Key Points
After treatment with rituximab, immunological responses to both polysaccharide and conjugated vaccines are impaired in patients with ITP.
Splenectomized patients who received rituximab may be at increased risk of infection because of compromised immune responses to vaccines.
Abstract
B-cell depletion may impair vaccine responses and increase infection risk in patients with immune thrombocytopenia (ITP). We investigated the effects of rituximab on antibody and cellular responses to Streptococcus pneumoniae polysaccharide and Haemophilus influenzae type b (Hib) vaccines in ITP patients. Of 60 patients in the main trial, 24 patients received both vaccines 6 months after rituximab (n = 17) or placebo (n = 7). Among 20 evaluable patients, 3 of 14 (21%) in the rituximab group and 4 of 6 (67%) in the placebo group achieved a fourfold increase in anti-pneumococcal antibodies (P = .12). For anti-Hib antibodies, 4 of 14 (29%) and 5 of 6 (83%), respectively, achieved a fourfold increase (P < .05). Fewer patients in the rituximab group demonstrated Hib killing (2 of 14 [14%], 5 of 6 [83%], P < .05). Three of 14 rituximab-treated patients failed to respond to vaccines by any criteria. After vaccinations, preplasma cell blasts and interferon-γ–secreting T cells were reduced in rituximab-treated patients. Antibody responses were impaired for at least 6 months after rituximab. Cellular immunity was reduced in parallel with depleted B-cell pools. These findings have implications for the timing of vaccinations and the mechanism of infection after rituximab in ITP patients.
Introduction
Immune thrombocytopenia (ITP) is an autoimmune disorder characterized by anti-platelet autoantibodies, thrombocytopenia, and bleeding. Approximately 80% of adult patients will develop a chronic form of ITP and many will require treatment; but oftentimes, the disease runs a benign course. B-cell depletion therapy with the monoclonal anti-CD20 antibody rituximab, is commonly used to induce remission in patients with ITP.1,2 In a systematic review, we reported that rituximab was associated with an initial overall response rate of ∼60% and a median response duration of 10 months. Additional observational data suggest that long-term remissions occur in ∼20% of patients.3-5 Our review also identified a potential signal of harm with rituximab: of 303 treated patients in published reports, 7 (2.3%) developed serious infections 4 of which were fatal.
Many patients with ITP including those who fail rituximab will ultimately require splenectomy, which improves platelet counts in 60% to 70% of patients.6 Splenectomy itself poses a risk of infection, mainly with encapsulated bacteria; thus, vaccination against Streptococcus pneumoniae, Haemophilus influenzae type b (Hib), and Neisseria meningitidis are recommended for all patients before splenectomy.7,8 Immune responses to these vaccines may be impaired in patients who have previously been exposed to rituximab because of its effect on antibody-producing cells.9-17 This raises particular concerns for ITP patients who have received rituximab and who eventually require splenectomy since the protection from presplenectomy vaccines may be compromised.
We designed a sub-study of a multicentered, randomized, placebo-controlled trial of nonsplenectomized patients with ITP to determine the impact of rituximab on vaccine responses. All participants received the pneumococcal polysaccharide vaccine and the Hib conjugate vaccine 6 months after rituximab or placebo infusions. We measured antibody responses after vaccinations and correlated the results with the frequency of T- and B-cell subsets. We found significant impairments in the ability of rituximab-treated patients to mount a specific antibody response with some patients demonstrating altered T-cell function. The data may explain how rituximab may predispose to infection, and provide a rationale for appropriate timing of vaccinations in this patient group.
Methods
Study design
We conducted a prospective cohort study in a subgroup of patients who were enrolled in a multicentered, randomized, double-blind, placebo-controlled trial of rituximab for nonsplenectomized patients with ITP receiving standard treatment.4 Three of 7 centers in Canada participated in this substudy. Six months after rituximab or placebo infusions, patients received both the 23-valent pneumococcal polysaccharide vaccine (Pneumovax-23; Merck) and the Hib conjugate vaccine (ActHIB; Aventis). The pneumococcal vaccine was administered by subcutaneous injection and contained a mixture of purified pneumococcal capsular polysaccharides from the 23 most common serotypes associated with invasive pneumococcal infection. The Hib vaccine was administered by intramuscular injection and contained the capsular polysaccharide polyribosyl-ribitol phosphate antigen, the major virulence factor, conjugated to tetanus toxoid. Blood samples were collected immediately before rituximab or placebo infusion, immediately before vaccinations (6 months after rituximab), and 1 week, 4 weeks, and 6 months after vaccinations. Serum samples were frozen and batched for antibody testing. Whole blood samples for B- and T-cell quantification were shipped fresh to the central laboratory for immediate processing and testing.
Patients
Patients who were eligible for the main trial were adults with ITP who had a platelet count below 30 × 109/L and required treatment.4 Exclusions were secondary causes of thrombocytopenia, splenectomy, previous rituximab treatment, significant cardiac or pulmonary disease, active infection or known allergies to vaccines. Patients provided separate consent to participate in the vaccine substudy in accordance with the Declaration of Helsinki, which was approved by the research ethics board at each participating site.
Antibody response to vaccines
The antibody response to vaccines was measured using quantitative and functional methods. We measured anti-pneumococcal capsular polysaccharide antibodies (specific to S pneumoniae) and anti-polyribosyl-ribitol phosphate antibodies (specific to Hib) by enzyme-linked immunosorbent assays (The Binding Site: VaccZyme Anti-PCP Immunoglobulin G [IgG] Enzyme Immunoassay; and VaccZyme Human Anti-Hib Enzyme Immunoassay). IgG-specific anti-pneumococcal antibodies were directed against a mixture of the capsular polysaccharide serotypes 1, 2, 3, 4, 5, 6B, 7F, 8, 9N, 9V, 10A, 11A, 12F, 14, 15B, 17F, 18C, 19F, 19A, 20, 22F, 23F, and 33F, which are contained in the 23-valent vaccine. Interfering antibodies against the pneumococcal cellular antigen were absorbed in a preincubation step. Samples were added to precoated wells, incubated for 30 minutes and washed. Specific antibodies were detected using a colorimetric substrate detected at 450 nm.
A serum bactericidal assay was used to assess the functional effect of anti-Hib antibodies on bacterial growth.18 For the test, twofold serial dilutions of test sera were incubated with 10 000 bacterial cells per well of Hib (strain GB3292) with 10 μL of Hanks buffer with Ca2+ and Mg2+ (Life Technologies), 2% Fildes enrichment (BBL; Becton Dickinson and Co.), and complement (sterile serum from 3- to 4-week-old baby rabbits; Pel-Freez). Bactericidal activity was assessed using Alamarblue, a fluorescent metabolic indicator (Life Technologies). Bactericidal titers were reported as the reciprocal of the serum dilution capable of killing 50% of bacterial cells compared with complement controls.
An adequate response to either the pneumococcal vaccine or the Hib vaccine was defined according to previously established criteria, as a fourfold increase in antibody concentration from baseline within the first month after vaccinations.19 For Hib, a specific IgG titer of 1 μg/mL or higher was considered protective.20 For the Hib bactericidal assay, a fourfold increase in bacterial killing within the first month after vaccinations was considered an adequate response.21
B-cell subsets and T-cell function in response to vaccines
Total B cells, B-cell subsets, and T cells were quantified by flow cytometry and reported as proportions of CD19+ B cells and CD3+ T cells among CD45+ leukocytes in whole blood. Subsets of naive B cells (CD19+CD27−), resting memory B cells (CD19+CD27+CD38−/low) and preplasma cell blasts (CD19lowCD27hiCD38hi) were determined by 3-color flow cytometry, as a proportion of peripheral blood mononuclear cells (PBMCs) isolated from whole blood by density gradient centrifugation. We calculated absolute preplasma cell blasts from the proportion of total CD19+ B cells and total lymphocyte count.
A human interferon-γ (IFN-γ) enzyme-linked immunosorbent spot (ELISPOT) assay was used to measure the frequency of tetanus toxoid-specific T cells (Mabtech). PBMCs were isolated from whole blood by density gradient centrifugation. B cells were depleted from PBMC samples by automated positive magnetic selection with anti-CD19 nanobeads (RoboSep and EasySep CD19 positive selection kit; StemCell Technologies) to normalize the number of antigen-presenting cells in rituximab and placebo samples. PBMCs, depleted of B cells, were added to wells (5 × 105/well) containing the recall antigen tetanus toxoid in RPMI media supplemented with 10% human serum, 100 IU/mL penicillin, 100 μg/mL streptomycin and 2nM l-glutamine (Invitrogen). Plates were incubated at 37°C, in a humidified environment containing 5% CO2 for 48 to 64 hours prior to washing with PBS. Captured IFN-γ was detected with an enzyme-conjugated antibody and positive spots were developed with 3,3′,5,5′-Tetramethylbenzidine substrate, enumerated (Bioreader 5000 ELISPOT reader; Bio-Sys GmbH) and expressed as spots per 5 × 105 PBMCs.
Statistical analysis
Descriptive statistics of continuous measurements included means, standard deviations, standard errors, medians and lower and upper quartiles. Comparisons of means between rituximab and placebo groups were performed using a 2-sample t test. The proportion of patients who achieved an adequate vaccine response (defined as a fourfold increase in antibody titer or fourfold increase in bactericidal activity) was compared between rituximab and placebo groups using the Fisher 2-sided exact test and reported as the difference in proportions with 95% confidence intervals (CIs). Tests were considered statistically significant at the 5% level.
Results
Patient demographics
Of the 60 patients (from 7 centers) enrolled in the main trial, 24 patients (from 3 centers) participated in this vaccine substudy. Seventeen patients had received rituximab and 7 patients had received placebo (Table 1). Median age was 40 years, and 17 of 24 (71%) were females. At the time of vaccinations, median platelet count was 115 × 109/L in the rituximab group and 42 × 109/L in the placebo group. Three patients in the rituximab group had received other ITP treatments during the 4 weeks before vaccinations (intravenous immune globulin, n = 1; corticosteroids, n = 1; and danazol, n = 1); and 1 patient in the placebo group had received danazol before vaccinations (Table 1).
Baseline characteristics of patients with ITP at the time of vaccinations with S pneumonia and Hib vaccines
| Characteristics . | Rituximab (n = 17) . | Placebo (n = 7) . |
|---|---|---|
| Median age, y (Q1, Q3)* | 40 (37, 66) | 40 (35, 63) |
| Female, N (%) | 12 (71) | 5 (71) |
| Platelet count, × 109/L median (Q1, Q3) | 115 (26, 221) | 42 (30, 208) |
| ITP treatments during the 4 weeks before vaccinations | ||
| Intravenous immune globulin, N | 1 | 0 |
| Prednisone, N | 1 | 1 |
| Danazol, N | 1 | 1 |
| Characteristics . | Rituximab (n = 17) . | Placebo (n = 7) . |
|---|---|---|
| Median age, y (Q1, Q3)* | 40 (37, 66) | 40 (35, 63) |
| Female, N (%) | 12 (71) | 5 (71) |
| Platelet count, × 109/L median (Q1, Q3) | 115 (26, 221) | 42 (30, 208) |
| ITP treatments during the 4 weeks before vaccinations | ||
| Intravenous immune globulin, N | 1 | 0 |
| Prednisone, N | 1 | 1 |
| Danazol, N | 1 | 1 |
Patients had received rituximab or placebo 6 months earlier as part of a randomized clinical trial.
Q1 and Q3 are the first and third quartiles.
Antibody response to vaccines
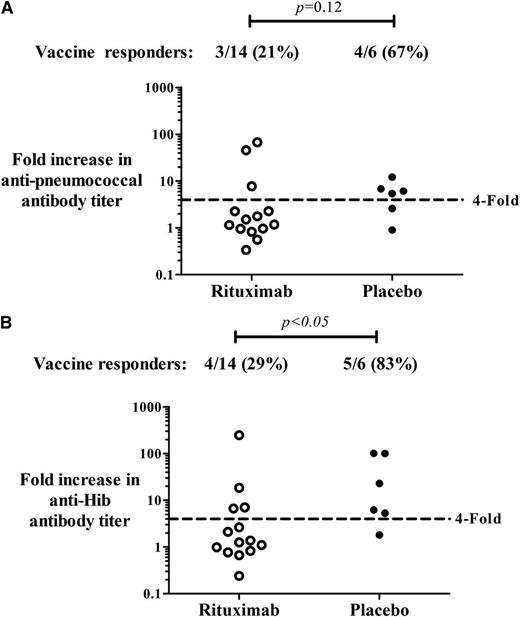
Antibody responses were assessed in 14 patients in the rituximab group, and 6 patients in the placebo group (4 patients were not evaluable because of missing baseline samples). Within 1 month of receiving the pneumococcal and Hib vaccines, 3 of 14 (21%) patients who had received rituximab 6 months earlier achieved a fourfold increase in specific anti-pneumococcal antibody titers compared with 4 of 6 (67%) patients in the placebo group (P = .12; difference in proportions = 46%; 95% CI, 2%-89%). For anti-Hib, 4 of 14 (29%) patients in the rituximab group and 5 of 6 (83%) patients in the placebo group achieved a fourfold increase in antibody titers (P < .05; difference in proportions = 54%; 95% CI, 17%-93%) (Figure 1).
Antibody response to vaccinations. IgG specific antibodies to (A) S pneumoniae and (B) Hib detected in the first month after the pneumococcal polysaccharide vaccine and the conjugate Hib vaccine. Patients had ITP and had been treated with rituximab (n = 14) or placebo (n = 6) 6 months before vaccinations. Vaccine responders were defined as patients who achieved at least a fourfold increase in antibody titer compared with baseline values.
Antibody response to vaccinations. IgG specific antibodies to (A) S pneumoniae and (B) Hib detected in the first month after the pneumococcal polysaccharide vaccine and the conjugate Hib vaccine. Patients had ITP and had been treated with rituximab (n = 14) or placebo (n = 6) 6 months before vaccinations. Vaccine responders were defined as patients who achieved at least a fourfold increase in antibody titer compared with baseline values.
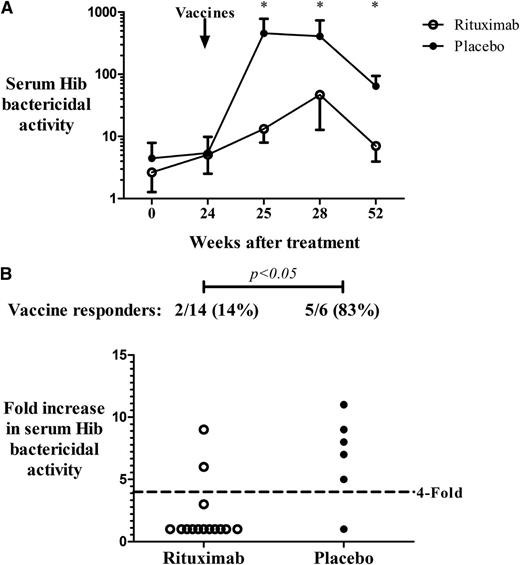
Hib bactericidal activity was reduced for rituximab-treated patients compared with placebo especially in the first week after vaccinations (Figure 2A). Two of 14 (14%) patients in the rituximab group achieved a fourfold increase in bactericidal activity from baseline values compared with 5 of 6 (83%) patients in the placebo group (P < .05; difference in proportions = 69%; 95% CI, 34%-100%) (Figure 2B). Two patients in the rituximab group who had achieved a fourfold increase in anti-Hib antibody titer by enzyme-linked immunosorbent assay and who had antibody concentrations above the threshold that is considered protective (1 µg/mL) did not demonstrate bactericidal activity. In contrast, all patients in the placebo group who achieved a fourfold increase in anti-Hib titers also demonstrated a significant increase in bactericidal activity. Results of antibody responses to pneumococcal and Hib vaccines did not change when the 4 patients who had received ITP therapy within 4 weeks before vaccinations were removed from the analysis.
Serum bactericidal activity against Hib. (A) Titer (reciprocal of the serum dilution capable of killing 50% of bacterial cells compared with complement controls) of serum bactericidal activity against Hib post vaccinations in ITP patients treated with rituximab (n = 14) or placebo (n = 6) (*P < .05; bars represent SEM). (B) Vaccine responders, defined as patients who achieved a fourfold increase in functional Hib killing in the first month after vaccinations.
Serum bactericidal activity against Hib. (A) Titer (reciprocal of the serum dilution capable of killing 50% of bacterial cells compared with complement controls) of serum bactericidal activity against Hib post vaccinations in ITP patients treated with rituximab (n = 14) or placebo (n = 6) (*P < .05; bars represent SEM). (B) Vaccine responders, defined as patients who achieved a fourfold increase in functional Hib killing in the first month after vaccinations.
B-cell mobilization after vaccines
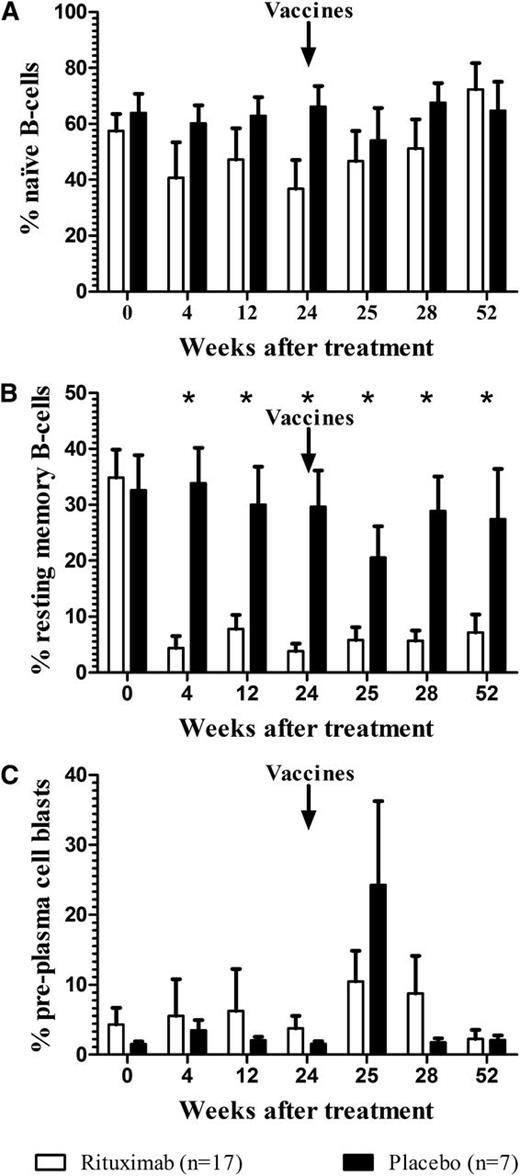
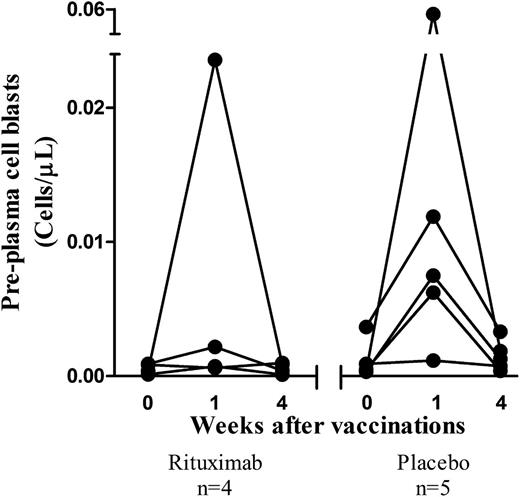
Enumeration of B and T cells was performed in all patients (rituximab, n = 17; placebo, n = 7). Peripheral blood CD19+ B cells were rapidly depleted by rituximab and remained depleted 12 months after treatment; whereas CD3+ T-cell levels were unaffected (data not shown). Naive B cells (CD19+CD27−) were slightly reduced 1 month after rituximab and recovered to baseline levels by 1 year (Figure 3A). In contrast, resting memory B cells (CD19+CD27+CD38−/low) were significantly lower than baseline values after rituximab treatment and remained 80% depleted 1 year later (P < .05) (Figure 3B). In rituximab-treated patients, preplasma cell blasts (CD19−CD27+CD38hi) were at near-normal levels 6 months after the infusion (Figure 3C); however a high degree of between-patient variability was observed. Absolute numbers of preplasma cell blasts were evaluable in a subset of patients: 4 of 5 patients in the placebo group demonstrated a sharp burst 1 week after vaccinations as expected22,23 ; whereas, only 1 of 4 evaluable patients in the rituximab group demonstrated a rise in preplasma cell blasts immediately after vaccinations (Figure 4).
Effect of rituximab on peripheral blood B-cell subsets. (A) Naive B cells (CD19+CD27−); (B) resting memory B cells (CD19+CD27+CD38−/low); and (C) preplasma cell blasts (CD19−CD27+CD38hi) expressed as percentage of total CD19+ cells (*P < .05; bars represent SEM). Rituximab (n = 17) or placebo (n = 7) was administered at time 0; S pneumoniae and Hib vaccines were administered on week 24.
Effect of rituximab on peripheral blood B-cell subsets. (A) Naive B cells (CD19+CD27−); (B) resting memory B cells (CD19+CD27+CD38−/low); and (C) preplasma cell blasts (CD19−CD27+CD38hi) expressed as percentage of total CD19+ cells (*P < .05; bars represent SEM). Rituximab (n = 17) or placebo (n = 7) was administered at time 0; S pneumoniae and Hib vaccines were administered on week 24.
Preplasma cell blasts before and after vaccinations. Absolute cell counts of peripheral blood preplasma cell blasts in patients with ITP who received rituximab (n = 4) or placebo (n = 5) 6 months earlier. All patients received vaccinations with S pneumoniae and Hib vaccines.
Preplasma cell blasts before and after vaccinations. Absolute cell counts of peripheral blood preplasma cell blasts in patients with ITP who received rituximab (n = 4) or placebo (n = 5) 6 months earlier. All patients received vaccinations with S pneumoniae and Hib vaccines.
T-cell responses after vaccinations
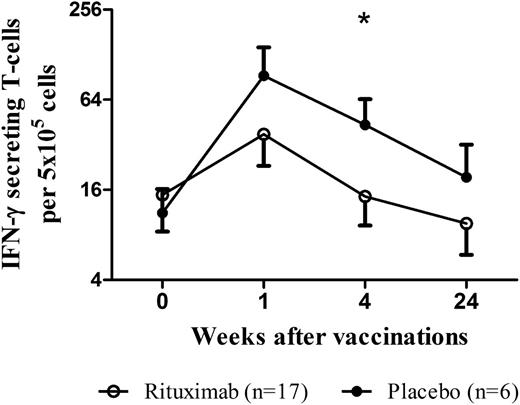
Functional T-cell responses were assessed in 17 patients in the rituximab group and 6 patients in the placebo group (one patient on placebo could not be analyzed due to missing samples). Following vaccinations, the mean number of IFN-γ–producing T cells was reduced in patients who had received rituximab compared with placebo at 1 week (38 cells vs 93 cells per 5 × 105 total cells) and 1 month (14 cells vs 43 cells per 5 × 105 total cells) (Figure 5).
Effect of rituximab on T-cell function after vaccinations. IFN-γ–secreting T cells were determined by ELISPOT at 0, 1, 4, and 24 weeks after vaccination with S pneumoniae and Hib vaccines in patients with ITP who received rituximab (n = 17) or placebo (n = 6) 6 months earlier (*P < .05; bars represent SEM).
Effect of rituximab on T-cell function after vaccinations. IFN-γ–secreting T cells were determined by ELISPOT at 0, 1, 4, and 24 weeks after vaccination with S pneumoniae and Hib vaccines in patients with ITP who received rituximab (n = 17) or placebo (n = 6) 6 months earlier (*P < .05; bars represent SEM).
Vaccine failures after rituximab
Three of 14 evaluable patients in the rituximab group failed to respond to both vaccines by all of the following established criteria: (1) They did not achieve a fourfold increase in specific antibodies against S pneumoniae and Hib; (2) the concentration of anti-Hib antibodies was below protective levels after vaccinations; and (3) they did not demonstrate an increase in bactericidal activity against Hib. No vaccine failures were observed among the 6 evaluable patients on placebo.
Discussion
Rituximab results in a platelet count response in ∼60% of ITP patients3 and 20% of patients will achieve long-term remissions.5 The increasing use of rituximab in ITP, an autoimmune disorder that often runs a benign course, calls for an urgent need to better understand the potential harms in this patient population. Most concerning is the possibility of prolonged alterations in normal immunity, which may predispose to infection or impair vaccine responsiveness. This is the first analysis of the impact of rituximab on vaccine responses in patients with ITP. We found that specific antibody responses to both the pneumococcal polysaccharide vaccine (representing a T-cell independent response) and the Hib conjugate vaccine (representing a T-cell dependent response) were significantly impaired for at least 6 months after rituximab treatment. We also demonstrated a reduction in bacterial killing using a functional assay, which was often less pronounced than specific IgG levels. The dissociation between quantitative and functional immunity has been previously reported after Hib and N meningitidis immunizations24-26 and may be attributable to differences in antibody avidity26 or the presence of other isotypes, such as IgM. Immunophenotypic analysis indicated that, while preplasma cell blasts were relatively resistant to depletion by rituximab since they express weak or no CD20, the “burst” in preplasma cell blasts that is expected after vaccinations22,23 was frequently lost.
Infection, although rare, is a serious concern with rituximab in patients with ITP3 and other autoimmune diseases.27,28 Rituximab has been associated with a risk of reactivation of latent viruses, especially active or occult hepatitis B virus (HBV) infection.29,30 In a study of 394 lymphoma patients with isolated hepatitis B core (HBc) antibody, the frequency of hepatitis B reactivation was 3 times higher in patients who received rituximab compared with standard chemotherapy (2.7% vs 0.8%).31 HBV reactivation is characterized by rapid viral replication due to a loss of immune surveillance, and a loss of CD4+ T-helper and CD8+ cytotoxic T-cell activity.32 Rituximab has also been associated with rare cases of progressive multifocal leukoencephalopathy, a lethal neurological condition caused by reactivation of latent JC virus,33,34 which prompted a safety warning by the FDA. Our data may offer some insight into the mechanism of viral reactivation: We documented a significant impairment in T-cell function, which was likely the result of a reduced pool of antigen presenting B cells.35 An indirect effect of rituximab on T cells is consistent with other data showing an improvement of T-cell dysregulation after treatment.36-38
A significant proportion of adults with chronic ITP will eventually undergo splenectomy to control their disease.39,40 Splenectomy itself is associated with a risk of infection that is ∼2.6-fold higher than nonsplenectomized ITP patients in the first 90 days after splenectomy, and 1.4-fold higher beyond 1 year.41 In a pooled analysis of splenectomized ITP patients (n = 484; median follow-up for the entire cohort, 6.9 years), 2.1% developed invasive infection, and 1.2% died.42 Encapsulated bacteria have been implicated in overwhelming postsplenectomy infections,40 thus routine vaccination against S pneumoniae, H influenzae, and N meningitidis is recommended before splenectomy.7,8 In longitudinal studies, vaccination generally resulted in a protective antibody response in most patients43 ; however, antibody levels are not routinely measured in practice. Splenectomized patients who have received rituximab may be at an even greater risk of infection because the protection afforded by vaccines may be compromised.
The impact of rituximab on the immune response to vaccination has been examined in patients with malignancy and rheumatic disease. In patients with lymphoma previously treated with high-dose chemotherapy, rituximab was associated with an inadequate antibody response to pneumococcal vaccine but a preserved response to tetanus toxoid and Hib vaccines.44 The addition of rituximab to chemotherapy in 11 patients with hematological malignancies resulted in a failure to respond to the H1N1 vaccine,14 and low rates of seroconversion were observed in 14 patients with lymphoid malignancies; however, responses were not significantly different from control patients who did not receive rituximab.45 In one study of patients with rheumatoid arthritis (n = 14), rituximab was not associated with an impaired antibody response to 2 of 3 influenza virus antigens even during profound B-cell depletion15 ; whereas in another study (n = 17) a poor response to the influenza vaccine was observed.10 Cellular immune responses to influenza vaccination was preserved in rituximab-treated patients with rheumatoid arthritis while humoral immunity was severely impaired.46 In another report, both humoral and cellular responses were found to be compromised.47 In summary, previous studies have focused on patients with underlying hematological malignancies who had received rituximab in addition to myelosuppressive chemotherapy14,17,44,45 or patients with rheumatoid arthritis10,11,15 in the context of biologic agents and other immunosuppressant medications. In general, these studies suggested that antibody responses were compromised. Ours is the first study to assess the impact of rituximab on immune responses in ITP controlling for disease stage and timing of rituximab exposure.
Strengths of this study were the controlled design, which took advantage of a prospective randomized trial, the use of complementary immunological methods and carefully controlled experiments. Small sample size was a limitation. This was due in part to the need for fresh samples for cell-based assays which were sometimes compromised by transit delays, and nonparticipation of some of the primary study centers due to resource constraints. The lack of an established correlation between antibody titers and protective immunity24,26 limits the inferences of our findings on infection avoidance. Although no adverse events were observed in our study, a longer follow-up period would be required to determine the true risk of infection in patients who did not respond to vaccines.
In light of our findings, we provide the following recommendations: (1) for patients with ITP, vaccines for S pneumoniae, H influenzae, and N meningitidis should be administered before rituximab, in anticipation that some patients will go on to require splenectomy; (2) patients should be counseled on the potential risk of vaccine unresponsiveness after rituximab; and (3) as with other patient groups,48 ITP patients should be screened for active or occult HBV infection before receiving rituximab, given the alterations in cellular immunity and the risk of viral reactivation. Other investigators have made similar recommendations about vaccinations before rituximab in ITP49 and rheumatic disease.50
In summary, rituximab was associated with an impaired antibody response to pneumococcal and Hib vaccines during the period of B-cell depletion. Antibody levels against Hib did not indicate functional bactericidal activity for all patients. B-cell depletion resulted in a loss of the “burst” in preplasma cell blasts that is expected after vaccinations, which may explain why specific antibody production was reduced. The overall reduction in antigen presenting cells may result in impaired T-cell function. Our findings provide insight into the mechanisms of humoral and cellular immune suppression caused by rituximab in patients with ITP.
There is an Inside Blood commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors are indebted to Rumi Clare, Diana Moffatt, Jane Moore, James Smith, and Cheryl Kipling for their expert technical work on the assays. The authors thank Julie Carruthers for coordinating the multicentered study and Yang Liu for statistical assistance. The authors thank Dr Romero-Steiner for providing the H influenzae type b strain for our experiments. This study would not have been possible without the dedication of Lianna Butler, Ruth Cameron, Laura Meraw, and Elizabeth Chatelain, site research coordinators, and the patients who participated in this study.
This was supported by the Canadian Institutes for Health Research (Operating Grant #89897) and by Canadian Blood Services. The main trial was an investigator-initiated study led by D.M.A. from McMaster University and funded by Hoffmann-LaRoche. The company had no role in the design, conduct or reporting of this study.
Authorship
Contribution: I.N. designed and performed the research, analyzed the data, and wrote the manuscript; J.G.K., M.L., and D.P.S. designed the research, analyzed the data, and edited and approved the manuscript; N.M.H., M.A.C., and R.J.C. conceived the research, analyzed the data, and edited and approved the manuscript; D.M.A. conceived the research, designed the research, analyzed the data, and wrote the manuscript; and A.T.T. and J.M. collected the data and edited and approved the final manuscript.
Conflict-of-interest disclosure: D.M.A. obtained research funding for investigator-initiated studies in ITP from Amgen, GlaxoSmithKline (GSK) and Hoffman-LaRoche, and has been a member of advisory boards for Amgen and GSK. J.G.K. has received research funding from Roche and Amgen and served as an advisory board member and chair for Amgen and GlaxoSmithKline. M.L. is founder, shareholder, and consultant for Circassia, Ltd., scientific founder and consultant for Adiga Life Sciences, and a consultant for Sanofi. The remaining authors declare no competing financial interests.
Correspondence: Donald M. Arnold, Department of Medicine, Michael G. DeGroote School of Medicine, McMaster University, HSC 3V-50, 1280 Main St West, Hamilton, ON, Canada L8N 4K1; e-mail: arnold@mcmaster.ca.