours poststimulation. The means of 5 independent experiments ± SEM are shown. (G) TNF-α (closed squares; black line), IL-12/23p40 (open squares; black line), and IL-10 (closed squares; gray line) secreted by wild-type BMDMs stimulated with LPS in the presence of increasing concentrations of ATP. Cytokine levels were determined after 8 hours by enzyme-linked immunosorbent assay (ELISA). The means ± SD of triplicate determinations are shown and are representative of 3 independent experiments. (H) The percent of TLR-induced cytokines produced in the presence of 100 μM ATP. Cytokine levels were determined after 8 hours by ELISA and are relative to TLR stimulation alone (dashed line). The means of TNF-α (black bars), IL-12/23p40 (striped bars), and IL-10 (gray bars) production ± SD of triplicate determinations are shown and are representative of 3 independent experiments. Statistical analysis is relative to samples stimulated with TLR stimuli alone. (I) LPS-induced TNF-α (black bars), IL-12/23p40 (striped bars), and IL-10 (gray bars) production by human macrophages exposed to 100 μM ATP alone or in the presence of 10 μM POM-1 was determined by ELISA at 8 hours poststimulation. The means ± SD of triplicate determinations are shown and are representative of 2 independent experiments.
ATP promotes regulatory macrophage development. (A-B) Tnfα (A) and Il-12/23p40 (B) mRNA expression in naïve wild-type BMDMs or BMDMs stimulated with LPS in the absence or presence of 100 μM ATP was determined after 4 hours of stimulation. The means of 3 independent experiments ± SEM are shown. (C) The percent increase of regulatory macrophage-associated transcripts in wild-type BMDMs in the presence of LPS and 100 μM ATP are shown at 4 hours poststimulation by qRT-PCR. The dashed line represents the expression of regulatory transcripts induced by LPS alone. The means of at least 3 independent experiments ± SEM are shown. (D-F) Secreted levels of TNF-α (D), IL-12/23p40 (E), and IL-10 (F) by wild-type BMDMs stimulated with LPS in the absence or presence of 100μM ATP at 8 hours poststimulation. The means of 5 independent experiments ± SEM are shown. (G) TNF-α (closed squares; black line), IL-12/23p40 (open squares; black line), and IL-10 (closed squares; gray line) secreted by wild-type BMDMs stimulated with LPS in the presence of increasing concentrations of ATP. Cytokine levels were determined after 8 hours by enzyme-linked immunosorbent assay (ELISA). The means ± SD of triplicate determinations are shown and are representative of 3 independent experiments. (H) The percent of TLR-induced cytokines produced in the presence of 100 μM ATP. Cytokine levels were determined after 8 hours by ELISA and are relative to TLR stimulation alone (dashed line). The means of TNF-α (black bars), IL-12/23p40 (striped bars), and IL-10 (gray bars) production ± SD of triplicate determinations are shown and are representative of 3 independent experiments. Statistical analysis is relative to samples stimulated with TLR stimuli alone. (I) LPS-induced TNF-α (black bars), IL-12/23p40 (striped bars), and IL-10 (gray bars) production by human macrophages exposed to 100 μM ATP alone or in the presence of 10 μM POM-1 was determined by ELISA at 8 hours poststimulation. The means ± SD of triplicate determinations are shown and are representative of 2 independent experiments.
The addition of ATP to macrophages also attenuated TNF-α (Figure 3D) and IL-12/23p40 (Figure 3E) protein secretion and increased IL-10 production (Figure 3F). These cytokine modulations were dose dependent and were achievable within the range of ATP released intrinsically from TLR-activated macrophages (Figure 3G). Furthermore, the decrease of inflammatory cytokine production and the induction of IL-10 release were not specific to LPS stimulation, because eATP also promoted regulatory macrophage differentiation in response to a variety of other TLR agonists (Figure 3H). We further tested whether CD39 contributed to the development of ATP-induced regulatory macrophages by activating macrophages in the presence of POM-1, which resulted in complete reversal of ATP-mediated immunosuppression and blocked the induction of a regulatory activation state in mouse (data not shown) and human macrophages (Figure 3I). Together, these results demonstrate that macrophages use CD39 and eATP to direct their activation status toward a regulatory phenotype.
ATP-derived adenosine modulates inflammatory macrophage activation
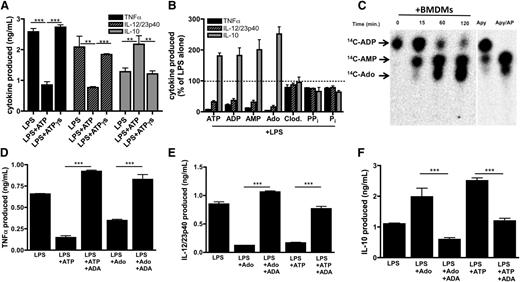
Having demonstrated that low levels of eATP have a previously unrecognized influence in directing regulatory macrophage development, we next sought to determine the molecular mechanism underlying this phenomenon. To address whether eATP hydrolysis contributed to regulatory macrophage induction by eATP, TNF-α, IL-12/23p40, and IL-10 production by macrophages stimulated in the presence of the nonhydrolyzable ATP analog, ATPγs were measured. In contrast to ATP, ATPγs did not inhibit LPS-induced TNF-α or IL-12/23p40 production, nor did it enhance IL-10 secretion (Figure 4A). These data therefore suggest that hydrolysis of eATP was required for the modulation of TNF-α and IL-12 production. To investigate which ATP metabolite(s) was responsible for attenuating TLR-induced inflammatory macrophage responses, we exposed macrophages to LPS in the presence of ADP, AMP, and adenosine and observed that each adenine metabolite suppressed TLR-induced TNF-α and IL-12/23p40 production while enhancing IL-10 production (Figure 4B). Although pyrophosphate has been shown to influence IL-1β production from macrophages,43 neither pyrophosphate nor its nonhydrolyzable analog clodronate altered TNF-α or IL-12/23p40 or induced IL-10 production from macrophages (Figure 4B). Because adenosine has been associated with immunosuppression,44,45 we examined whether macrophages were capable of generating adenosine from adenine nucleotides by thin-layer chromatography (TLC). Macrophages produced AMP within 15 minutes of exposure to ADP, and by 1 hour, substantial adenosine generation was evident by TLC (Figure 4C). Within 2 hours, virtually all the ADP provided to the cells was converted to AMP and adenosine. Thus, the TLC data are consistent with the NMR results (above) and indicate that macrophages convert extracellular adenine nucleotides to adenosine.
ATP-derived adenosine modulates inflammatory macrophage activation. (A) Wild-type BMDMs were stimulated with LPS in the absence or presence of 100 μM ATP or nonhydrolyzable ATPγs and TNF-α (black bars), IL-12/23p40 (striped bars), and IL-10 (gray bars) production was measured by ELISA 8 hours later. The means of 3 independent experiments ± SEM are shown. (B) Levels of TNF-α (black bars), IL-12/23p40 (striped bars), and IL-10 (gray bars) produced by wild-type BMDMs stimulated with LPS in the absence or presence of 100 μM ATP, ADP, AMP, adenosine (Ado), clodronate (Clod.), pyrophosphate, or phosphate. Cytokine levels were measured by ELISA after 8 hours. The means ± SD of triplicate determinations are shown and are representative of 3 independent experiments. Statistical analysis is relative to samples stimulated with LPS alone. (C) The kinetics of 14C-ADP hydrolysis by resting macrophages over time (left) was determined by TLC. Hydrolysis controls consist of apyrase alone for 5 min (Apy) or apyrase in the presence of alkaline phosphatase (Apy/AP) and 14C-ADP (right). Data are representative of at least 3 independent experiments. (D-F) Levels of TNF-α (D), IL-12/23p40 (E), and IL-10 (F) produced by wild-type BMDMs stimulated with LPS in the absence or presence of 100 μM ATP or adenosine in the absence or presence of 1 U/mL ADA. Cytokines were measured 8 hours after stimulation by ELISA. The means ± SD of triplicate determinations are shown and are representative of 3 independent experiments.
ATP-derived adenosine modulates inflammatory macrophage activation. (A) Wild-type BMDMs were stimulated with LPS in the absence or presence of 100 μM ATP or nonhydrolyzable ATPγs and TNF-α (black bars), IL-12/23p40 (striped bars), and IL-10 (gray bars) production was measured by ELISA 8 hours later. The means of 3 independent experiments ± SEM are shown. (B) Levels of TNF-α (black bars), IL-12/23p40 (striped bars), and IL-10 (gray bars) produced by wild-type BMDMs stimulated with LPS in the absence or presence of 100 μM ATP, ADP, AMP, adenosine (Ado), clodronate (Clod.), pyrophosphate, or phosphate. Cytokine levels were measured by ELISA after 8 hours. The means ± SD of triplicate determinations are shown and are representative of 3 independent experiments. Statistical analysis is relative to samples stimulated with LPS alone. (C) The kinetics of 14C-ADP hydrolysis by resting macrophages over time (left) was determined by TLC. Hydrolysis controls consist of apyrase alone for 5 min (Apy) or apyrase in the presence of alkaline phosphatase (Apy/AP) and 14C-ADP (right). Data are representative of at least 3 independent experiments. (D-F) Levels of TNF-α (D), IL-12/23p40 (E), and IL-10 (F) produced by wild-type BMDMs stimulated with LPS in the absence or presence of 100 μM ATP or adenosine in the absence or presence of 1 U/mL ADA. Cytokines were measured 8 hours after stimulation by ELISA. The means ± SD of triplicate determinations are shown and are representative of 3 independent experiments.
To further implicate macrophage-generated adenosine as a key immunomodulatory mediator, we assessed cytokine production by macrophages stimulated in the presence of adenosine deaminase (ADA), which rapidly converts adenosine into inosine, thereby effectively removing adenosine from the culture. The addition of ADA to macrophages prevented the ability of adenosine or ATP to inhibit LPS-induced TNF-α (Figure 4D) and IL-12/23p40 production (Figure 4E). ADA also blocked adenosine and ATP-enhancement of IL-10 production (Figure 4F). Thus, induction of the regulatory macrophage phenotype by eATP requires macrophage hydrolysis of ATP to adenosine.
ATP-derived adenosine attenuates inflammatory cytokine production via A2bR signaling
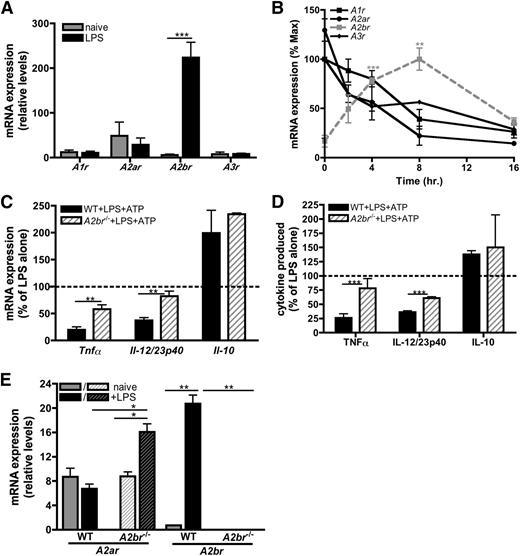
There are 4 known receptors for adenosine: A1R, A2aR, A2bR, and A3R. Quantitative reverse-transcription polymerase chain reaction (qRT-PCR) indicated that macrophages express low levels of transcripts for all 4 adenosine receptors (Figure 5A), and analysis of their expression over time revealed that adenosine receptor expression was dynamically regulated during macrophage activation (Figure 5B). Naïve macrophages express A2aR most highly (Figure 5A), whereas the A2bR increased in response to LPS. Transcript levels of the other adenosine receptors declined over time (Figure 5A-B). These data suggest that TLR stimulation selectively upregulates A2bR expression to increase the sensitivity of the macrophage to available adenosine within the extracellular milieu.
ATP-derived adenosine attenuates inflammatory cytokine production via A2bR signaling. (A) Adenosine receptor mRNA expression in naïve (gray bars) or LPS-stimulated (black bars) BMDMs at 4 hours poststimulation. The means ± SD of duplicates are shown and are representative of 3 independent experiments. (B) mRNA expression of A1r (black, closed squares; black line), A2ar (black, closed circles; black line), A2br (gray closed squares; gray, dashed line), and A3r (black, closed diamonds; black line) in BMDMs stimulated with LPS over time. The means ± SD of duplicates are shown and are representative of 3 independent experiments. (C) The percent of LPS-induced cytokine transcript expression modulated by 100 μM ATP in wild-type (solid bars) or A2br−/− (striped bars) BMDMs 4 hours poststimulation. The dashed line represents mRNA expression in BMDMs stimulated with LPS alone. The means of 3 independent experiments ± SEM are shown. (D) The percent of LPS-induced cytokine produced in the presence of 100 μM ATP in wild-type (solid bars) or A2br−/− (striped bars) BMDMs was determined by ELISA at 16 hours poststimulation. The dashed line represents cytokine production induced by LPS alone. The means ± SD of triplicates are shown and are representative of 2 independent experiments. (E) A2ar and A2br mRNA expression in wild-type (solid bars) and A2br−/− (striped bars) BMDMs unstimulated (gray bars) or stimulated with LPS (black bars) was determined by qRT-PCR at 4 hours poststimulation. The means ± SD of duplicates are shown and are representative of 3 independent experiments. WT, wild-type.
ATP-derived adenosine attenuates inflammatory cytokine production via A2bR signaling. (A) Adenosine receptor mRNA expression in naïve (gray bars) or LPS-stimulated (black bars) BMDMs at 4 hours poststimulation. The means ± SD of duplicates are shown and are representative of 3 independent experiments. (B) mRNA expression of A1r (black, closed squares; black line), A2ar (black, closed circles; black line), A2br (gray closed squares; gray, dashed line), and A3r (black, closed diamonds; black line) in BMDMs stimulated with LPS over time. The means ± SD of duplicates are shown and are representative of 3 independent experiments. (C) The percent of LPS-induced cytokine transcript expression modulated by 100 μM ATP in wild-type (solid bars) or A2br−/− (striped bars) BMDMs 4 hours poststimulation. The dashed line represents mRNA expression in BMDMs stimulated with LPS alone. The means of 3 independent experiments ± SEM are shown. (D) The percent of LPS-induced cytokine produced in the presence of 100 μM ATP in wild-type (solid bars) or A2br−/− (striped bars) BMDMs was determined by ELISA at 16 hours poststimulation. The dashed line represents cytokine production induced by LPS alone. The means ± SD of triplicates are shown and are representative of 2 independent experiments. (E) A2ar and A2br mRNA expression in wild-type (solid bars) and A2br−/− (striped bars) BMDMs unstimulated (gray bars) or stimulated with LPS (black bars) was determined by qRT-PCR at 4 hours poststimulation. The means ± SD of duplicates are shown and are representative of 3 independent experiments. WT, wild-type.
To further study the role of the A2bR in mediating regulatory macrophage development, we examined A2br−/− macrophages following stimulation with LPS and ATP. A2br−/− macrophages expressed significantly more inflammatory transcripts compared with wild-type macrophages (Figure 5C), and LPS-stimulated A2br−/− macrophages were functionally more inflammatory than their wild-type counterparts, secreting higher amounts of TNF-α and IL-12/23p40 (Figure 5D). These data indicate that the A2bR was involved in the modulation of inflammatory cytokines. The A2bR was not essential for the regulation of IL-10, because the expression of Il-10 transcripts was not significantly different between A2br−/− and wild-type macrophages (Figure 5C). Interestingly, we observed an increase in A2ar expression in A2br−/− macrophages upon activation (Figure 5E), suggesting macrophages may compensate for the absence of A2bR signaling by preferentially upregulating the A2aR. Together, these results demonstrate that in response to LPS, macrophages upregulate the A2bR to respond to (ATP-derived) adenosine to limit inflammatory macrophage responses.
CD39 blockade renders macrophages hyperinflammatory
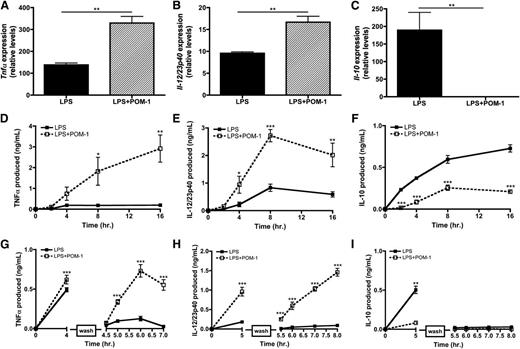
Because CD39 initiates the hydrolysis cascade necessary to generate adenosine, we hypothesized that CD39 may function as an intrinsic regulator of the macrophage inflammatory response. To address the functional significance of ATP release by macrophages on their own activation state, we examined the expression of cytokine mRNA from TLR-activated macrophages in the presence of POM-1. Blocking ATP hydrolysis with POM-1 augmented LPS-mediated inflammatory cytokine expression while significantly decreasing the production of Il-10 (Figure 6A-C). We also evaluated the kinetics of cytokine production by macrophages stimulated with LPS in the presence of POM-1 and found they secreted significantly more TNF-α and IL-12/23p40 and less IL-10 over time compared with macrophages activated in the absence of POM-1 (Figure 6D-F). It is important to highlight that these data were observed in the absence of exogenous ATP, thus revealing that CD39 blockade results in the failure to convert macrophage-released ATP into adenosine, thereby enhancing macrophage inflammatory responses.
CD39 blockade renders macrophages hyperinflammatory. (A-C) Tnfα (A), Il-12/23p40 (B), and Il-10 (C) mRNA expression at 4 hours poststimulation in wild-type BMDMs stimulated with LPS in the absence (solid bars) or presence of 10 μM POM-1 (striped bars). The means ± SD of triplicate determinations are shown and are representative of 3 independent experiments. (D-F) Cytokine production by BMDMs stimulated with LPS in the absence (closed squares; solid line) or presence (open squares; dashed line) of 10 μM POM-1. TNF-α (A), IL-12/23p40 (B) and IL-10 (C) levels were detected by ELISA. The means of at least 3 independent experiments ± SEM are shown. (G-I) BMDMs were stimulated with LPS in the absence (closed squares; solid line) or presence (open squares; dashed line) of 10 μM POM-1 and then washed with phosphate-buffered saline (break in y-axis) and replaced with LPS-free media and monitored over the next 5 hours for TNFα (D), IL-12/23p40 (E), and IL-10 (F) production. Protein levels was measured by ELISA. The means ± SD of triplicates are shown and are representative of 3 independent experiments.
CD39 blockade renders macrophages hyperinflammatory. (A-C) Tnfα (A), Il-12/23p40 (B), and Il-10 (C) mRNA expression at 4 hours poststimulation in wild-type BMDMs stimulated with LPS in the absence (solid bars) or presence of 10 μM POM-1 (striped bars). The means ± SD of triplicate determinations are shown and are representative of 3 independent experiments. (D-F) Cytokine production by BMDMs stimulated with LPS in the absence (closed squares; solid line) or presence (open squares; dashed line) of 10 μM POM-1. TNF-α (A), IL-12/23p40 (B) and IL-10 (C) levels were detected by ELISA. The means of at least 3 independent experiments ± SEM are shown. (G-I) BMDMs were stimulated with LPS in the absence (closed squares; solid line) or presence (open squares; dashed line) of 10 μM POM-1 and then washed with phosphate-buffered saline (break in y-axis) and replaced with LPS-free media and monitored over the next 5 hours for TNFα (D), IL-12/23p40 (E), and IL-10 (F) production. Protein levels was measured by ELISA. The means ± SD of triplicates are shown and are representative of 3 independent experiments.
We next examined whether CD39 activity could also influence the duration of inflammatory macrophage activation. To do this, macrophages were briefly stimulated with LPS in the presence or absence of POM-1. The LPS was then removed and the macrophages were recultured in fresh LPS-free media and cytokine release was measured over time. In the absence of POM-1, LPS-stimulated macrophages decreased TNF-α and IL-12/23p40 production shortly after the removal of LPS (Figure 6G-H). In contrast, POM-1 treatment resulted in the continued production of TNF-α and IL-12/23p40, despite the removal of LPS from macrophage cultures (Figure 6G-H). IL-10 secretion was not similarly affected, suggesting that LPS-induced IL-10 transcripts are inherently unstable (Figure 6I).
CD39-deficient macrophages exhibit impaired regulatory macrophage induction
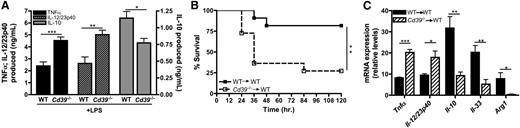
The function of macrophage-CD39 during inflammatory diseases remains poorly understood. Therefore, inflammatory responses by Cd39−/− macrophages were compared with wild-type macrophages. Similar to POM-1–treated macrophages, CD39-deficient macrophages produced significantly more TNF-α and IL-12/23p40 and less IL-10 than their wild-type counterparts when exposed to LPS in vitro (Figure 7A). To investigate the role of macrophage-specific expression of CD39 in orchestrating a proinflammatory response in vivo, we adoptively transferred naïve Cd39−/− macrophages into the peritoneum of wild-type mice. These mice were then challenged with low levels of LPS and their survival was monitored over the next 5 days. Seventy five percent of mice that received Cd39+/+ macrophages survived, whereas only 25% of the mice that received Cd39−/− macrophages survived the endotoxin challenge (Figure 7B). Regulatory macrophage-specific biomarkers were also assessed in peritoneal cells from wild-type mice following adoptive transfer of either wild-type or CD39−/− macrophages after endotoxin challenge. Consistent with the results generated in vitro (Figure 6A-C), CD39-deficient macrophages promoted significant enhancement of Tnfα and Il-12/23p40 expression in vivo (Figure 7C), while the induction of genes associated with regulatory macrophages, including Il-10, Il-33, and Arg1, was significantly impaired (Figure 7C). These results therefore suggest that normal transition from an inflammatory to regulatory macrophage activation state is important in preventing lethal septic pathology. Taken together, these data demonstrate that CD39 expressed on macrophages is necessary to limit endotoxin-induced lethality in vivo and that the presence of relatively few Cd39−/− macrophages are capable of dysregulating the course of an inflammatory response.
CD39-deficient macrophages exhibit impaired regulatory macrophage induction and enhance LPS toxicity in vivo. (A) Levels of TNF-α (black bars), IL-12/23p40 (striped bars), and IL-10 (gray bars) produced by wild-type and Cd39−/− BMDMs stimulated with LPS for 8 hours. Cytokine production was determined by ELISA. The means ± SEM are shown. (B) Kaplan-Meier survival curves of wild-type mice challenged with 300 μg LPS (intraperitoneal) 3 hours after adoptive transfer (intraperitoneal) of wild-type (closed squares; solid line) or Cd39−/− BMDMs (open squares; dashed line). Data represent the means of 10 mice per group. (C) The expression of cytokine transcripts from ex vivo peritoneal cells was determined 1 hour post-LPS challenge from wild-type mice receiving 1 × 106 naïve wild-type (solid bars) or Cd39−/− (striped bars) BMDMs. The means of samples from 4 mice per group ± SEM are shown. WT, wild-type.
CD39-deficient macrophages exhibit impaired regulatory macrophage induction and enhance LPS toxicity in vivo. (A) Levels of TNF-α (black bars), IL-12/23p40 (striped bars), and IL-10 (gray bars) produced by wild-type and Cd39−/− BMDMs stimulated with LPS for 8 hours. Cytokine production was determined by ELISA. The means ± SEM are shown. (B) Kaplan-Meier survival curves of wild-type mice challenged with 300 μg LPS (intraperitoneal) 3 hours after adoptive transfer (intraperitoneal) of wild-type (closed squares; solid line) or Cd39−/− BMDMs (open squares; dashed line). Data represent the means of 10 mice per group. (C) The expression of cytokine transcripts from ex vivo peritoneal cells was determined 1 hour post-LPS challenge from wild-type mice receiving 1 × 106 naïve wild-type (solid bars) or Cd39−/− (striped bars) BMDMs. The means of samples from 4 mice per group ± SEM are shown. WT, wild-type.
Discussion
In response to infection, macrophages produce inflammatory mediators that, if not appropriately regulated, have the potential to harm the host. This is often manifested during sepsis,3,5,46 where a switch from inflammatory to immunosuppressive macrophages has been suggested to play a critical role in protecting the host from lethal septic shock.4,7,8 However, the molecular mechanisms governing this transition remain poorly understood. In this study, we investigated the potential for macrophages to self-regulate their activation state during inflammation. We demonstrate that the induction of inflammatory macrophage responses is accompanied by intrinsic purinergic regulatory mechanisms to limit the development of a hyperinflammatory activation state. First, TLR-induced glycolysis results in the generation of ATP, a fraction of which is released through pannexin-1 membrane channels. Second, following its release into the extracellular milieu, eATP is rapidly hydrolyzed via CD39 on macrophages. Third, over the next 4 hours, TLR-activated macrophages convert ATP to adenosine and selectively upregulate endogenous A2bR expression, thus enhancing their sensitivity to ATP-derived adenosine. This results in the transition from an inflammatory to a regulatory activation state. Therefore, in contrast to IL-1β–dependent inflammation driven by high levels of exogenous ATP,9-11,39 our data demonstrate that endogenous ATP can negatively regulate macrophage activation. The novelty of this work is the demonstration that TLR-mediated macrophage activation is normally transient and that blockade of any steps of this newly defined macrophage-intrinsic regulatory cascade results in hyperinflammatory macrophage responses.
This work reveals, for the first time, an essential role of macrophage-specific CD39 in directing the course of inflammatory disease progression in vivo. To date, there are no animal models in which macrophages are the only cell type deficient in CD39; therefore, the specific contribution of macrophage-CD39 to disease pathology has been unclear. As demonstrated here, the transfer of small numbers of Cd39−/− macrophages into wild-type mice resulted in increased lethality in a murine model of septic shock. Furthermore, CD39-deficient macrophages maintained a hyperinflammatory state and failed to transition into regulatory macrophages in vitro or in vivo. These data support previous reports suggesting that the ability of inflammatory macrophages to transition to an anti-inflammatory state is vital to the survival of septic individuals4,6,7 and reveals a novel role for macrophage-CD39 in mediating this transition.
Although it has been well documented that macrophages exposed to exogenous adenosine exhibit immunomodulatory responses,45,47 the present work expands this idea and demonstrates that (1) TLR-activated macrophages themselves represent an important source of eATP, (2) macrophages rapidly and efficiently convert eATP to adenosine, (3) this conversion is dependent on CD39, and (4) macrophages respond to eATP-derived adenosine by assuming a transcriptional profile that is characteristic of regulatory macrophages. Our data are consistent with previous reports suggesting a role for A2bR-mediated immunosuppression48-50 and implicating the A2aR as the major adenosine receptor involved in IL-10 induction in macrophages.51 Interestingly, both the A2aR and A2bR are G-protein coupled receptors associated with the Gαs subunit and are capable of activating adenylate cyclase and enhancing intracellular cyclic AMP (cAMP) levels.44 We previously demonstrated that stimulating macrophages in the presence of cAMP led to the development of the regulatory phenotype25 and the activation of G-protein coupled receptors resulted in extracellular signal-regulated kinase (ERK)-dependent IL-10 hyperinduction.52,53 Indeed, LPS-stimulated macrophages exposed to adenosine resulted in ERK activation (data not shown), thereby supporting a role for ERK in driving adenosine-induced regulatory macrophage development.
Importantly, in addition to IL-10, macrophages exposed to ATP upregulate a number of transcripts that are preferentially upregulated in regulatory macrophages compared with classically (M1), including IL-33, Sphk, and HB-EGF.23-26 Some of these regulatory macrophage-specific transcripts have been associated with lung inflammation, but their expression is likely to be tissue dependent. Indeed, the negative correlation between LPS toxicity and expression of these regulatory macrophage biomarkers in mice strongly suggests that these molecules promote immunosuppression (Figure 7). Although Arg1 was upregulated in response to ATP, Arg1 may not be a reliable AAM-specific biomarker, because it can be induced through IL-4/IL-13–independent mechanisms. Arg1 expression via STAT3-dependent54 and cAMP-mediated pathways55,56 have been previously reported. Furthermore, although it was recently reported that adenosine augments IL-4–induced alternative activation in macrophages,57 we did not observe enhanced expression of any AAM-specific biomarkers in TLR-activated macrophages exposed to ATP. Together, these observations indicate that TLR-activated macrophages exposed to eATP represent a state of activation distinct from AAMs.
Homeostatic concentrations of eATP within tissues have been estimated to be within the nanomolar to micromolar range,58,59 but these values likely underestimate the level of eATP within the local, pericellular environment. Furthermore, eATP levels have been shown to increase 10- to 100-fold during pathophysiological states.59,60 Therefore, it will be interesting to understand the molecular control of ATP release from macrophages. We did not observe any change in pannexin-1 expression over time in macrophages (data not shown), suggesting that the induction of pannexin-1 expression is not a point of regulating ATP release from macrophages. Recently, caspase-3 and caspase-7 have been implicated in controlling pannexin-1 function in apoptotic cells,35 but the macrophages used in this study were not apoptotic. Thus, ATP release is likely not due to caspase activation in these cells. Interestingly, however, we observed Hif1α expression and intracellular ATP levels increased substantially over the first 2 hours poststimulation (data not shown). Therefore, we predict that the activation of glycolysis needed to synthesize intracellular stores of ATP prior to secretion may represent an important checkpoint governing the kinetics of ATP release from macrophages.
There are currently a number of adenosine-directed therapeutics under development to treat various inflammatory diseases,61 but none that specifically target macrophages. Our data reveal that the release and subsequent hydrolysis of ATP is a previously unrecognized mechanism controlling the magnitude and duration of macrophage activation. The ability for CD39-mediated ATP hydrolysis to direct regulatory macrophage development was shown to also be applicable to human monocyte and macrophages, thus highlighting the translational potential of this work. The results of this study therefore broaden the spectrum of therapeutics that could be designed to treat diseases in which macrophage activation plays a contributing role.4
In summary, we have identified a novel macrophage-intrinsic regulatory pathway that may provide a mechanism to explain how macrophages are capable of playing disparate roles in the progression and the resolution of inflammation. This work further demonstrates that TLR-induced macrophage activation is a transient state that can be efficiently reversed and that CD39 serves a central role in orchestrating the transition from a proinflammatory to an immunoregulatory state of activation in macrophages.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Vincent Lee for guidance with TLC experiments, Dr Nese Sari for assistance with NMR experiments, and Drs Thomas McCaughtry, Volker Briken, Najib El-Sayid, Andrew Stewart, and Patricia Darrah for critical review of the manuscript.
This work was supported by grants from the National Institutes of Health, National Institute of General Medical Sciences (grant R01 GM102589) (D.M.M.) and the National Institute of Allergy and Infectious Disease (training grant T32-A1089621) (H.B.C.).
Authorship
Contribution: H.B.C. designed and performed the experiments, analyzed the data, prepared figures, and wrote the manuscript; K.T.B. performed and analyzed NMR experiments; J.P.M. analyzed and supervised NMR experiments; K.R. provided A2br−/− femurs and provided valuable input on the writing of the manuscript; S.C.R. provided Cd39−/− femurs; and D.M.M. designed and supervised the research and contributed to the writing of the manuscript.
Conflict-of-interest disclosure: D.M.M. declares partial ownership in LeukoSight, Inc., a company developing a line of anti-inflammatory therapeutics. S.C.R. declares research funding by Pfizer and Helmsley Trust/Harvard Institute Translational Immunology, National Institutes of Health, and the Juvenile Diabetes Research Foundation (Australia); has lecturer fees from ACP and ATC; royalties from Biolegend, eBioscience, EMD Millipore, and Mersana; and owns equity in Puretech/Nanopharma, biopharmaceutical companies working in antibody:drug conjugates. The remaining authors declare no competing financial interests.
Certain commercial equipment, instruments, and materials are identified in this paper in order to specify the experimental procedure. Such identification does not imply recommendation or endorsement by the National Institute of Standards and Technology, nor does it imply that the material or equipment identified is necessarily the best available for the purpose.
Correspondence: David M. Mosser, Department of Cell Biology and Molecular Genetics, Room 3102 Bioscience Research Building, University of Maryland, College Park, MD 20742; e-mail: dmosser@umd.edu.