Abstract
Patients with chronic myeloproliferative neoplasms, including essential thrombocythemia (ET), polycythemia vera (PV), and chronic myeloid leukemia (CML), are at increased risk of new hematologic malignancies, but their risk of nonhematologic malignancies remains unknown. In the present study, we assessed the risk of both types of malignancies after an ET, PV, or CML diagnosis. We linked 2 population-based nationwide registries, the Danish National Registry of Patients, covering all Danish hospitals and the Danish Cancer Registry, and assessed subsequent cancer risk in a cohort of all 7229 patients diagnosed with a chronic myeloproliferative neoplasm during 1977-2008. We compared the incidence of subsequent cancer in this cohort with that expected on the basis of cancer incidence in the general population (standardized incidence ratio). Overall, ET, PV, and CML patients were at increased risk of developing both new hematologic and nonhematologic cancers. The standardized incidence ratio for developing a nonhematologic cancer was 1.2 (95% confidence interval [95% CI]): 1.0-1.4) for patients with ET, 1.4 (95% CI: 1.3-1.5) for patients with PV, and 1.6 (95% CI: 1.3-2.0) for patients with CML. We conclude that patients with chronic myeloproliferative neoplasms are at increased risk of developing a new malignant disease.
Introduction
Chronic myeloproliferative neoplasms are clonal diseases of the BM arising from a pluripotent hematopoietic stem cell.1 Traditionally, the diseases are classified based on chromosomal abnormalities into the Philadelphia-chromosome–negative disorders (CMPNs) and chronic myeloid leukemia (CML).1 The classic CMPNs are essential thrombocythemia (ET), polycythemia vera (PV), and primary myelofibrosis (PMF).1 Although CMPNs are considered relatively indolent diseases, patients are at lifelong increased risk of thrombosis, hemorrhage, and myelofibrotic or leukemic transformation.2,3
The potential for CMPNs to evolve into more malignant and aggressive myeloid neoplasms has been known for more than 60 years,4 but the epidemiologic evidence is limited. A recent Italian study based on 820 PV and ET patients from a single institution reported an increased risk for lymphoid neoplasms in patients with CMPNs.5 The patients were followed for incident lymphoid neoplasms through retrospective review of their clinical records from the same institution. The investigators compared the incidence of lymphomas among the CMPN patients with that of the general population living in the same area as the hospital. The resulting standardized incidence ratios (SIRs) for chronic lymphocytic leukemia and nonHodgkin lymphoma in CMPN patients compared with the general population were 3.4 (95% confidence interval [95% CI]: 1.9-6.2) and 12.4 (95% CI: 4.7-33.6), respectively.5
Two other recent studies have investigated the association between CMPNs and carcinomas. Rebora et al conducted a registry-based study in Sweden focusing on the incidence of new primary malignancies diagnosed during 1970-1995 among CML patients compared with the general population.6 They reported SIRs of 2.8 (95% CI: 1.3-5.1), 5.4 (95% CI: 3.2-8.5), and 1.6 (95% CI: 1.2-2.2) for stomach cancer, nonmelanoma skin cancer, and urogenital cancer, respectively.6 Fallah et al reported an increased incidence of new primary cancers among patients diagnosed with PV based on a Swedish registry study, and found SIRs above unity for parathyroid tumors, as well as for kidney and skin cancers, after a PV diagnosis.7
The risk of developing a subsequent cancer among patients with different types of CMPNs compared with the general population has not been studied previously on a large scale. In the present study, we report the risk of developing nonhematologic and hematologic cancers among adult patients with ET, PV, or CML using population-based data from the Danish health care system.
Methods
Data sources and patients
The Danish National Registry of Patients (DNRP) and the Danish Cancer Registry (DCR) provided data for this cohort study. Since 1968, all residents of Denmark have received a unique, permanent civil registration number, allowing unambiguous individual-level linkage among all Danish registries. The civil registration number is a prerequisite for obtaining any form of social benefit in Denmark, including health care.8
The DNRP, established in 1977,9 contains information on virtually all discharges from public hospitals since 1977 and on outpatient clinic visits since 1995. Denmark has very few private hospitals (all with no acute care). Data recorded in the DNRP include civil registration number, dates of outpatient visits, hospital admission and discharge dates, and up to 20 diagnoses coded by physicians according to the World Health Organization's (WHO's) International Classification of Diseases, 8th revision (ICD-8), which covered 1977-1993 and the 10th revision (ICD-10) thereafter. We identified all patients diagnosed with a chronic myeloproliferative neoplasm during 1977-2008 by their ICD-8 or ICD-10 diagnosis code. Because there is no specific diagnosis code for primary myelofibrosis in the ICD-10, we created 3 cohorts of patients based on available diagnosis codes as follows: patients with ET (ICD-8 code 287.29 or ICD-10 codes D473 or D752), patients with PV (ICD-8 code 208.99 or ICD-10 code D459), and patients with CML (ICD-8 code 205.19 or ICD-10 code C921). Diagnostic coding of hematologic malignancies in the DNRP has been reported to be valid.10 The diagnosis date was defined as the date of first hospital admission or the first visit to an outpatient clinic with a CMPN diagnosis code. Because CMPN is rare among children and adolescents, we restricted the study population to patients 20 years of age or older.
Subsequent diagnoses of cancer among CMPN patients were identified by linkage to the DCR, which has recorded all cancer diagnoses at an individual level since 1943.11 Cancers are classified according to the ICD-10. Registration is based on cancer diagnoses reported by hospitals, practicing physicians, and departments of pathology and forensic medicine at the time of original diagnosis or when changes are made to the diagnosis. In addition, if a cancer diagnosis is made at autopsy, the DCR obtains information through linkage to the death certificate registry. In comprehensive assessments, the DCR has been found to be 95%-98% valid and complete.11
As a first step, we used the DCR to identify all cancer diagnoses in our 3 study cohorts (patients with ET, patients with PV, and patients with CML). To exclude other prevalent cancer diagnoses, we then eliminated CMPN patients with a previous cancer diagnosis or a cancer diagnosis within the first year after their ET, PV, or CML diagnosis (other than nonmelanoma skin cancer or carcinoma in situ cervix uteri). Follow-up status was determined through linkage to the Danish Civil Registration System. Follow-up began on the date of first hospital admission or first visit to an outpatient clinic resulting in a CMPN or CML diagnosis, and continued until death, emigration, or December 31, 2008, whichever came first. Patients diagnosed with CMPN or CML during follow-up were not considered to have a new cancer. This study was approved by the Danish Data Protection Agency.
Statistical analysis
We calculated the expected number of cancers after diagnosis of a CMPN or CML using national incidence rates of cancer by age, sex, and year of diagnosis in 1-year intervals. The number of cancers that would be expected if patients with a CMPN had the same risk of cancer as the general Danish population was calculated by multiplying the number of person-years of follow-up by the incidence rates for each cancer category. The association between a CMPN diagnosis and the risk of developing a subsequent cancer was assessed by the SIR, the ratio of the observed number of cancers to the expected number of cancers. We calculated 95% CIs for the estimate of the SIR by assuming a Poisson distribution of the observed number of cancers during the follow-up period in a specific cancer category. Exact 95% CIs were used when the observed number of cancers was < 10; otherwise, the Byar approximation was used.12
We stratified our data by sex, age, calendar period of diagnosis, and follow-up time in all 3 cohorts. In addition, we used chronic obstructive pulmonary disease (COPD) as an indicator of smoking. Because blood test results of heavy smokers can resemble those of CMPN patients, diagnostic misclassification could occur if heavy smokers were erroneously given an ET, PV, or CML diagnosis code. Because we lacked direct information on smoking status, the COPD diagnosis served as a proxy measure. To estimate the possible effect of diagnostic misclassification of ET, PV, and CML diagnoses, we therefore stratified analyses according to whether a diagnosis of known COPD (ICD-8 codes 490-492; ICD-10 codes J40-J44)13 had been made before or concurrently with these diagnoses. For patients diagnosed in 1995 or later, we also stratified analyses according to hospital contact (ie, whether patients were admitted to the hospital at any time or were followed solely as outpatients).
Treatment options changed during the period in which these patients were diagnosed. In Denmark, IFN-α treatment was introduced in approximately 1985 and imatinib began to be used to treat CML in 2000. To evaluate the possible effect of these new treatments on subsequent cancer occurrence, we stratified analyses according to these 2 time points.
We present specific cancer diagnoses only for the PV cohort, and only if 5 or more cases were recorded in one of the diagnostic groups.
Results
We identified 1578 patients diagnosed with ET, 4625 patients with PV, and 1026 patients with CML during 1977-2008 (Table 1). The median age was 65.2 years (interquartile range [IQR] = 52.6-75.3 years) among ET patients, 65.5 years (IQR = 55.9-74.1 year) among PV patients, and 59.7 years (IQR = 45.2-71.0 years) among patients with CML. In the 3 cohorts, median follow-up time was 4.0 years (IQR = 1.8-7.5 years) among ET patients, 5.0 years (IQR = 2.2-9.8 years) among PV patients, and 2.4 years (IQR = 1.0-5.2 years) among patients with CML.
Characteristics for 3 cohorts of Danish patients diagnosed with ET, PV, or CML during 1977-2008 and followed for new primary cancers
| . | ET patients, n (%) . | PV patients, n (%) . | CML patients, n (%) . |
|---|---|---|---|
| All | 1578 (100.0) | 4625 (100.0) | 1026 (100.0) |
| Women | 1050 (66.5) | 2017 (43.6) | 446 (43.5) |
| Men | 528 (33.5) | 2608 (56.4) | 580 (56.5) |
| Age, y | |||
| 20-49 | 325 (20.6) | 717 (15.5) | 329 (32.1) |
| 50-69 | 634 (40.2) | 2190 (47.4) | 422 (41.1) |
| 70+ | 619 (39.2) | 1718 (37.1) | 275 (26.8) |
| Year of diagnosis | |||
| 1977-1994 | 233 (14.8) | 2753 (59.5) | 569 (55.5) |
| 1995-2008 | 1345 (85.2) | 1872 (40.5) | 457 (44.5) |
| Follow-up time | |||
| Year 2 | 245 (15.5) | 560 (12.1) | 256 (25.0) |
| Years 3-5 | 550 (34.9) | 1398 (30.2) | 422 (41.1) |
| Years 6+ | 783 (49.6) | 2667 (57.7) | 348 (33.9) |
| Outpatient contact only (for diagnoses made in 1995 and later) | 834 (62.0) | 1087 (58.1) | 157 (34.4) |
| Chronic obstructive pulmonary disease | 107 (7.0) | 427 (9.2) | 30 (2.9) |
| . | ET patients, n (%) . | PV patients, n (%) . | CML patients, n (%) . |
|---|---|---|---|
| All | 1578 (100.0) | 4625 (100.0) | 1026 (100.0) |
| Women | 1050 (66.5) | 2017 (43.6) | 446 (43.5) |
| Men | 528 (33.5) | 2608 (56.4) | 580 (56.5) |
| Age, y | |||
| 20-49 | 325 (20.6) | 717 (15.5) | 329 (32.1) |
| 50-69 | 634 (40.2) | 2190 (47.4) | 422 (41.1) |
| 70+ | 619 (39.2) | 1718 (37.1) | 275 (26.8) |
| Year of diagnosis | |||
| 1977-1994 | 233 (14.8) | 2753 (59.5) | 569 (55.5) |
| 1995-2008 | 1345 (85.2) | 1872 (40.5) | 457 (44.5) |
| Follow-up time | |||
| Year 2 | 245 (15.5) | 560 (12.1) | 256 (25.0) |
| Years 3-5 | 550 (34.9) | 1398 (30.2) | 422 (41.1) |
| Years 6+ | 783 (49.6) | 2667 (57.7) | 348 (33.9) |
| Outpatient contact only (for diagnoses made in 1995 and later) | 834 (62.0) | 1087 (58.1) | 157 (34.4) |
| Chronic obstructive pulmonary disease | 107 (7.0) | 427 (9.2) | 30 (2.9) |
The number of patients is shown, with percentages within in each stratum in parentheses.
In the ET cohort, 152 nonhematologic and 37 hematologic cancers were observed during 8087 person-years of follow-up (Table 2). In the PV cohort, 704 nonhematologic and 115 hematologic cancers were observed during 31 270 person-years of follow-up (Table 3). Among CML patients, the corresponding numbers were 75 nonhematologic and 14 hematologic cancers observed during 3960 person-years of follow-up (Table 4).
Observed and expected cancers and SIRs among Danish patients diagnosed with ET during 1977-2008 and followed for new primary cancers
| . | Person-years of patients with ET . | ET patients with nonhematologic cancers, n . | Nonhematologic cancers expected, n . | SIR (95% CI) . | ET patients with hematologic cancers, n . | Hematologic cancers expected, n . | SIR (95% CI) . |
|---|---|---|---|---|---|---|---|
| All | 8087 | 152 | 125.7 | 1.2 (1.0-1.4) | 37 | 7.4 | 5.0 (3.5-6.9) |
| Women | 5516 | 89 | 83.3 | 1.1 (0.9-1.3) | 23 | 4.5 | 5.1 (3.2-7.6) |
| Men | 2571 | 63 | 42.3 | 1.5 (1.1-1.9) | 14 | 2.8 | 5.0 (2.7-8.3) |
| Age at ET diagnosis, y | |||||||
| 20-49 | 2280 | 13 | 12.9 | 1.0 (0.5-1.7) | 2 | 0.5 | 3.8 (0.5-13.7) |
| 50-69 | 3574 | 72 | 57.4 | 1.3 (1.0-1.6) | 22 | 3.2 | 6.9 (4.3-10.4) |
| 70+ | 2234 | 67 | 55.3 | 1.2 (0.9-1.5) | 13 | 3.6 | 3.6 (1.9-6.4) |
| Year of ET diagnosis | |||||||
| 1977-1994 | 2198 | 34 | 29.9 | 1.1 (0.8-1.6) | 10 | 1.7 | 5.8 (2.8-10.7) |
| 1995-2008 | 5889 | 118 | 95.8 | 1.2 (1.0-1.5) | 27 | 5.6 | 4.8 (3.2-7.0) |
| Follow-up time | |||||||
| Year 2 | 1455 | 32 | 22.7 | 1.4 (1.0-2.0) | 8 | 1.3 | 6.0 (2.6-11.8) |
| Years 3-5 | 3142 | 60 | 48.9 | 1.2 (0.9-1.6) | 15 | 2.9 | 5.2 (2.9-8.6) |
| Years 6+ | 3490 | 60 | 54.1 | 1.1 (0.9-1.4) | 14 | 3.1 | 4.5 (2.5-7.5) |
| Hospital contact type (from 1995 on) | |||||||
| Outpatient only | 3350 | 63 | 53.1 | 1.2 (0.9-1.5) | 12 | 3.1 | 3.9 (2.0-6.7) |
| Inpatient admission | 2539 | 55 | 42.6 | 1.3 (1.0-1.7) | 15 | 2.5 | 5.9 (3.3-9.8) |
| . | Person-years of patients with ET . | ET patients with nonhematologic cancers, n . | Nonhematologic cancers expected, n . | SIR (95% CI) . | ET patients with hematologic cancers, n . | Hematologic cancers expected, n . | SIR (95% CI) . |
|---|---|---|---|---|---|---|---|
| All | 8087 | 152 | 125.7 | 1.2 (1.0-1.4) | 37 | 7.4 | 5.0 (3.5-6.9) |
| Women | 5516 | 89 | 83.3 | 1.1 (0.9-1.3) | 23 | 4.5 | 5.1 (3.2-7.6) |
| Men | 2571 | 63 | 42.3 | 1.5 (1.1-1.9) | 14 | 2.8 | 5.0 (2.7-8.3) |
| Age at ET diagnosis, y | |||||||
| 20-49 | 2280 | 13 | 12.9 | 1.0 (0.5-1.7) | 2 | 0.5 | 3.8 (0.5-13.7) |
| 50-69 | 3574 | 72 | 57.4 | 1.3 (1.0-1.6) | 22 | 3.2 | 6.9 (4.3-10.4) |
| 70+ | 2234 | 67 | 55.3 | 1.2 (0.9-1.5) | 13 | 3.6 | 3.6 (1.9-6.4) |
| Year of ET diagnosis | |||||||
| 1977-1994 | 2198 | 34 | 29.9 | 1.1 (0.8-1.6) | 10 | 1.7 | 5.8 (2.8-10.7) |
| 1995-2008 | 5889 | 118 | 95.8 | 1.2 (1.0-1.5) | 27 | 5.6 | 4.8 (3.2-7.0) |
| Follow-up time | |||||||
| Year 2 | 1455 | 32 | 22.7 | 1.4 (1.0-2.0) | 8 | 1.3 | 6.0 (2.6-11.8) |
| Years 3-5 | 3142 | 60 | 48.9 | 1.2 (0.9-1.6) | 15 | 2.9 | 5.2 (2.9-8.6) |
| Years 6+ | 3490 | 60 | 54.1 | 1.1 (0.9-1.4) | 14 | 3.1 | 4.5 (2.5-7.5) |
| Hospital contact type (from 1995 on) | |||||||
| Outpatient only | 3350 | 63 | 53.1 | 1.2 (0.9-1.5) | 12 | 3.1 | 3.9 (2.0-6.7) |
| Inpatient admission | 2539 | 55 | 42.6 | 1.3 (1.0-1.7) | 15 | 2.5 | 5.9 (3.3-9.8) |
Observed and expected cancers and SIRs among Danish patients diagnosed with PV during 1977-2008 and followed for new primary cancers
| . | Person-years of patients with PV, n . | PV patients with nonhematologic cancers, n . | Nonhematologic cancers expected, n . | SIR (95% CI) . | PV patients with hematologic cancers, n . | Hematologic cancers expected, n . | SIR (95% CI) . |
|---|---|---|---|---|---|---|---|
| All | 31 270 | 704 | 493.5 | 1.4 (1.3-1.5) | 115 | 30.3 | 3.8 (3.1-4.6) |
| Women | 12 493 | 278 | 189.7 | 1.5 (1.3-1.7) | 47 | 10.6 | 4.4 (3.2-5.9) |
| Men | 18 776 | 426 | 303.8 | 1.4 (1.3-1.5) | 68 | 19.7 | 3.5 (2.7-4.4) |
| Age at PV diagnosis, y | |||||||
| 20-49 | 8460 | 97 | 50.9 | 1.9 (1.6-2.3) | 15 | 3.1 | 4.9 (2.7-8.1) |
| 50-69 | 15 685 | 387 | 263.5 | 1.5 (1.3-1.6) | 63 | 15.8 | 4.0 (3.1-5.1) |
| 70+ | 7124 | 220 | 179.0 | 1.2 (1.1-1.4) | 37 | 11.5 | 2.3 (2.3-4.4) |
| Year of PV diagnosis | |||||||
| 1977-1994 | 22 693 | 506 | 343.3 | 1.5 (1.4-1.6) | 82 | 21.1 | 3.9 (3.1-4.8) |
| 1995-2008 | 8577 | 198 | 150.1 | 1.3 (1.1-1.5) | 33 | 9.3 | 3.6 (2.5-5.0) |
| Follow-up time | |||||||
| Year 2 | 4339 | 79 | 65.6 | 1.2 (1.0-1.5) | 11 | 4.0 | 2.8 (1.4-5.0) |
| Years 3-5 | 10 005 | 212 | 153.9 | 1.4 (1.2-1.6) | 35 | 9.4 | 3.7 (2.6-5.2) |
| Years 6+ | 16 926 | 413 | 273.9 | 1.5 (1.4-1.7) | 69 | 17 | 4.1 (3.2-5.1) |
| Hospital contact type (from 1995 on) | |||||||
| Outpatient only | 4395 | 89 | 74.1 | 1.2 (1.0-1.5) | 14 | 4.5 | 3.1 (1.7-5.2) |
| Inpatient admission | 4182 | 109 | 76.1 | 1.4 (1.2-1.7) | 19 | 4.7 | 4.0 (2.4-6.3) |
| . | Person-years of patients with PV, n . | PV patients with nonhematologic cancers, n . | Nonhematologic cancers expected, n . | SIR (95% CI) . | PV patients with hematologic cancers, n . | Hematologic cancers expected, n . | SIR (95% CI) . |
|---|---|---|---|---|---|---|---|
| All | 31 270 | 704 | 493.5 | 1.4 (1.3-1.5) | 115 | 30.3 | 3.8 (3.1-4.6) |
| Women | 12 493 | 278 | 189.7 | 1.5 (1.3-1.7) | 47 | 10.6 | 4.4 (3.2-5.9) |
| Men | 18 776 | 426 | 303.8 | 1.4 (1.3-1.5) | 68 | 19.7 | 3.5 (2.7-4.4) |
| Age at PV diagnosis, y | |||||||
| 20-49 | 8460 | 97 | 50.9 | 1.9 (1.6-2.3) | 15 | 3.1 | 4.9 (2.7-8.1) |
| 50-69 | 15 685 | 387 | 263.5 | 1.5 (1.3-1.6) | 63 | 15.8 | 4.0 (3.1-5.1) |
| 70+ | 7124 | 220 | 179.0 | 1.2 (1.1-1.4) | 37 | 11.5 | 2.3 (2.3-4.4) |
| Year of PV diagnosis | |||||||
| 1977-1994 | 22 693 | 506 | 343.3 | 1.5 (1.4-1.6) | 82 | 21.1 | 3.9 (3.1-4.8) |
| 1995-2008 | 8577 | 198 | 150.1 | 1.3 (1.1-1.5) | 33 | 9.3 | 3.6 (2.5-5.0) |
| Follow-up time | |||||||
| Year 2 | 4339 | 79 | 65.6 | 1.2 (1.0-1.5) | 11 | 4.0 | 2.8 (1.4-5.0) |
| Years 3-5 | 10 005 | 212 | 153.9 | 1.4 (1.2-1.6) | 35 | 9.4 | 3.7 (2.6-5.2) |
| Years 6+ | 16 926 | 413 | 273.9 | 1.5 (1.4-1.7) | 69 | 17 | 4.1 (3.2-5.1) |
| Hospital contact type (from 1995 on) | |||||||
| Outpatient only | 4395 | 89 | 74.1 | 1.2 (1.0-1.5) | 14 | 4.5 | 3.1 (1.7-5.2) |
| Inpatient admission | 4182 | 109 | 76.1 | 1.4 (1.2-1.7) | 19 | 4.7 | 4.0 (2.4-6.3) |
Observed and expected cancers and SIRs among patients Danish patients diagnosed with CML during 1977-2008 and followed for new primary cancers
| . | Person-years of CML patients . | CML patients with nonhematologic cancers, n . | Nonhematologic cancers expected, n . | SIR (95% CI) . | CML patients with hematologic cancers, n . | Hematologic cancers expected, n . | SIR (95% CI) . |
|---|---|---|---|---|---|---|---|
| All | 3960 | 75 | 46.0 | 1.6 (1.3-2.0) | 14 | 2.7 | 5.2 (2.8-8.7) |
| Women | 1788 | 27 | 21.4 | 1.3 (0.8-1.8) | 5 | 1.1 | 4.7 (1.5-11.0) |
| Men | 2172 | 48 | 24.5 | 2.0 (1.4-2.6) | 9 | 1.6 | 5.5 (2.5-10.4) |
| Age at CML diagnosis, y | |||||||
| 20-49 | 1772 | 20 | 7.6 | 2.6 (1.6-4.1) | 2 | 0.4 | 5.3 (0.6-19.0) |
| 50-69 | 1563 | 38 | 22.5 | 1.7 (1.2-2.3) | 2 | 1.3 | 1.5 (0.2-5.5) |
| 70+ | 625 | 17 | 15.9 | 1.1 (0.6-1.7) | 10 | 1.0 | 9.9 (4.7-18.1) |
| Year of CML diagnosis | |||||||
| 1977-1994 | 2250 | 39 | 23.8 | 1.6 (1.2-2.2) | 10 | 1.4 | 7.2 (3.5-13.3) |
| 1995-2008 | 1710 | 36 | 22.2 | 1.6 (1.1-2.3) | 4 | 1.3 | 3.1 (0.8-7.0) |
| Follow-up time | |||||||
| Year 2 | 896 | 14 | 11.0 | 1.3 (0.7-2.1) | 6 | 0.7 | 9.2 (3.3-20.0) |
| Years 3-5 | 1561 | 27 | 18.8 | 1.4 (1.0-2.1) | 5 | 1.1 | 4.5 (1.5-10.5) |
| Years 6+ | 1503 | 34 | 16.2 | 2.1 (1.5-2.9) | 3 | 0.9 | 3.2 (0.7-9.3) |
| Hospital contact type (from 1995 on) | |||||||
| Outpatient only | 1109 | 15 | 8.6 | 1.7 (1.0-2.9) | 0 | 0.5 | |
| Inpatient admission | 601 | 21 | 13.5 | 1.6 (1.0-2.4) | 4 | 0.8 | 5.0 (1.4-12.9) |
| . | Person-years of CML patients . | CML patients with nonhematologic cancers, n . | Nonhematologic cancers expected, n . | SIR (95% CI) . | CML patients with hematologic cancers, n . | Hematologic cancers expected, n . | SIR (95% CI) . |
|---|---|---|---|---|---|---|---|
| All | 3960 | 75 | 46.0 | 1.6 (1.3-2.0) | 14 | 2.7 | 5.2 (2.8-8.7) |
| Women | 1788 | 27 | 21.4 | 1.3 (0.8-1.8) | 5 | 1.1 | 4.7 (1.5-11.0) |
| Men | 2172 | 48 | 24.5 | 2.0 (1.4-2.6) | 9 | 1.6 | 5.5 (2.5-10.4) |
| Age at CML diagnosis, y | |||||||
| 20-49 | 1772 | 20 | 7.6 | 2.6 (1.6-4.1) | 2 | 0.4 | 5.3 (0.6-19.0) |
| 50-69 | 1563 | 38 | 22.5 | 1.7 (1.2-2.3) | 2 | 1.3 | 1.5 (0.2-5.5) |
| 70+ | 625 | 17 | 15.9 | 1.1 (0.6-1.7) | 10 | 1.0 | 9.9 (4.7-18.1) |
| Year of CML diagnosis | |||||||
| 1977-1994 | 2250 | 39 | 23.8 | 1.6 (1.2-2.2) | 10 | 1.4 | 7.2 (3.5-13.3) |
| 1995-2008 | 1710 | 36 | 22.2 | 1.6 (1.1-2.3) | 4 | 1.3 | 3.1 (0.8-7.0) |
| Follow-up time | |||||||
| Year 2 | 896 | 14 | 11.0 | 1.3 (0.7-2.1) | 6 | 0.7 | 9.2 (3.3-20.0) |
| Years 3-5 | 1561 | 27 | 18.8 | 1.4 (1.0-2.1) | 5 | 1.1 | 4.5 (1.5-10.5) |
| Years 6+ | 1503 | 34 | 16.2 | 2.1 (1.5-2.9) | 3 | 0.9 | 3.2 (0.7-9.3) |
| Hospital contact type (from 1995 on) | |||||||
| Outpatient only | 1109 | 15 | 8.6 | 1.7 (1.0-2.9) | 0 | 0.5 | |
| Inpatient admission | 601 | 21 | 13.5 | 1.6 (1.0-2.4) | 4 | 0.8 | 5.0 (1.4-12.9) |
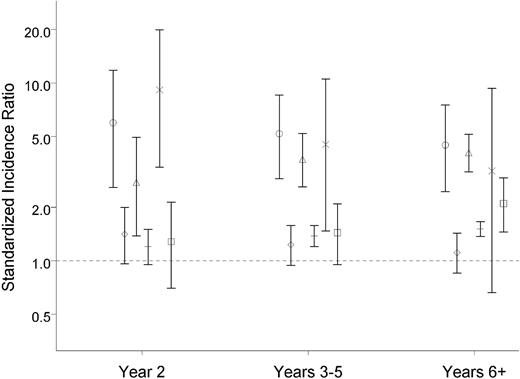
Overall, patients in the 3 cohorts were at increased risk of developing both new hematologic and new nonhematologic cancers (Tables 2,Table 3–4). SIRs for developing a nonhematologic cancer were very similar in the 3 cohorts: 1.2 (95% CI: 1.0-1.4) for ET patients, 1.4 (95% CI: 1.3-1.5) for PV patients, and 1.6 (95% CI: 1.3-2.0) for patients with CML (Tables 2,Table 3–4). The same overall tendency was observed when results were stratified by sex; age; year of ET, PV, or CML diagnosis (Tables 2,Table 3–4); and follow-up period (Figure 1). The SIRs for developing a hematologic cancer in the 3 cohorts were 5.0 (95% CI: 3.6-6.9) for ET patients, 3.8 (95% CI: 3.1-4.6) for PV patients, and 5.2 (95% CI: 2.8-8.7) for patients with CML. After stratification by sex, age, year, and follow-up time, the findings remained virtually unchanged. We found lower SIRs in the latest calendar period among CML patients (Table 4).
SIR for new primary cancers according to year of follow-up for patients with ET, PV, or CML. ○ indicates ET with hematologic cancer; ♢, ET with nonhematologic cancer; Δ, PV with hematologic cancer; +, PV with nonhematologic cancer; ×, CML with hematologic cancer; and □, CML with nonhematologic cancer.
SIR for new primary cancers according to year of follow-up for patients with ET, PV, or CML. ○ indicates ET with hematologic cancer; ♢, ET with nonhematologic cancer; Δ, PV with hematologic cancer; +, PV with nonhematologic cancer; ×, CML with hematologic cancer; and □, CML with nonhematologic cancer.
In Table 5, the observed cancers among PV patients are stratified by type, indicating an elevated risk of cancer of the oropharynx, esophagus, liver, pancreas, lung, prostate, kidney, urinary tract, eye, and skin compared with the general population. SIRs ranged between 1.2 and 3.7. The risk of carcinoma not specified by site was also increased, with a SIR of approximately 1.6 (Table 5). Among hematologic neoplasms, the risk was increased mainly for myeloid neoplasms. However, the risk for developing nonHodgkin lymphoma was also increased (SIR = 1.8; 95% CI: 1.1-2.7). The SIRs for the ET and CML cohorts were very similar to those shown in Table 5 for the PV cohort. They were estimated with less precision, however, because of the smaller number of patients with these diagnoses (data not shown).
Observed and expected cancers and SIRs among Danish patients diagnosed with PV during 1977-2008 and followed for new primary cancers
| . | Cancers observed, n . | Cancers expected, n . | SIR (95% CI) . |
|---|---|---|---|
| Oropharyngeal | 14 | 8.9 | 1.6 (0.9-2.6) |
| Esophagus | 15 | 6.4 | 2.4 (1.4-4.0) |
| Stomach | 12 | 12.0 | 1.0 (0.5-1.7) |
| Colon | 41 | 43.6 | 0.9 (0.7-1.3) |
| Rectum | 21 | 23.0 | 0.9 (0.6-1.4) |
| Liver, primary | 11 | 5.1 | 2.2 (1.1-3.9) |
| Gall bladder | 4 | 3.6 | 1.1 (0.3-2.9) |
| Pancreas | 21 | 13.9 | 1.5 (0.9-2.3) |
| Lung, primary | 127 | 67.9 | 1.9 (1.6-2.2) |
| Breast | 43 | 37.7 | 1.1 (0.8-1.5) |
| Cervix uteri | 6 | 3.5 | 1.7 (0.6-3.8) |
| Corpus uteri | 14 | 8.7 | 1.6 (0.9-2.7) |
| Ovary | 4 | 6.7 | 0.6 (0.2-1.5) |
| Prostate | 64 | 50.2 | 1.3 (1.0-1.6) |
| Kidney | 17 | 9.0 | 1.9 (1.1-3.0) |
| Urinary tract | 50 | 35.3 | 1.4 (1.1-1.9) |
| Malignant melanoma | 17 | 10.1 | 1.7 (1.0-2.7) |
| Nonmelanoma skin cancer | 146 | 88.5 | 1.7 (1.4-1.9) |
| Eye | 3 | 1.0 | 3.1 (0.6-8.9) |
| Brain | 10 | 11.4 | 0.9 (0.4-1.6) |
| Carcinoma unspecified | 33 | 20.7 | 1.6 (1.1-2.2) |
| NonHodgkin lymphoma | 20 | 11.4 | 1.8 (1.1-2.7) |
| Lymphoid leukemia | 4 | 6.7 | 0.6 (0.2-1.5) |
| Multiple myeloma | 8 | 5.3 | 1.5 (0.7-3.0) |
| Myeloid leukemia | 55 | 3.4 | 16.0 (12.0-20.8) |
| Unspecified leukemia | 17 | 0.5 | 33.1 (19.3-53.0) |
| Myelodysplastic syndrome | 10 | 2.0 | 5.0 (2.4-9.2) |
| . | Cancers observed, n . | Cancers expected, n . | SIR (95% CI) . |
|---|---|---|---|
| Oropharyngeal | 14 | 8.9 | 1.6 (0.9-2.6) |
| Esophagus | 15 | 6.4 | 2.4 (1.4-4.0) |
| Stomach | 12 | 12.0 | 1.0 (0.5-1.7) |
| Colon | 41 | 43.6 | 0.9 (0.7-1.3) |
| Rectum | 21 | 23.0 | 0.9 (0.6-1.4) |
| Liver, primary | 11 | 5.1 | 2.2 (1.1-3.9) |
| Gall bladder | 4 | 3.6 | 1.1 (0.3-2.9) |
| Pancreas | 21 | 13.9 | 1.5 (0.9-2.3) |
| Lung, primary | 127 | 67.9 | 1.9 (1.6-2.2) |
| Breast | 43 | 37.7 | 1.1 (0.8-1.5) |
| Cervix uteri | 6 | 3.5 | 1.7 (0.6-3.8) |
| Corpus uteri | 14 | 8.7 | 1.6 (0.9-2.7) |
| Ovary | 4 | 6.7 | 0.6 (0.2-1.5) |
| Prostate | 64 | 50.2 | 1.3 (1.0-1.6) |
| Kidney | 17 | 9.0 | 1.9 (1.1-3.0) |
| Urinary tract | 50 | 35.3 | 1.4 (1.1-1.9) |
| Malignant melanoma | 17 | 10.1 | 1.7 (1.0-2.7) |
| Nonmelanoma skin cancer | 146 | 88.5 | 1.7 (1.4-1.9) |
| Eye | 3 | 1.0 | 3.1 (0.6-8.9) |
| Brain | 10 | 11.4 | 0.9 (0.4-1.6) |
| Carcinoma unspecified | 33 | 20.7 | 1.6 (1.1-2.2) |
| NonHodgkin lymphoma | 20 | 11.4 | 1.8 (1.1-2.7) |
| Lymphoid leukemia | 4 | 6.7 | 0.6 (0.2-1.5) |
| Multiple myeloma | 8 | 5.3 | 1.5 (0.7-3.0) |
| Myeloid leukemia | 55 | 3.4 | 16.0 (12.0-20.8) |
| Unspecified leukemia | 17 | 0.5 | 33.1 (19.3-53.0) |
| Myelodysplastic syndrome | 10 | 2.0 | 5.0 (2.4-9.2) |
Data are stratified according to cancer type.
We repeated all analyses stratified by gender to investigate gender-specific effects. Overall, the SIRs for men and women were very similar (data not shown). We also examined the effect of time elapsed between the ET, PV, or CML diagnoses and the diagnosis of a subsequent malignancy for all specific cancer diagnoses listed in Table 5 by stratifying according to follow-up time. No clear trends were observed, except for myeloid leukemia, which showed a stepwise pattern toward increasing risk with longer follow-up time among patients with PV: SIR = 8.5 (96% CI: 2.3-21.7), SIR = 14.6 (96% CI: 8.3-23.7), and SIR = 18.6 (96% CI: 13.0-25.9) for 1-2 years, 2-4 years, and 5 years of follow-up, respectively.
To evaluate the possible effects of changes in treatment options, we also performed stratified analyses at 2 time points, 1985 and 2000. No clear changes in cancer occurrence were apparent over this time period (data not shown). However, the SIRs for new hematologic malignancies for CML patients may have decreased, because the SIR for 1977-1984 was 13.4 (95% CI: 5.4-27.6) and the SIR for 1985-2008 was 3.2 (95% CI: 1.3-6.6).
To estimate the possible effect of diagnostic misclassification of ET, PV, and CML diagnoses, we stratified analyses according to whether a diagnosis of COPD had been made. For nonhematologic cancers, the observed SIRs by COPD diagnosis status were as follows: ET without COPD = 1.2 (95% CI: 1.0-1.4), ET with COPD = 1.5 (95% CI: 0.8-2.7), PV without COPD = 1.4 (95% CI: 1.3-1.5), PV with COPD = 1.6 (95% CI: 1.2-2.1), CML without COPD = 1.6 (95% CI: 1.2-2.0), and CML with COPD = 3.4 (95% CI: 1.1-8.0). Among patients with PV, the SIRs for a subsequent diagnosis of lung cancer according to COPD status were as follows: PV without COPD = 1.8 (95% CI: 1.4-2.1) and PV with COPD = 3.4 (95% CI: 2.0-5.4).
Discussion
The results of the present study show that patients with ET, PV, and CML are at increased risk of developing another malignancy compared with the general population. This finding applies to various solid tumors and to hematologic myeloid and lymphoid malignancies. The cancer risk was much larger for another hematologic malignancy (SIR ≅ 5), than for a nonhematologic malignancy (SIR ≅ 1.5).
Our registry-based data do not provide patient-specific information that would allow us to investigate factors that could underlie increased cancer risk. However, cancer risk in patients with ET, PV, and CML might be increased for several reasons. The well-known increased risk of new hematologic myeloid malignancies such as AML4 has been attributed to the disease itself as well as to the treatment with cytotoxic drugs. The clonal origin of the disease and the resulting cell proliferation predisposes patients to new genetic abnormalities and therefore possibly to new cancers.14
Drugs previously used widely for the treatment of CMPNs (eg, busulfan, P32, and chlorambucil) have well-established carcinogenic potential,15-17 especially when used in combination or sequentially.18,19 Because the increased incidence and risk of AML among CMPN patients treated with these drugs has been recognized, their use has declined. In the past several years, the most widely prescribed drug to treat patients with CMPNs has been hydroxyurea (HU). The risk of leukemia attributed to HU when used as a single cytotoxic drug treatment for CMPN is under discussion, because studies conducted thus far have been unable to distinguish the leukemogenic effects of the CMPN disease from those of the treatment.15,17,18
Previously, the incidence of solid tumors among CMPN patients compared with the general population was examined according to specific treatment. In a secondary analysis of a randomized controlled trial, 461 PV patients 65 years of age or more were initially treated with P32.19 Those who were in complete remission after 4 months were then randomized to either observation or maintenance therapy with HU.19 When the rate of hematologic and nonhematologic neoplasms was compared in the 2 treatment arms, their overall rate was reported to be comparable to the general population. However, the rate of both hematologic neoplasms and carcinomas remained considerably higher in patients treated with both P32 and HU.19 In another randomized trial, 293 PV patients were randomized to either P32 or busulfan treatment. At follow-up, no difference in rates of leukemia or carcinomas was observed.20 In an uncontrolled study, 331 ET patients from a single hematology department were followed for new malignancies.18 No difference in the incidence of carcinomas was observed across different treatment arms, including untreated patients; however, patients treated with alkylating agents had a higher incidence of hematologic neoplasms.18
For specific cancers, associations between a PV diagnosis and kidney cancer risk21 and HU treatment and skin cancer risk22 have been suggested previously. Some of our results are in agreement with the recent Swedish registry study by Fallah et al, which reported SIRs for kidney cancer of 2.2, for nonmelanoma skin cancer of 2.0, and for melanoma of 1.9.7 However, unlike our study, Fallah et al did not report on increased risk of cancer of the esophagus, liver, lung, prostate, urinary tract, or nonHodgkin lymphoma. The cause of this discrepancy is unknown.
Some characteristics of the patients in our study may have affected their subsequent observed cancer incidence. For example, patients were identified using nationwide population-based registries. With this approach, no ET, PV, or CML patients were lost to follow-up. In addition, diagnoses of ET, PV, or CML and incident cancers were not required to be made in the same department, hospital, or region. This may have led to a higher incidence of cancer than observed when patients are followed in the same department.
During the1977-1994 period, patients could be included in our study only if they were admitted to the hospital, because registration of outpatient visits was not established in Denmark until 1995. This may have affected cancer risk estimates at least in 2 ways. Because patients were not identified during the earlier study period until hospital admission, they may have been more severely affected than patients diagnosed in the later period, possibly influencing subsequent cancer risk. In addition, the diagnosis date in the first study period was defined as the date of the first hospital admission, and the inclusion of prevalent and incident CMPN cases is therefore likely because previous outpatient visits were not captured. This could cause selection bias toward ET, PV, or CML patients with longer survival, who also might have a different cancer risk. The direction of the influence of the bias introduced by these 2 issues is not evident; however, we observed no tendency toward higher or lower cancer risk across the time periods studied.
The low number of ET patients diagnosed during the first study period was probably due to the definition of diagnosis date, because ET patients are rarely admitted to the hospital. In addition, the definition of diagnosis date in the first study period explains the relatively short median follow-up time observed. The shorter follow-up among CML patients was possibly influenced by the higher mortality observed previously.23 Furthermore, we have no information about the disease phase at which the CML diagnosis was made. Some patients might have already been in blast crisis at diagnosis, with the accompanying adverse prognosis.24
Another limitation is that ET, PV, and CML cases were identified by ICD codes. Because new malignancies for patients with a known malignant disease are not always coded, this could lead to underestimation of the risk of a new cancer. In addition, we cannot rule out the possibility of diagnostic misclassification of CMPN (eg, reactive thrombocytosis25 erroneously coded as ET), which might inflate cancer risk. To address this issue, we analyzed SIRs for patients diagnosed with a cancer during the first 3 months after their CMPN diagnosis. The higher SIRs observed in this period possibly reflect some diagnostic misclassification of CMPN, as well as increased diagnostic intensity immediately after diagnosis of a chronic disease (data not shown). In addition, we stratified analyses according to COPD diagnosis status and, as expected, observed higher lung cancer risk in patients with PV and COPD than in patients with PV but without COPD. Nevertheless, lung cancer risk and cancer risk in general remained above unity for PV patients without COPD. We also found elevated cancer incidence among CML patients, for whom misclassification of a CML diagnosis seems unlikely because of the presence of the Philadelphia chromosome. Some patients with a diagnosis of ET may instead have been in an early stage of myelofibrosis with a more aggressive hematologic course.26 In our study, we were unable to include a specific group of CMPN patients with myelofibrosis, and the effect of possible diagnostic misclassification on the nonhematologic cancer risk of some ET patients remains unclear.
Another factor for consideration is that most patients with CMPNs are treated with aspirin for thrombosis prophylaxis. A recent meta-analysis of 4 randomized trials comparing aspirin with placebo treatment as primary prevention for vascular events found lower colon cancer incidence and mortality among users of low-dose aspirin compared with placebo.27 Our study showed no increased incidence of colorectal cancer among CMPN patients.
The increased SIR for lymphomas in our study is in agreement with the finding previously reported by Vannucchi et al.5 The observed increased risk for lymphoid malignancies among CMPN patients might be related to treatment, because immunosuppressive drugs used for other indications have been shown previously to increase the incidence of lymphoma.28,29
With the introduction of imatinib for the treatment of CML in the past decade, the prognosis for this disease has changed dramatically.30,31 This might influence cancer incidence in 2 directions. Improved survival, providing longer time-at-risk for other health-related events, could result in higher cancer incidence among CML patients. However, cancer incidence also may decrease because of less use of cytotoxic drugs, stem cell transplantation, and immunosuppressive drugs. We observed no definite differences in cancer risk across different time periods.
In conclusion, our data suggest that risk of a new cancer is increased in patients with myeloproliferative neoplasms.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: H.F., H.C.H., and H.T.S. conceptualized the idea for the study; D.K.F. performed the data analyses; H.F., C.F.C., and H.T.S. directed the statistical analyses; H.F. wrote the first draft of the manuscript; and all authors participated in study design and in writing subsequent drafts of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests. The Department of Clinical Epidemiology, Aarhus University Hospital, receives funding for other studies from several companies in the form of research grants to (and administered by) Aarhus University. None of these studies has any relation to the present study.
Correspondence: Henrik Frederiksen, MD, PhD, Department of Clinical Epidemiology, Aarhus University Hospital, Olof Palmes Allé 43-45, DK-8200 Aarhus N, Denmark; e-mail: hef@dadlnet.dk.