Abstract
Dasatinib is a potent BCR-ABL inhibitor effective in chronic myeloid leukemia and Ph+ acute lymphoblastic leukemia (ALL) resistant/intolerant to imatinib. In the GIMEMA LAL1205 protocol, patients with newly diagnosed Ph+ ALL older than 18 years (with no upper age limit) received dasatinib induction therapy for 84 days combined with steroids for the first 32 days and intrathecal chemotherapy. Postremission therapy was free. Fifty-three patients were evaluable (median age, 53.6 years). All patients achieved a complete hematologic remission (CHR), 49 (92.5%) at day 22. At this time point, 10 patients achieved a BCR-ABL reduction to < 10−3. At 20 months, the overall survival was 69.2% and disease-free survival was 51.1%. A significant difference in DFS was observed between patients who showed at day 22 a decrease in BCR-ABL levels to < 10−3 compared with patients who never reached these levels during induction. In multivariate analysis, BCR-ABL levels of < 10−3 at day 85 correlated with disease-free survival. No deaths or relapses occurred during induction. Twenty-three patients relapsed after completing induction. A T315I mutation was detected in 12 of 17 relapsed cases. Treatment was well tolerated; only 4 patients discontinued therapy during the last phase of the induction when already in CHR. In adult Ph+ ALL, induction treatment with dasatinib plus steroids is associated with a CHR in virtually all patients, irrespective of age, good compliance, no deaths, and a very rapid debulking of the neoplastic clone. This trial was registered at www.clinicaltrials.gov as #NCT00391989.
Introduction
Despite an improved understanding of the biology of acute lymphoblastic leukemia (ALL), the overall prognosis of adult patients remains unsatisfactory.1-3 The Philadelphia (Ph) chromosome is the most frequent genetic abnormality in adult ALL; its prevalence increases with age, accounting for 12% to 30% in patients 18 to 35 years of age, 40% to 45% in patients 36 to 50 years of age,4,5 and > 50% in patients older than 60 years.6 The Ph chromosome and BCR-ABL fusion gene have been associated with a highly unfavorable prognosis, independent of age,7,8 and elderly patients were often treated only with supportive therapy.
The tyrosine kinase inhibitor (TKI) imatinib has profoundly altered the management of patients with chronic myeloid leukemia and impacted on the natural course of the disease.9,10 Imatinib has also been effectively used in Ph+ ALL, both in adults and children.11-13
In a GIMEMA study, all 29 Ph+ ALL patients aged 60 years of age or older treated with imatinib plus prednisone without chemotherapy as first-line treatment obtained a complete hematologic remission (CHR).14 The GMALL study group also showed that, in elderly patients with de novo Ph+ ALL, induction with imatinib resulted in a significantly higher CR rate and lower toxicity than with chemotherapy.15
Dasatinib is a second-generation TKI with a 300-fold greater activity than imatinib in vitro.16,17 Dasatinib has demonstrated a marked efficacy in patients with CML after relapse or resistance to imatinib18-21 and, more recently, as first-line treatment.22 In particular, dasatinib is active in patients who have developed imatinib-resistant BCR-ABL mutations, except for T315I. In relapsed/refractory adult Ph+ ALL, > 30% of patients treated with dasatinib achieved a CHR within 6 months of treatment.15,23
The GIMEMA cooperative group initiated in 2006 a phase 2 study to assess the activity, in terms of hematologic CR rate, of first-line induction treatment with dasatinib without systemic chemotherapy for adult Ph+ ALL patients of any age. The final results are hereby reported.
Methods
Patients
Protocol LAL1205 was approved by the ethics committee of all centers. Patients 18 years of age or older (with no upper age limit) were eligible if they had been diagnosed with Ph+/BCR-ABL+ ALL, had not received anti–leukemic drugs except for steroids, had a WHO performance status of 2 or less, and had no central nervous system leukemia. All patients provided written informed consent in accordance with the Declaration of Helsinki.
Study design and therapy
Patients received a 7-day steroid prephase of oral prednisone at increasing doses (10-60 mg/m2 per day), during which the presence of the BCR-ABL transcript was centrally confirmed. Patients then received induction therapy with oral dasatinib 70 mg twice daily for 84 days. Prednisone 60 mg/m2 per day (capped at 120 mg daily) was administered until day 24 and then tapered and stopped at day 32. Intrathecal methotrexate was administered on days 22 and 43. Dasatinib dose reduction/discontinuation was permitted after nonhematologic toxicity only and discontinuation was permitted after recurrence of a grade 3 or 4 toxicity only. Postinduction treatment was not specified.
The primary endpoint was the CHR rate after dasatinib induction. Secondary objectives were toxicity, immunophenotypic response rate, molecular response rate, disease-free survival (DFS), relapse rate, and overall survival (OS).
Response assessment and toxicity monitoring
Hematologic parameters were closely monitored. BM evaluations, including immunophenotypic and molecular monitoring, were performed at days 22, 43, 57, and 85 (end of induction), with additional assessments if necessary. CHR was defined as a reduction in BM blasts to < 5%, absence of blasts in the PB, and no extramedullary involvement from leukemia, CHRi was defined as CHR without complete recovery of the blood count (neutrophils ≥ 1.5 × 109/L and platelets ≥ 100 × 109/L). After CHR achievement, hematologic relapse was defined as presence of blasts in PB or any nonhematologic site, or ≥ 5% BM blasts. Steroid response was based on the PB blast reduction rate (threshold, 75%) at the end of the pre–phase.24 Adverse events were graded according to the Common Toxicity Criteria Grading System of the National Cancer Institute Version 3.0.
Molecular diagnosis and MRD monitoring
All molecular and immunophenotypic analyses were performed at the “Sapienza” University of Rome, with molecular methods standardized according to the European Union BIOMED-1 Concerted Action program.25 For diagnosis, total RNA was extracted26 from BM, and RT-PCR was performed using primers specific for the p190 or p210 forms of the protein.27 Minimal residual disease (MRD) monitoring was performed by quantitative RT-PCR.28,29 BCR-ABL transcript levels were expressed as a percentage compared with the ABL control gene, with cut-off values of ≥ 10−3 or < 10−3.
Immunophenotypic diagnosis and MRD monitoring
BM samples were evaluated by different combinations of 4 fluorochrome-conjugated (FITC/PE/PerCP/APC) antibodies: CD58/CD10/CD34/CD19; CD38/CD10/CD34/CD19; CD45/CD10/CD34/CD19; CD10/CD20/CD34/CD19; TdT/CD10/CD34/CD19; TdT/IgM/CD34/CD19; CD10/CD22/CD34/CD19; CD66c/CD10/CD34/CD19; CD13/CD10/CD34/CD19; and CD33/CD10/CD34/CD19. All reagents came from BD Biosciences, except for CD13-, CD33-, TdT-FITC, and CD10-PE, from Dako Denmark, CD38-, CD58-, CD66c-FITC, CD20-, and CD22-PE from Immunotech Beckman Coulter, and IgM-PE from Southern Biotechnology. Nuclear TdT and intracytoplasmic IgM were evaluated after cell fixation and permeabilization using the Fix and Perm Cell Permeabilization kit (BD Biosciences). MRD was evaluated by flow cytometry only in patients characterized at diagnosis, at the same time points of the molecular monitoring (days 22, 43, 57, and 85).
The flow cytometer FACSCalibur (BD Biosciences) acquired 30 000 events at the time of diagnosis and at least 800 000 for the detection of MRD. Data were analyzed using the CELLQuest and PAINT-A-GATE software (BD Biosciences).
Mutation analysis
Mutation analysis was performed on relapse samples. Total RNA was extracted from mononuclear cells and reverse transcribed. Scanning of the ABL KD for the presence of mutations was performed as reported.30,31 Briefly, after a first amplification of a fragment spanning, both the BCR-ABL breakpoint and the ABL KD, 3 overlapping amplicons covering the KD (amino acids 206-335, 262-421, and 371-524) were generated by nested PCR and prescreening for the presence of sequence variations by D-HPLC (WAVE 3500-HT; Transgenomic). Assay sensitivity was 5% to 10%. To ensure that mutations present in ≥ 90% of BCR-ABL+ cells could not escape D-HPLC detection, a mixture of wild-type and patient PCR products in a 1:1 ratio was also run. In D-HPLC+ cases, subsequent sequencing of both strands for each fragment was done on an ABI PRISM 3730 (Applied Biosystems). The sensitivity of the method was 20% to 25%.
Statistical methods
To assess differences in PCR levels between the various time points during induction treatment (ie, day 0 vs day 22, day 22 vs day 43, and so on), the Friedman test was used. OS was defined as the time from diagnosis to death from any cause and DFS as the time from day 85 to relapse, death, or date of last follow-up for patients alive in first CHR. In case of patients without a day 85 evaluation, the last evaluation date was considered (last observation carried forward method). The OS and DFS probabilities were estimated using the Kaplan-Meier method32 and the median follow-up time by reversing the codes for the censoring indicator in a Kaplan-Meier analysis.
Cumulative incidence of relapse (CIR) was calculated from day 85 to relapse or date of last follow-up for patients alive in first CHR, using the cumulative incidence method and considering death in CHR as competing risk.33
The log-rank test was used to compare risk factor categories for the Kaplan-Meier curves and the Gray test for the incidence curves.
Multivariate analysis on DFS was performed by the Cox model34 ; results were expressed as hazard ratios (HRs) ± 95% CI. Only variables considered clinically relevant were included. We have included in the model missing values for BCR-ABL reduction at day 85 and immunophenotypic reduction at day 85 considering them as a distinct category (BCR-ABL reduction d + 85, missing values vs ≥ 10−3; and immunophenotypic reduction d + 85, missing values vs ≥ 10−2); these comparisons were not significant (data not shown). Analyses were performed using the SAS Version 9.1.3 statistical software.
Results
Patients
Fifty-five adult patients with Ph+ ALL were enrolled. One patient discontinued treatment after 14 days after gastrointestinal toxicity, which subsequently resolved, and 1 patient refused treatment (Jehovah's Witness). Of 53 evaluable patients, 28 were females and 25 males. The median age was 53.6 years (range, 23.8-76.5 years), with 12 patients older than 60 years. Because of the inclusion of the patients in their 60s and older, the study was characterized by an overall population of older patients. The median WBC count was 18.8 × 109/L (range, 2.2-132.9 × 109/L). Thirty-three patients had the p190 form of BCR-ABL, 13 the p210 form, and 7 both p190 and p210 (for the analysis, these latter 2 groups were considered together).
Treatment responses
After the steroid pre–phase, 86% of patients showed a blast reduction ≥ 75% and 14% < 75%. During dasatinib treatment, 53 of 53 evaluable patients (100%) achieved a CHR (one CHRi): 49 patients (92.5%) achieved a CHR at the first BM determination at day 22, 3 at the second determination at day 43, and one at the third determination at day 57. The median time to CHR was 23 days.
Compliance and toxicity
Treatment was well tolerated. Four patients discontinued treatment during the last phase of the induction: 2 between days 64 and 70 because of toxicity (1 weight gain, fever of unknown origin, and pleural effusion G1/G2 and superficial edema G2; 1 fever of unknown origin G2, nausea and vomiting G3); 1 at day 72 for proteinuria (G3) and one between days 77 and 84 for personal decision (this patient had a serious adverse event during treatment: one hypertransaminasemia G4). All 4 patients discontinued treatment from day 64 onwards and were already in CHR. Eleven patients discontinued therapy temporarily for a median of 4 days (range, 1-16 days) and thereafter resumed the scheduled full dose. The causes of the discontinuation, as reported by the investigators, were: mild increase of liver function tests (3), gastrointestinal toxicity (2), clinical decision (2), infection (2), mood alteration (1), and hyperkalemia (1).
Of the 38 patients who did not discontinue treatment, only 2 presented a serious adverse event related to dasatinib: 1 pleural effusion (moderate) and 1 fever of unknown origin (mild). No deaths occurred during the dasatinib induction treatment.
MRD monitoring
BCR-ABL transcript levels decreased rapidly during dasatinib induction (Figure 1A); patients who achieved BCR-ABL levels < 10−3 were 10 of 44 (22.7%) at day 22, 15 of 45 (33.3%) at day 43, 18 of 45 (40.0%) at day 57, and 25 of 48 (52.1%) at day 85. Mean BCR-ABL levels remained higher in patients who subsequently relapsed compared with those who remained free from relapse at the last follow-up, with the difference between the groups most apparent on days 22, 43, and 57 (Figure 1B). p190 patients had a more rapid molecular response compared with p210 patients, as shown by the difference between days 22 and 43 (P = .0412 vs P = .0956, respectively; Figure 1C).
BCR-ABL transcript levels during dasatinib therapy. (A) All patients. (B) Patients who subsequently relapsed (red) or remained in remission at the last follow-up (blue). (C) Patients with p190 (red) or p210 (blue) forms of BCR-ABL. For display purposes, undetectable BCR-ABL transcript levels are plotted at 0.00001.
BCR-ABL transcript levels during dasatinib therapy. (A) All patients. (B) Patients who subsequently relapsed (red) or remained in remission at the last follow-up (blue). (C) Patients with p190 (red) or p210 (blue) forms of BCR-ABL. For display purposes, undetectable BCR-ABL transcript levels are plotted at 0.00001.
Twelve patients reached undetectable BCR-ABL values at least at 1 of 4 different induction points evaluated; however, only 8 maintained the PCR negativity also at the completion of the induction at day 85.
Forty-nine patients were monitored for MRD by flow cytometry. At diagnosis, all cases were positive for cyCD79a, CD19, CD22, CD10, CD34, and TdT, and negative for MPO, CD3, CD7, CD2, and CD5. CD13 was expressed in > 20% of cells in 27 of 48 patients (56.2%), CD33 in 13 of 48 patients (29.1%), CD66c in 28 of 47 patients (61.7%), and CD20 in 12 of 48 patients (25%). More than 10% of leukemic cells expressed cyIgμ in 15 of 46 patients (32.6%). No correlation was found between immunophenotype and therapeutic response.
After treatment with dasatinib, a marked BM reduction of the leukemic clone was recorded: at day 22, 9 of 44 patients (20.4%) were negative and 12 of 44 patients (27.3%) showed < 0.01% of disease; at day 43, 19 of 47 patients (40.4%) were negative and 15 of 47 (31.9%) showed < 0.01% of disease; at day 57, 26 of 42 patients (61.9%) were negative and 7 of 42 (16.6%) showed < 0.01% of disease; and at day 85, 30 of 44 patients (68.3%) were negative and 3 of 44 (6.8%) showed < 0.01% of disease.
The leukemic cell reduction was significant between days 0 and 22, days 22 and 43 (P < .0001), and days 43 and 57 (P = .0143). No further significant reduction was observed between days 57 and 85.
Postinduction treatment
The LAL1205 protocol did not include any planned treatment scheme after day 84. Based on the postinduction management decided at the different centers, the 53 patients could be further subdivided as follows: no further treatment, 2 patients; TKI alone, 19 patients (16 dasatinib, 2 imatinib, and 1 imatinib-dasatinib); TKI plus chemotherapy (10) and/or autografting (4), 14 patients; and allografting, 18 patients. All of these treatment approaches were carried out while the patients were in first CHR.
OS and DFS
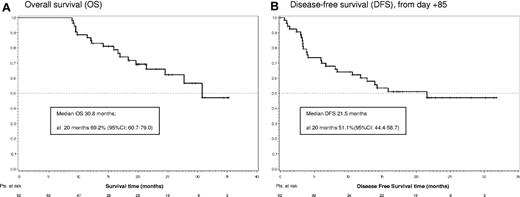
The median follow-up of the study is 24.8 months (range, 8.9-35.3 months). The median OS is 30.8 months, with an OS at 20 months of 69.2% (95% CI, 60.7%-79.0%; Figure 2A). To date, 19 patients have died, all after completing the 84-day dasatinib induction phase. Sixteen died after relapse and 3 died in CHR because of allogeneic stem cell transplantation complications. The median DFS is 21.5 months, and DFS at 20 months is 51.1% (95% CI, 44.4%-58.7%; Figure 2B).
Relapse
At 20 months, 42.9% of patients remained free from relapse (95% CI, 41.9%-43.9%). At the last follow-up, 23 relapses have occurred, with no relapses recorded during the 84-day dasatinib induction. Median time to relapse from achievement of first CHR was 5.9 months (range, 2.8-23.6 months), which was equivalent to 109.5 days from day 84 (range, 18-655 days). Relapses occurred in 11 of 33 p190 patients, in 10 of 13 p210 patients, and in 2 of 7 p190/p210 patients. CIR estimations at 20 months were 30.9% (95% CI, 29.6%-32.3%) and 76.9% (95% CI, 73.7%-80.3%) for p190 and p210 patients, respectively (P = .0176). Relapses occurred in 14 of 19 patients treated only with a TKI, in 2 of 2 patients who received no further treatment, in 5 of 14 patients treated with a TKI plus chemotherapy and/or autografting, and in 2 of 18 allografted patients.
Mutations were frequently detected at relapse. Among 17 patients analyzed, a T315I mutation was detected in 12, E255K in 1, and no BCR-ABL mutations in 4. Eight of 12 mutations (66.7%) occurred in patients who continued treatment with a TKI alone (7 dasatinib, 1 imatinib-dasatinib) and 4 (33.3%) in patients who underwent further therapy other than only a TKI. No correlation was found between the mutational status at diagnosis and at relapse.
Correlation between molecular and immunophenotypic responses with DFS
Molecular response was predictive for DFS. A subgroup analysis between patients who achieved at day 22 a BCR-ABL level < 10−3 (n = 10) and patients who never achieved levels < 10−3 during dasatinib treatment (n = 7) showed a significant difference in DFS (P = .0381; Figure 3A). When DFS was analyzed according to the molecular response at day 85, after induction completion, estimations at 20 months were 67.5% (95% CI, 56.1%-81.3%) for patients with BCR-ABL levels < 10−3 and 29.8% (95% CI, 24.1%-36.7%) for patients with levels ≥ 10−3 (P = .0094; Figure 3B). Of the 8 patients who had at day 85 undetectable BCR-ABL values, 2 have relapsed at 10.1 and 12.7 months from first CHR, whereas 6 remain alive in CHR at a median of 24.4 months from first CHR (range, 18.8-28.1 months from the first CHR). One of these 8 patients with undetectable BCR-ABL values received TKI alone, 6 TKI plus chemotherapy, and/or autografting and 2 were allografted.
DFS according to BCR-ABL transcript reduction during dasatinib treatment. (A) BCR-ABL levels < 10−3 at day 22 (n = 10) versus patients who never achieved levels < 10−3 during dasatinib induction treatment (n = 7). (B) BCR-ABL levels < 10−3 (n = 25) or ≥ 10−3 (n = 23) at day 85.
DFS according to BCR-ABL transcript reduction during dasatinib treatment. (A) BCR-ABL levels < 10−3 at day 22 (n = 10) versus patients who never achieved levels < 10−3 during dasatinib induction treatment (n = 7). (B) BCR-ABL levels < 10−3 (n = 25) or ≥ 10−3 (n = 23) at day 85.
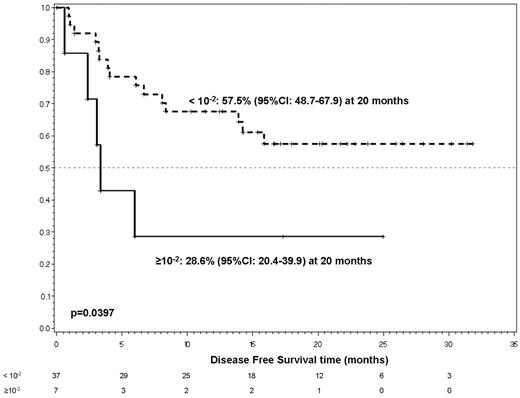
Immunophenotypic response (< 0.01% of leukemic cells in the BM) was associated with DFS only at day 85 (P = .0397) in univariate analysis (Figure 4).
Univariate and multivariate DFS analysis
Taking into account in univariate analysis the WBC count at diagnosis (as continuous variable), age (as continuous variable), sex, and response to steroid pre–phase, only the WBC count (HR = 1.011, 95% CI, 1.001-1.021, P = .0393) correlated with DFS.
In a multivariate model involving WBC count, age, sex, type of BCR-ABL, BCR-ABL reduction at day 85, and immunophenotypic reduction at day 85, only BCR-ABL reduction correlated with DFS (HR = 0.336, 95% CI, 0.126-0.895, P = .0291; Table 1).
Multivariate analysis of factors on DFS (from day 85)
| Variable . | Hazard ratio (95% CI) . | P . |
|---|---|---|
| WBC at diagnosis, × 109/L | 1.005 (0.993-1.017) | .4358 |
| Age, y (as continuous variable) | 1.003 (0.971-1.037) | .8414 |
| Sex (female vs male) | 2.501 (1.010-6.193) | .0476 |
| BCR-ABL reduction day 85, < 10−3 vs ≥ 10−3 | 0.336 (0.126-0.895) | .0291 |
| Immunophenotypic reduction day 85, < 10−2 vs ≥ 10−2 | 0.411 (0.120-1.405) | .1562 |
| BCR-ABL type, p190 vs p210 | 0.446 (0.181-1.100) | .0797 |
| BCR-ABL type, p190/p210 vs p210 | 0.518 (0.129-2.070) | .3517 |
| Other comparisons | ||
| BCR-ABL type: p190/p210 vs p190 | 1.159 (0.293-4.592) | .8335 |
| Variable . | Hazard ratio (95% CI) . | P . |
|---|---|---|
| WBC at diagnosis, × 109/L | 1.005 (0.993-1.017) | .4358 |
| Age, y (as continuous variable) | 1.003 (0.971-1.037) | .8414 |
| Sex (female vs male) | 2.501 (1.010-6.193) | .0476 |
| BCR-ABL reduction day 85, < 10−3 vs ≥ 10−3 | 0.336 (0.126-0.895) | .0291 |
| Immunophenotypic reduction day 85, < 10−2 vs ≥ 10−2 | 0.411 (0.120-1.405) | .1562 |
| BCR-ABL type, p190 vs p210 | 0.446 (0.181-1.100) | .0797 |
| BCR-ABL type, p190/p210 vs p210 | 0.518 (0.129-2.070) | .3517 |
| Other comparisons | ||
| BCR-ABL type: p190/p210 vs p190 | 1.159 (0.293-4.592) | .8335 |
Discussion
The results obtained demonstrate the activity and safety of a first-line treatment strategy with dasatinib plus steroids for adult Ph+ ALL patients of any age. Dasatinib treatment resulted in a marked and rapid debulking of the neoplastic clone. All 53 evaluable patients achieved a CHR, 49 (92.5%) already by day 22. At the last evaluation on day 85, the disease persisted in the majority of patients only at very low levels; indeed, 52.1% of patients had BCR-ABL levels < 10−3. At the last follow-up, most patients were alive and 23 patients had relapsed. All relapses occurred after completing the dasatinib induction phase, at a median of 109.5 days postinduction. The protocol was designed to define the power of an induction treatment with a second-generation TKI alone plus steroids and no chemotherapy for adult Ph+ ALL patients of all ages, whereas postremission treatment was not dictated by the protocol. Interestingly, postinduction treatment in relapsing patients frequently composed only a TKI (14 patients) or no further treatment (2 patients), for a total of 16 of 23 relapsed patients (69.6%), whereas 5 of 23 patients (21.7%) had received a TKI plus chemotherapy and/or an autograft, and 2 of 18 (11.1%) an allograft. Although not part of the protocol, these observations underline the requirement of postconsolidation treatment other than only a TKI. They also indicate that an allogeneic transplantation can be successfully carried out after obtaining a CHR with dasatinib plus steroids. Mean BCR-ABL levels appeared to be more rapidly reduced in p190+ patients, suggesting a greater susceptibility of BCR-ABLp190–expressing cells to dasatinib. A similar finding was reported by our group in Ph+ patients treated with chemotherapy.35
Patients who maintained their remission state after dasatinib had achieved lower levels of MRD during induction than patients who subsequently relapsed, with the difference most evident on days 22 and 43. Patients who achieved a BCR-ABL reduction to < 10−3 at day 22 had a significantly higher DFS rate than those who failed to achieve a BCR-ABL status < 10−3 at any time during induction. To date, only 2 of 10 patients who had achieved at day 22 a BCR-ABL reduction to < 10−3 have relapsed. In multivariate analysis, the BCR-ABL transcript level at day 85 correlated with DFS. Indeed, only 2 of 8 patients who at day 85 had undetectable levels of BCR-ABL have so far relapsed. These observations, in agreement with previous findings with conventional chemotherapy,35 indicate that patients with Ph+ ALL have a heterogeneous sensitivity to dasatinib and suggest that a delayed or less profound molecular response during dasatinib induction identifies patients with an increased relapse likelihood.
The occurrence of BCR-ABL mutations is a key element in managing patients during TKI therapy. Because of the higher genetic instability occurring in Ph+ ALL, this is a greater issue than in CML. Dasatinib is active against the majority of imatinib-resistant BCR-ABL mutations, although T315I is resistant to all available TKIs. In our study, T315I was detected in 12 of 17 relapsing patients, indicating that a T315I mutation may be the primary cause of dasatinib-associated relapse. This is a key finding given that many relapsing patients had received only a TKI as postinduction treatment. Both our group36 and others37 have found BCR-ABL mutations, including T315I, at diagnosis. In our series, low levels of T315I mutations could also be detected at diagnosis, but this does not seem to correlate with a subsequent relapse or persistence of remission.36 Taken together, mutation and molecular response data strongly argue in favor of treatment targeted at consolidating remission and at controlling/reducing MRD after dasatinib-induced CHR, particularly for patients with a less profound molecular response.
In the GIMEMA network, all ALL patients are centrally processed at diagnosis and during the follow-up. BCR-ABL can thus be detected during the 1-week steroid prephase. This framework also allows a uniform monitoring of MRD and detection of BCR-ABL mutations at relapse. Despite the advantages of PCR-based methods for MRD monitoring, flow cytometry enables BCR-ABL protein detection at diagnosis with a 100% accuracy.38,39 This may allow a rapid diagnosis of Ph+ ALL in regions where PCR-based technologies are not available and oral treatment is more manageable.
Combining the results of the present study (53 patients) and the previous one with imatinib monotherapy in elderly patients (48 patients, 19 enrolled after the interim analysis already published),14 > 100 adult Ph+ ALL patients (median age, 62.7 years; range, 23.8-89 years) have so far been treated with a TKI alone as first-line treatment within the GIMEMA network. This has resulted in a CHR in all patients and no deaths in induction. Thus, virtually all adult Ph+ ALL patients, irrespective of age, can achieve a CHR with an oral treatment that is associated with a very favorable tolerability and safety profile, partly administrable at home. Taken together, these observations question the role of chemotherapy in combination with a TKI as induction treatment in Ph+ ALL. In all studies, imatinib and chemotherapy have resulted in CR rates between 90% and 98%, with early deaths always associated with the combination.40-45 Dasatinib with chemotherapy has also been associated with notable toxicities and treatment discontinuation.46,47 The rates of OS and DFS of 69.2% and 51.1% at 20 months of our study, clearly influenced by the postremission treatment, compare favorably with the data reported in the literature, particularly in view of the overall older age of our patients' series.
Because of relapses occurring after completing the TKI-based induction therapy, the optimal postinduction treatment after CHR needs to be defined. It should be stressed that not all patients relapse and the speed and degree of molecular response may be crucial. Our study shows that most patients witnessing a rapid and profound molecular response are still alive in CHR. Furthermore, a follow-up analysis of the GIMEMA LAL0201-B protocol of elderly patients treated with imatinib monotherapy14 has shown that 8 of 48 evaluable patients (median age, 68 years; range, 60-89 years) remain in persistent remission at a median follow-up of 4.5 years, including 4 patients with > 6 years of remission (R.F., and M.V., unpublished data, January 2011). However, determining an optimal treatment strategy for patients with persistent MRD positivity, particularly younger patients, remains a challenge. In the new GIMEMA study, patients 60 years of age or younger will be treated with dasatinib induction followed by alloSCT where a donor is available or chemotherapy consolidation. The goal is to improve on the results obtained in the present study and further impact on the prognosis of Ph+ ALL patients.
In conclusion, we wish to underline the requirement that all newly diagnosed ALL patients should be rapidly investigated for the presence of the BCR-ABL rearrangement/protein. This needs to be extended to the elderly where this abnormality accounts for > 50% of cases.6 The analysis should be done by PCR or flow cytometry within one week from diagnosis, during which steroids should be initiated. BCR-ABL+ patients should be started on a TKI. Based on the feasibility, safety, and efficacy of dasatinib, we advocate the use of the inhibitor alone (plus steroids) to obtain a rapid and profound debulking of the disease in adult Ph+ ALL, including the elderly, sparing the toxicity associated with induction protocols combining TKI and chemotherapy. Ph+ ALL should no longer be considered the most lethal hematologic malignancy, as long as the abnormality is detected upfront and a TKI promptly initiated at all ages. The situation is reminiscent of that encountered years ago for acute promyelocytic leukemia, in which the rapid identification of the genetic abnormality and immediate implementation of specific treatment have changed the natural course of the disease in both children and adults.48-50 The current challenge is to identify the best postremission treatment for Ph+ ALL patients. The ongoing protocols are specifically addressing this key issue.
Presented in part at the 49th American Society of Hematology Congress (2007), the 13th European Hematology Association Congress (2008), and the 50th ASH Congress (2008).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Sandra De Simone for administrative support and Data Management.
This work was supported by Associazione Italiana per la Ricerca sul Cancro, Milan; Ministero Istruzione, Università e Ricerca, Progetti di Ricerca di Interesse Nazionale, Associazione Italiana contro le Leucemie, and Progetto “Oncologia,” Ministero della Salute, Rome; and Compagnia di San Paolo, Turin, Italy as well as European LeukemiaNet, FIRB 2006. The GIMEMA Foundation, sponsor of the study, received unrestricted research grants from Novartis and BMS.
Authorship
Contribution: R.F., M.V., G. Meloni, G.C., F.M., G. Martinelli, and M.B. conceived and designed the study; A.V., G. Meloni, A.G., M.S.D.P., L.E., G.C., F.N., F.F., C.C., S. Sica, P.L., E.Z., C.F., M.L., A.C., I.I., S. Soverini, and G. Martinelli provided study materials or patients; F.P., P.F., and M.V. analyzed data; R.F., A.V., M.V., and A.G. wrote the manuscript; and all authors gave final approval of the manuscript.
Conflict-of-interest disclosure: R.F. received honoraria for participation to advisory boards and as speaker at scientific meetings from BMS. M.V. received honoraria for participation to advisory boards and as speaker at scientific meetings from BMS. G. Martinelli received honoraria for participation to advisory boards and as speaker at scientific meetings from BMS. M.B. received honoraria for participation to advisory boards and as speaker at scientific meetings from BMS. A.V. received honoraria as speaker at scientific meetings and travel support from BMS. S. Soverini received honoraria as speaker at scientific meetings and travel support from BMS. The remaining authors declare no competing financial interests.
For a list of the institutions participating in the LAL 1205 protocol, please see the supplemental Appendix (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Correspondence: Robin Foà, Hematology, Department of Cellular Biotechnologies and Hematology, “Sapienza,” University of Rome, Via Benevento 6, 00161 Rome, Italy; e-mail: rfoa@bce.uniroma1.it.