Abstract
Host hematopoietically derived APCs play a vital role in the initiation of GVH responses. However, the APC autonomous molecular mechanisms that are critical for the induction of GVHD are not known. We report here that the Ikaros-Notch axis in host hematopoietically derived APCs regulates the severity of acute GVHD across multiple clinically relevant murine models of experimental bone marrow transplantation. In the present study, Ikaros deficiency (Ik−/−) limited to host hematopoietically derived APCs enhanced donor T-cell expansion and intensified acute GVHD, as determined by survival and other GVHD-specific parameters. The Ik−/− conventional CD8+ and CD8−CD11c+ dendritic cells (DCs), the most potent APCs, showed no increase in the expression of activation markers or in response to TLR stimulation compared with wild-type controls. However, Ik−/− DCs demonstrated an enhanced stimulation of allogeneic T cells. Deficiency of Ikaros in the conventional CD8+ and CD8−CD11c+ DCs was associated with an increase in Notch signaling, the blockade of which mitigated the enhanced in vitro and in vivo allostimulatory capacity. Therefore, the Ikaros-Notch axis is a novel pathway that modulates DC biology in general, and targeting this pathway in host hematopoietically derived APCs may reduce GVHD.
Introduction
Acute GVHD, a major complication of allogeneic bone marrow transplantation (BMT), has limited the efficacy and application of this potent therapy.1 Alloreactive donor T lymphocytes cause GVHD. Modulation of the molecular mechanisms of T lymphocyte functions by calcineurin inhibitors and other drugs have been the traditional targets for reducing GVHD.1 Emerging data demonstrate that host APCs are critical for the induction of GVHD.1 However, little is known about the APC autonomous molecular mechanisms that are critical for the modulation of GVHD.
Ikaros is a member of a family of zinc-finger nuclear proteins2 that is expressed in hematopoietic progenitors and is an essential transcription factor for the development of lymphoid lineages.2,3 Ikaros has been implicated in the progress and development of T-cell leukemia and lymphoma.4 The relevance of Ikaros in the modulation of T-cell responses is becoming increasingly appreciated.5-11 The role of Ikaros in modulating dendritic cell (DC) responses is less clear, but is critical for the development and differentiation of DC subsets such as plasmacytoid DCs (pDCs).12-14 The molecular mechanism and in vivo relevance of Ikaros in modulating DCs are not known.
Notch proteins belong to a family of highly conserved receptors that are proteolytically cleaved by the γ-secretase complex after the binding of its ligands,5 which translocates into the nucleus and activates the transcription of several target genes.15,16 Notch controls cell fate differentiation decisions during hematopoiesis and lymphoid development5 and is an important modulator of mature T-cell responses under certain contexts.5,17 Emerging data demonstrate an important role for Notch signaling in the development and functional responses of DCs,5,18,19 and it has also been shown to be relevant for the activation of T cells by DCs.5,20,21 However, the cell-intrinsic factors that enhance Notch signaling in DCs are not known.
Ikaros is known to negatively regulate the response to Notch signaling,22-24 and activation of Notch signaling has been shown to occur in the absence of Ikaros. The converging roles of Ikaros-Notch have been demonstrated on leukemogenesis25-28 and T-cell development,22,29 but whether the Ikaros-Notch axis is involved in the regulation of DC responses is unknown.
In the present study, we investigated the impact of Ikaros in host hematopoietic cell–derived APCs on the severity of GVHD. We show that deficiency of Ikaros in host APCs exacerbated GVHD. Mechanistic studies demonstrated that Ikaros regulated the in vitro and in vivo responses of DCs, which was dependent, at least in part, on enhanced Notch signaling. We demonstrate a critical role for a novel molecular pathway, the Ikaros-Notch axis, in host APCs as an important modulator of GVHD.
Methods
Mice
C57BL/6 (B6, H-2b, CD45.2+), C3H.sw (H-2b), BALB/c (H-2d), and B6 Ly5.2 (H-2b, CD45.1+) mice were purchased from The Jackson Laboratory and the National Cancer Institute. Ik−/− and IkDN+/− mice were backcrossed to B6 mice for > 8 generations.8 B6 CD4-Cre and Mx-Cre transgenic (Tg) ROSA26DNMAMLf (DNMAML) mice expressing a Cre-inducible, dominant-negative, Mastermind-like pan-Notch inhibitor have been described previously.15,30 BMT recipient mice were housed and maintained as described in supplemental Methods (available on the Blood Web site; see the Supplemental Materials link at the top of the online article). All animals were cared for under regulations reviewed and approved by the University of Michigan Committee on Use and Care of Animals based on University Laboratory Animal Medicine guidelines.
Generation of BM chimeras
Bone marrow (BM) chimeras were generated as described previously31 and as detailed in supplemental Methods.
BMT
Systemic and histopathologic analysis of GVHD
Survival after BMT was assessed daily. The degree of clinical and histopathologic GVHD was assessed as described previously and as detailed in supplemental Methods.31
DC cultures
BM cells from wild-type B6 (WT-B6), Ik−/−, IkDN+/−, and BALB/c mice were cultured with murine recombinant GM-CSF (PeproTech) and isolated as described previously33 and as detailed in supplemental Methods.
FACS analysis
Isolation of hepatic lymphocytes
Hepatic lymphocytes were isolated as described previously.34 Briefly, livers from recipient male B6 mice were minced and suspended in PBS containing 1mM EDTA. Percoll was added to the cell suspension (Amersham Pharmacia Biotech) to a final concentration of 33%. After centrifugation for 30 minutes at room temperature, hepatic lymphocytes were collected from the bottom and contaminating RBCs were lysed.
MLR
Mixed-leukocyte reaction (MLR) cultures with splenic T cells and DCs and macrophages were performed as detailed in supplemental Methods.
Ex vivo suppression assay
CD4+CD25− and CD4+CD25+ T cells were isolated from hepatic cells pooled from 5 allogeneic BM chimeras using a CD4+CD25+ regulatory T-cell isolation kit (Miltenyi Biotec) with auto MACS following the manufacturer's protocol. The purity of each type of cell was > 85%. 2.5 × 103 CD4+CD25+ T cells from these pooled allogeneic BM chimeras were incubated with 1 × 104 CD4+CD25− T cells obtained from the same animals. They were stimulated with 5 × 104 irradiated (137Cs source) WT-B6 splenocytes for 120 hours. In the titration assay, CD4+CD25+ T cells from WT-BALB/c BM serially diluted from 2 × 104 to 2500 cells/well and incubated with 2 × 104 CD4+CD25− T cells and 5 × 103 irradiated B6 BM-derived DCs (BMDCs) for 120 hours. Incorporation of 3H-thymidine (1 μCi/well) by the proliferating cells was measured during the last 18 hours of culture.
Cytokine ELISA
TNF-α, IL-6, IL-1β, IL-17A, and IFN-γ were measured in serum and culture supernatants by ELISA according to the manufacturer's protocol (see supplemental Methods).
CFSE and apoptosis analysis
CD90.2+ T cells were isolated from BALB/c mice, stained for CFSE, and subsequent apoptosis was identified based on staining for annexin V, as detailed in supplemental Methods.
Expression analysis by Q-PCR
Quantitative PCR (Q-PCR) was performed using SYBR Green dye as described in supplemental Methods. The primer sequence is shown in supplemental Table 1.
Treatment with DAPT
BMDCs were treated with the γ-secretase inhibitor N-[N-(3,5-difluorophenacetyl)-alanyl]-S-phenylglycine t-butyl ester (DAPT, 5μM) overnight and then stimulated with MLR. MLR was performed as detailed in supplemental Methods. For the in vivo experiments, the [B6 → B6Ly5.2] and [Ik−/− → B6Ly5.2] chimeras were transplanted as described in supplemental Methods. DAPT (50 μg/kg) or the control diluents were administered by IP injection on days −1, +1, and +3 after BMT.18
Statistical analysis
The Mann-Whitney U test was used for statistical analysis of in vitro data, and the Wilcoxon rank test was used to analyze survival data. P < .05 was considered statistically significant.
Results
Ikaros deficiency in host APCs exacerbates GVHD in an experimental GVHD model
We first evaluated the role of Ikaros in host hematopoietically derived APCs on the severity of GVHD1 by generating bone marrow chimeras. WT-B6 Ly5.2 animals were lethally irradiated with 11 Gy and infused with 5 × 106 BM cells and 5 × 106 splenocytes from syngeneic Ly5.1 WT B6 or Ik−/− B6 donors. Chimerism analyses of donor hematopoietic cells and donor CD11c+ demonstrated that these animals were complete donor chimeras (> 97%) 3 months after BMT (data not shown). The [B6 → B6Ly5.2] or [Ik−/− → B6Ly5.2] chimeras were then used as recipients 4 months after primary BMT in a secondary allogeneic BMT. These recipient chimeras received 9 Gy and were injected IV with 2 × 106 CD90+ T cells, along with 5 × 106 BM cells from either syngeneic B6 or the MHC-matched but multiple minor histocompatibility antigen (miHA)–mismatched allogeneic C3H.SW donors. All of the chimeras that received syngeneic T cells and BM cells survived the duration of the observation period with no signs of GVHD (Figure 1A). Most (60%) of the allogeneic [B6 → B6Ly5.2] chimeras died with signs of GVHD by day +100 after BMT. In contrast, 100% of the [Ik−/− → B6Ly5.2] chimeras that received allogeneic T cells and BM cells died rapidly with signs of severe clinical GVHD, demonstrating significantly greater mortality compared with the allogeneic [B6 → B6Ly5.2] animals (Figure 1A).
Ikaros deficiency in host APCs exacerbates GVHD. (A) WT B6 Ly5.2 animals were lethally irradiated with 11 Gy and infused with 5 × 106 BM cells and 5 × 106 splenocytes from syngeneic Ly5.1 WT B6 or Ik−/− B6 donors. Four months later, these animals [B6 → B6Ly5.2] or [Ik−/− → B6Ly5.2] chimeras received 9 Gy and 2 × 106 CD90+ T cells along with 5 × 106 BM cells from either syngeneic B6 or allogeneic MHC-matched, multiple miHA-mismatched C3H.sw donors and analyzed for survival. Shown are B6 → [B6 → B6Ly5.2] (n = 7) (■), B6 → [Ik−/− → B6Ly5.2] (n = 6) (♦), C3H.sw → [B6 → B6 Ly5.2] (n = 12) (▴), and C3H.sw → [Ik−/− → B6Ly5.2] (n = 11) (●). ***P < .001 for C3H.sw → [B6 → B6Ly5.2] compared with C3H.sw → [Ik−/− → B6Ly5.2]. Data are representative of 2 independent experiments. (B) Representative hematoxylin and eosin–stained images and histopathologic score of gastrointestinal tracts (small and large bowels), liver, and skin on days +14 and +21 after allogeneic BMT. Each group includes 5-9 mice. (C) Serum IFN-γ and IL-17A levels at day 7. Each group includes 5-9 mice. (D) Donor T-effector and Treg expansion and ex vivo suppression. Donor CD8+ T-cell expansion was analyzed in splenocytes harvested on day +14 from allogeneic [B6 → Ly5.2] or [Ik−/− → B6Ly5.2] chimeras (n = 5 animals/group) as shown by the black bars versus the gray bars, respectively (P < .05). Donor CD4+CD25+Foxp3+ Treg expansion was analyzed in the spleens harvested on days +14 and +21 and in the liver harvested on day +21 from the allogeneic [B6 → Ly5.2] or [Ik−/− → B6Ly5.2] animals (n = 5 animals/group) as shown by the black bars versus the gray bars, respectively (P = not significant at both day +14 and day +21). The donor CD4+CD25+ and the CD4+CD25− T cells were isolated from the livers (pooled from 5 mice/group) harvested on day +21 from the allogeneic [B6 → Ly5.2] or [Ik−/− → B6Ly5.2] animals. The suppressive function of CD4+CD25+ T cells was analyzed by coculturing the isolated 2.5 × 103 CD4+CD25+ T cells with 104 CD4+CD25− T cells in the presence of 5 × 104 irradiated WT B6 spleen cells for 120 hours. Incorporation of 3H-thymidine (1 μCi/well) by proliferating cells was measured during the last 6 hours of culture. Data show means ± SEM. P = not significant. Data are from 1 of 2 similar experiments. (E) WT B6 Ly5.2 animals were lethally irradiated with 11 Gy and infused with 5 × 106 BM cells and 5 × 106 splenocytes from syngeneic Ly5.1 WT B6 or Ik−/− B6 donors. Four months later, [B6 → B6Ly5.2] or [Ik−/− → B6Ly5.2]) animals were irradiated with 9 Gy and transplanted with 2 × 106 CD90+ T cells along with 5 × 106 BM cells from either syngeneic B6 or allogeneic MHC-mismatched BALB/c donors and analyzed for survival. Shown are B6 → [B6 → B6Ly5.2] (n = 7) (■), BALB/c → [B6 → B6Ly5.2] (n = 10) (▴), and BALB/c → [Ik−/− → B6Ly5.2] (n = 13) (●) animals. Data are representative of 2 independent experiments. (F) WT B6Ly5.2 animals were lethally irradiated with 11 Gy and infused with 5 × 106 BM cells and 5 × 106 splenocytes from syngeneic Ly5.1 WT B6 or IkDN+/− B6 donors. Four months later, [B6 → B6Ly5.2] or [IkDN+/− → B6Ly5.2]) animals were used as recipients, irradiated with 9 Gy, and transplanted with 2 × 106 CD90+ T cells along with 5 × 106 BM cells from either syngeneic B6 or allogeneic MHC-matched, miHA-mismatched C3H.sw donors, and the animals were evaluated for survival. Shown are B6 → [B6 → B6Ly5.2] (n = 6) (■), C3H.sw → [B6 → B6 Ly5.2] (n = 9) (▴), and C3H.sw → [IkDN+/− → B6Ly5.2] (n = 11) ●. Data are representative of 2 independent experiments.
Ikaros deficiency in host APCs exacerbates GVHD. (A) WT B6 Ly5.2 animals were lethally irradiated with 11 Gy and infused with 5 × 106 BM cells and 5 × 106 splenocytes from syngeneic Ly5.1 WT B6 or Ik−/− B6 donors. Four months later, these animals [B6 → B6Ly5.2] or [Ik−/− → B6Ly5.2] chimeras received 9 Gy and 2 × 106 CD90+ T cells along with 5 × 106 BM cells from either syngeneic B6 or allogeneic MHC-matched, multiple miHA-mismatched C3H.sw donors and analyzed for survival. Shown are B6 → [B6 → B6Ly5.2] (n = 7) (■), B6 → [Ik−/− → B6Ly5.2] (n = 6) (♦), C3H.sw → [B6 → B6 Ly5.2] (n = 12) (▴), and C3H.sw → [Ik−/− → B6Ly5.2] (n = 11) (●). ***P < .001 for C3H.sw → [B6 → B6Ly5.2] compared with C3H.sw → [Ik−/− → B6Ly5.2]. Data are representative of 2 independent experiments. (B) Representative hematoxylin and eosin–stained images and histopathologic score of gastrointestinal tracts (small and large bowels), liver, and skin on days +14 and +21 after allogeneic BMT. Each group includes 5-9 mice. (C) Serum IFN-γ and IL-17A levels at day 7. Each group includes 5-9 mice. (D) Donor T-effector and Treg expansion and ex vivo suppression. Donor CD8+ T-cell expansion was analyzed in splenocytes harvested on day +14 from allogeneic [B6 → Ly5.2] or [Ik−/− → B6Ly5.2] chimeras (n = 5 animals/group) as shown by the black bars versus the gray bars, respectively (P < .05). Donor CD4+CD25+Foxp3+ Treg expansion was analyzed in the spleens harvested on days +14 and +21 and in the liver harvested on day +21 from the allogeneic [B6 → Ly5.2] or [Ik−/− → B6Ly5.2] animals (n = 5 animals/group) as shown by the black bars versus the gray bars, respectively (P = not significant at both day +14 and day +21). The donor CD4+CD25+ and the CD4+CD25− T cells were isolated from the livers (pooled from 5 mice/group) harvested on day +21 from the allogeneic [B6 → Ly5.2] or [Ik−/− → B6Ly5.2] animals. The suppressive function of CD4+CD25+ T cells was analyzed by coculturing the isolated 2.5 × 103 CD4+CD25+ T cells with 104 CD4+CD25− T cells in the presence of 5 × 104 irradiated WT B6 spleen cells for 120 hours. Incorporation of 3H-thymidine (1 μCi/well) by proliferating cells was measured during the last 6 hours of culture. Data show means ± SEM. P = not significant. Data are from 1 of 2 similar experiments. (E) WT B6 Ly5.2 animals were lethally irradiated with 11 Gy and infused with 5 × 106 BM cells and 5 × 106 splenocytes from syngeneic Ly5.1 WT B6 or Ik−/− B6 donors. Four months later, [B6 → B6Ly5.2] or [Ik−/− → B6Ly5.2]) animals were irradiated with 9 Gy and transplanted with 2 × 106 CD90+ T cells along with 5 × 106 BM cells from either syngeneic B6 or allogeneic MHC-mismatched BALB/c donors and analyzed for survival. Shown are B6 → [B6 → B6Ly5.2] (n = 7) (■), BALB/c → [B6 → B6Ly5.2] (n = 10) (▴), and BALB/c → [Ik−/− → B6Ly5.2] (n = 13) (●) animals. Data are representative of 2 independent experiments. (F) WT B6Ly5.2 animals were lethally irradiated with 11 Gy and infused with 5 × 106 BM cells and 5 × 106 splenocytes from syngeneic Ly5.1 WT B6 or IkDN+/− B6 donors. Four months later, [B6 → B6Ly5.2] or [IkDN+/− → B6Ly5.2]) animals were used as recipients, irradiated with 9 Gy, and transplanted with 2 × 106 CD90+ T cells along with 5 × 106 BM cells from either syngeneic B6 or allogeneic MHC-matched, miHA-mismatched C3H.sw donors, and the animals were evaluated for survival. Shown are B6 → [B6 → B6Ly5.2] (n = 6) (■), C3H.sw → [B6 → B6 Ly5.2] (n = 9) (▴), and C3H.sw → [IkDN+/− → B6Ly5.2] (n = 11) ●. Data are representative of 2 independent experiments.
The enhanced mortality in the allogeneic [Ik−/− → B6Ly5.2] animals was associated with greater severity of GVHD on day +14 in the 3 GVHD-specific target organs—the liver, gastrointestinal tract (small and large bowels), and skin—than in the [B6 → B6Ly5.2] allogeneic controls (Figure 1B). The increased severity persisted in the liver and gastrointestinal tract, but was not significantly different in the skin at later time points (Figure 1B). We next examined serum levels of IFN-γ and IL-17 and donor T-cell expansion in the recipient spleens.1 Serum levels of IFN-γ and IL-17 and donor T cells, as shown in Figures 1C and 1D, respectively, were significantly higher in the allogeneic [Ik−/− → B6Ly5.2] compared with the allogeneic [B6 → B6Ly5.2] animals. We also examined whether the expansion of donor Tregs was affected after allogeneic BMT because of the known paucity of pDCs in the absence of Ikaros.35 We found similar expansion of donor CD4+Foxp3+Tregs on days +14 and +21 in the spleens of both the allogeneic [Ik−/− → B6Ly5.2] and [B6 → B6Ly5.2] animals (Figure 1D). We also examined donor Treg recovery from the liver, the GVHD target organ, which demonstrated a significant difference in GVHD-induced histopathologic damage. Consistent with recovery from the spleen, there was no significant difference in the recovery of donor CD4+Foxp3+Tregs from the host livers at day +21 (Figure 1D). These data suggest that the absence of Ik in the host hematopoietic cells enhanced the expansion of T-effector cells without significantly altering the expansion of donor Tregs. To further analyze whether the absence of Ikaros altered the function of the donor Tregs, we next harvested the host livers at day 21 and analyzed the function of the donor CD4+CD25+ T cells in an ex vivo suppression assay. The donor CD4+CD25+ T cells recovered from the livers of both Ik-deficient and WT chimeras demonstrated equivalent suppression of the isolated CD4+CD25− T effector cells (Figure 1D). These data indicate that the absence of Ik in host hematopoietic cells did not alter donor Treg expansion or function in the liver, the GVHD target organ.
To preclude strain-specific responses, we used the [B6 → B6Ly5.2] and [Ik−/− → B6Ly5.2] chimeras as recipients in an MHC-mismatched, BALB/c→B6 model of allogeneic BMT. Both the [B6 → B6Ly5.2] and [Ik−/− → B6Ly5.2] groups were irradiated with 9 Gy and transplanted with 2 × 106 CD90+ T cells, along with 5 × 106 BM cells from either syngeneic B6 or allogeneic BALB/c donors. As expected, all of the syngeneic recipients survived. Consistent with the data shown in Figure 1A, the allogeneic [Ik−/− → B6Ly5.2] animals showed greater mortality with more severe GVHD than the allogeneic [B6 → B6Ly5.2] recipients (Figure 1E).
To confirm the impact of Ikaros deficiency in host APCs on GVHD, we also used Ikaros dominant-negative (IkDN+/−) mice to generate the [IkDN+/− → B6Ly5.2] and the control [B6 → B6Ly5.2] chimeras. These chimeras were irradiated with 9 Gy and transplanted with 2 × 106 CD90+ splenic T cells along with 5 × 106 BM cells from either syngeneic B6 or allogeneic C3H.SW donors. As shown in Figure 1F, the allogeneic [IkDN+/− → B6Ly5.2] animals demonstrated significantly greater mortality than the allogeneic [B6 → B6Ly5.2] animals (100% vs 50%, respectively, P < .05).
Effect of Ikaros on the numbers and phenotype of conventional DCs
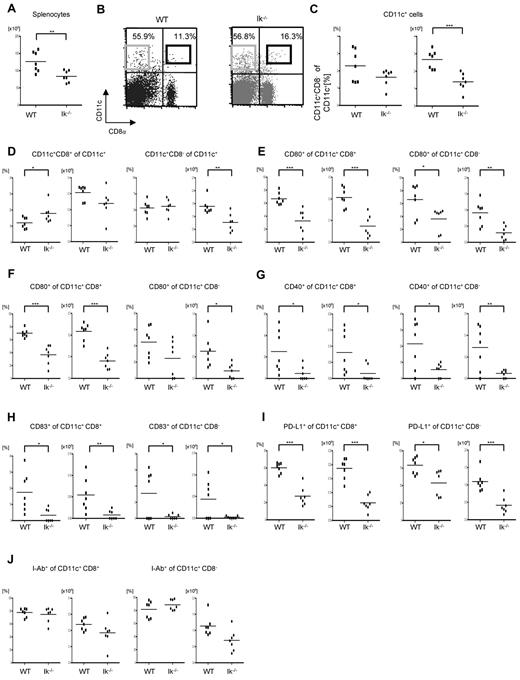
To evaluate the mechanism of enhanced GVHD, we focused on DCs because they are the most potent APCs and because host-type DCs have been shown to be sufficient for the induction of GVHD.32 We determined the effect of Ikaros deficiency on the numbers and phenotype of DCs. The total splenocyte count was significantly lower in the Ikaros-deficient, Ik−/− mice, compared with WT animals (Figure 2A). Although no differences were observed in the percentage of CD11c+ DCs, the absolute numbers were lower and the percentage of CD8+ DCs was higher in the Ik-deficient animals compared with WT animals (Figure 2B-D). However, in contrast to a previous study,12 we also found a similar percentage of CD8− DCs in the spleens of Ik−/− mice (Figure 2B-D). The absolute numbers and percentage expression of costimulatory molecules such as CD80, CD86, CD40, CD83, and PDL1 was much lower in both CD8+ and CD8−CD11c+ splenic DCs in Ik−/− mice compared with WT animals (Figure 2E-I). The expression of MHC class II was similar in the CD8+ and CD8−CD11c+ splenic DCs from the WT and Ik−/− animals (Figure 2J). We also examined the percentage and number of pDCs and, in agreement with a previous study, found no pDCs in the spleens of Ik−/− mice (data not shown).13
Splenic DC phenotype in the absence of Ikaros. The total splenocyte count and the frequency and the expression of costimulation molecules on CD11c+ cells were analyzed from naive Ik−/− and WT B6 animals that were not transplanted (n = 5-7/group). *P < .05; **P < .01; ***P < .001. (A) Total splenocyte count. (B) Expression of CD11c and CD8. The black and gray boxes signify CD8+CD11c+ DCs and CD8−CD11c+ DCs, respectively. (C) Frequency and absolute number of CD11c+ DCs. (D) Frequency and absolute number of CD8+CD11c+ DCs and CD8−CD11c+ DCs in spleen. The expression of the costimulatory molecules CD80 (E), CD86 (F), CD40 (G), CD83 (H), and B7H1 (PDL1) I-Ab (I) on CD8+CD11c+ DCs or CD8−CD11c+ DCs.
Splenic DC phenotype in the absence of Ikaros. The total splenocyte count and the frequency and the expression of costimulation molecules on CD11c+ cells were analyzed from naive Ik−/− and WT B6 animals that were not transplanted (n = 5-7/group). *P < .05; **P < .01; ***P < .001. (A) Total splenocyte count. (B) Expression of CD11c and CD8. The black and gray boxes signify CD8+CD11c+ DCs and CD8−CD11c+ DCs, respectively. (C) Frequency and absolute number of CD11c+ DCs. (D) Frequency and absolute number of CD8+CD11c+ DCs and CD8−CD11c+ DCs in spleen. The expression of the costimulatory molecules CD80 (E), CD86 (F), CD40 (G), CD83 (H), and B7H1 (PDL1) I-Ab (I) on CD8+CD11c+ DCs or CD8−CD11c+ DCs.
We next examined the phenotype of GM-CSF–induced BMDCs. The phenotype of GM-CSF induced BMDCs generated from Ik−/− mice was similar to that of WT BMDCs except for an increase in the surface of CD40 expression (supplemental Figure 1A-F).
After the generation of the chimeras, it was possible that the reconstitution of the host hematopoietically derived APCs from the WT and the Ik−/− donors were different. Therefore, we analyzed the DC numbers/phenotype in the Ik−/− → B6Ly5.2 and the B6Ly5.1 → B6Ly5.2 animals. We first evaluated the percentage and total numbers of the splenic CD11c+ DCs after the generation of BM chimeras. Consistent with the results from naive Ik-deficient and WT animals, the DC numbers and subsets were lower, whereas the percentage CD11c+ DCs and the CD8+ and CD8− DCs were similar in the [Ik−/− → B6Ly5.2] animals compared with the [WT B6 → B6Ly5.2] animals (Figure 3A-I).
Reconstitution of DCs, numbers, and phenotype in the [B6 → B6Ly5.2] and [Ik−/− → B6 Ly5.2] chimeras. WT B6 Ly5.2 animals were lethally irradiated with 11 Gy and infused with 5 × 106 BM cells and 5 × 106 splenocytes from syngeneic Ly5.1 WT B6 or Ik−/− B6 donors. Four months later, these animals (n = 4-5 animals/group) were killed and the splenocytes were harvested and analyzed for total splenocyte count (A), frequency and number of CD11c+ DCs (B), and frequency and absolute numbers of CD8+CD11c+ DCs and CD8−CD11c+ DCs (C). The CD8+CD11c+ and CD8−CD11c+ DCs were analyzed for expression of CD80 (D), CD86 (E), CD40 (F), CD83 (G), and B7H1 (PD-L1) (I) I-Ab. *P < .05, **P < .01.
Reconstitution of DCs, numbers, and phenotype in the [B6 → B6Ly5.2] and [Ik−/− → B6 Ly5.2] chimeras. WT B6 Ly5.2 animals were lethally irradiated with 11 Gy and infused with 5 × 106 BM cells and 5 × 106 splenocytes from syngeneic Ly5.1 WT B6 or Ik−/− B6 donors. Four months later, these animals (n = 4-5 animals/group) were killed and the splenocytes were harvested and analyzed for total splenocyte count (A), frequency and number of CD11c+ DCs (B), and frequency and absolute numbers of CD8+CD11c+ DCs and CD8−CD11c+ DCs (C). The CD8+CD11c+ and CD8−CD11c+ DCs were analyzed for expression of CD80 (D), CD86 (E), CD40 (F), CD83 (G), and B7H1 (PD-L1) (I) I-Ab. *P < .05, **P < .01.
Ikaros deficiency enhances the functional responses of conventional DCs
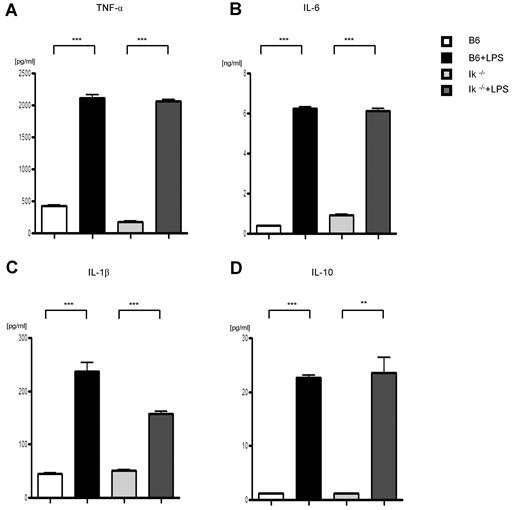
Because of the above results, we next evaluated the functional responses of conventional DCs from WT and Ikaros-deficient, Ik−/−, and IkDN+/− mice to investigate the source of the GVHD aggravation caused by the deficiency of Ikaros in host APCs. We first tested the effect of Ikaros deficiency on the innate immune responses of DCs that are triggered through TLRs, cell-surface molecules that recognize pathogen-associated molecular patterns. BMDCs from WT, Ik−/−, and IkDN+/− mice were stimulated with lipopolysaccharide (LPS), a potent stimulus of the innate immune response via TLR4. Ikaros-deficient DCs responded in a comparable manner to WT DCs, as determined by the secretion of TNF-α, IL-6, IL-1β, and IL-10 (Figure 4A-D). To rule out any potential impact of culture conditions used for the generation of BMDCs, we also determined the responses of freshly isolated splenic DCs from the WT, Ik−/−, and IkDN+/− animals to LPS stimulation. Consistent with BMDC responses, the deficiency of Ikaros did not alter the responses of splenic DCs to LPS stimulation (supplemental Figure 2A-C). These data demonstrate that intrinsic Ikaros deficiency did not alter conventional DC responses that are mediated through TLR-4 stimulation.
LPS stimulation of DCs. BMDCs were harvested and stimulated through TLR-4 with LPS (1 μg/mL) or diluent control for 16 hours. Supernatants were analyzed for TNF-α (A), IL-6 (B), IL-1β (C), and IL-10 (D) with ELISA. **P < .01, ***P < .001.
LPS stimulation of DCs. BMDCs were harvested and stimulated through TLR-4 with LPS (1 μg/mL) or diluent control for 16 hours. Supernatants were analyzed for TNF-α (A), IL-6 (B), IL-1β (C), and IL-10 (D) with ELISA. **P < .01, ***P < .001.
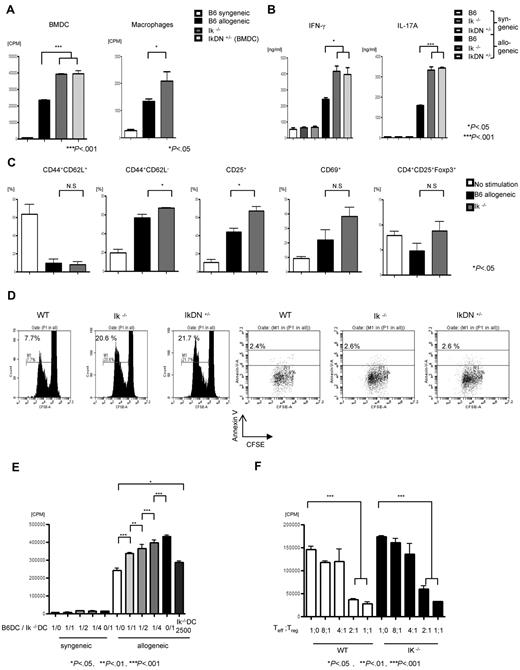
We next determined the effects of Ikaros deficiency in conventional DCs on alloresponses in an MLR assay. As shown in Figure 5A, deficiency of Ikaros in DCs significantly augmented allogeneic T-cell expansion. To determine whether the absence of Ikaros only modulated the function of CD11c+ DCs or that of other APC subsets as well, we also examined the ability of peritoneally derived F4/80+ macrophages to stimulate allogeneic T cells. Macrophages derived from Ik−/− animals stimulated greater proliferation of allogeneic T cells compared with those from WT B6 animals (Figure 5A). IFN-γ and IL-17 secretion in culture supernatants collected at 48 hours from the MLR was similarly increased (Figure 5B). Similar increases in the expansion of allogeneic T cells were also observed in the absence of Ikaros in conventional DCs when freshly isolated from the spleens of WT, Ik−/−, and IkDN+/− animals and used as stimulators in MLR (supplemental Figure 3).
Ikaros-deficient DCs show enhanced stimulation of allogeneic T cells. (A) BMDCs and peritoneal macrophages from WT (n = 3 mice/group), Ik−/− (n = 3 mice/group) and IkDN+/− B6 (n = 3 mice/group) animals were used as stimulators in an MLR with T cells from either BALB/c (allogeneic) or C57BL/6 (syngeneic) mice and analyzed for T-cell proliferation with 3H-thymidine incorporation at 96 hours. Data are means ± SEM of quadruplicate cultures. Results are from 1 of 3 similar experiments with the DCs and 1 of 4 for the macrophages. (B) Supernatants from the cultures were collected at 72 hours and analyzed for IFN-γ and IL-17A by ELISA. Data are means ± SEM of quadruplicate cultures. Results are from 1 of 3 similar experiments. (C) Analysis of T-cell phenotype after cultures with WT or Ik−/− DCs. CD4+ T cells were harvested after 96-hour culture with DCs and analyzed for expression of CD44, CD62L, CD25, CD69, and Foxp3 on CD4+ cells. Data are means ± SEM of quadruplicate cultures. Results are from 1 of 2 similar experiments. (D) CFSE-labeled splenic CD90+ T cells from BALB/c mice were cultured for 96 hours with B6 BMDCs from WT, Ik−/−, or IkDN+/− animals, harvested, and analyzed for CFSE and Annexin V on cells gated for CD3+. Data shown are representative of 1 of 3 similar experiments. (E) Ik−/− with WT BMDCs were mixed at the indicated ratios and used as stimulators in an MLR against BALB/c T cells. Shown is T-cell proliferation with 3H-thymidine incorporation at 96 hours. Data are means ± SEM of triplicate cultures. Results are from 1 of 2 similar experiments. (F) Treg suppression assay. BALB/c CD4+CD25− T cells were cultured with either WT or Ik−/− BMDCs along with BALB/c CD4+CD25+ Tregs at the indicated ratios. Proliferation was assessed after 5-day cultures with 3H-thymidine for the last 16 hours. Data are means ± SEM of quadruplicate cultures. Results are from 1 of 2 similar experiments.
Ikaros-deficient DCs show enhanced stimulation of allogeneic T cells. (A) BMDCs and peritoneal macrophages from WT (n = 3 mice/group), Ik−/− (n = 3 mice/group) and IkDN+/− B6 (n = 3 mice/group) animals were used as stimulators in an MLR with T cells from either BALB/c (allogeneic) or C57BL/6 (syngeneic) mice and analyzed for T-cell proliferation with 3H-thymidine incorporation at 96 hours. Data are means ± SEM of quadruplicate cultures. Results are from 1 of 3 similar experiments with the DCs and 1 of 4 for the macrophages. (B) Supernatants from the cultures were collected at 72 hours and analyzed for IFN-γ and IL-17A by ELISA. Data are means ± SEM of quadruplicate cultures. Results are from 1 of 3 similar experiments. (C) Analysis of T-cell phenotype after cultures with WT or Ik−/− DCs. CD4+ T cells were harvested after 96-hour culture with DCs and analyzed for expression of CD44, CD62L, CD25, CD69, and Foxp3 on CD4+ cells. Data are means ± SEM of quadruplicate cultures. Results are from 1 of 2 similar experiments. (D) CFSE-labeled splenic CD90+ T cells from BALB/c mice were cultured for 96 hours with B6 BMDCs from WT, Ik−/−, or IkDN+/− animals, harvested, and analyzed for CFSE and Annexin V on cells gated for CD3+. Data shown are representative of 1 of 3 similar experiments. (E) Ik−/− with WT BMDCs were mixed at the indicated ratios and used as stimulators in an MLR against BALB/c T cells. Shown is T-cell proliferation with 3H-thymidine incorporation at 96 hours. Data are means ± SEM of triplicate cultures. Results are from 1 of 2 similar experiments. (F) Treg suppression assay. BALB/c CD4+CD25− T cells were cultured with either WT or Ik−/− BMDCs along with BALB/c CD4+CD25+ Tregs at the indicated ratios. Proliferation was assessed after 5-day cultures with 3H-thymidine for the last 16 hours. Data are means ± SEM of quadruplicate cultures. Results are from 1 of 2 similar experiments.
We next determined whether Ik−/− DCs altered the phenotype and/or function of the naive allogeneic T cells that were cocultured with them. Allogeneic CD4+ T cells stimulated with Ik−/− DCs showed no difference in the expression of CD69 or Foxp3, but demonstrated greater expression of CD25 with a decrease in CD62L expression compared with WT DCs (Figure 5C).
Furthermore, as shown in Figure 5D, deficiency of Ikaros in DCs enhanced the proliferation of the allogeneic T cells, as determined by CFSE staining, without causing a significant change in the rate of apoptosis compared with WT DCs (Figure 5D).
To further analyze the cellular mechanisms for the enhanced allogeneic T-cell stimulation capacity of the Ikaros-deficient DCs, we examined the impact of dose escalation of Ik−/− DCs in mixing studies with WT DCs. Increasing the ratio of Ik−/− DCs to WT DCs caused increasing expansion of allogeneic T cells, suggesting that this is caused by a DC-autonomous defect in Ikaros (Figure 5E).
Because Tregs are known to suppress allogeneic T-cell responses,36 we next examined whether the allogeneic T-cell expansion mediated by the Ikaros-deficient DCs was also suppressible by natural Tregs. Consistent with in vivo expansion and suppression data (Figure 1D), Tregs equivalently suppressed the proliferation of alloreactive T cells regardless of whether they were stimulated by the Ik−/− or WT DCs (Figure 5F).
Absence of Ikaros enhances Notch signaling in DCs
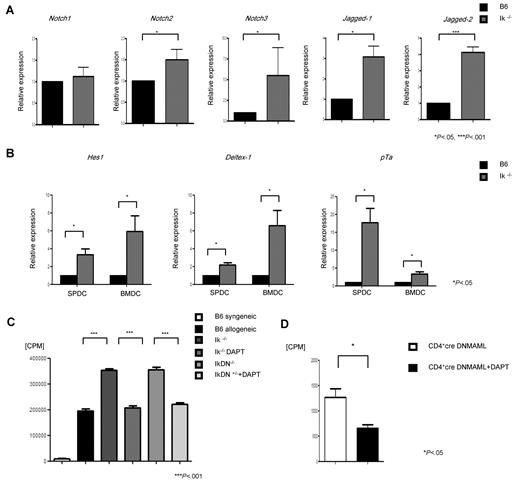
We next sought to determine the potential molecular mechanisms underpinning the regulation of DCs by Ikaros. Notch signaling is one of the major pathways modulating DC responses,18,19,37 and has been shown to be negatively regulated by Ikaros in certain contexts.23,24,26,28,29 Therefore, to elucidate the molecular mechanism of the effect of Ikaros on DC responses, we tested the hypothesis that deficiency of Ikaros in DCs enhances the Notch signaling pathway. DCs from the WT and Ik−/− animals were harvested and analyzed for their constitutive expression of Notch receptors, ligands, and the Notch target genes by Q-PCR, as described in “Expression analysis by Q-PCR.” As shown in Figure 6A, compared with WT DCs, the Ik−/− DCs demonstrated significantly higher expression of both the Notch2 and Notch3 receptors and the Jagged-1 and Jagged-2 ligands. We next confirmed the increase in Notch signaling by the enhanced expression of several Notch target genes, such as Deltex-1, Hes-1, and pTa, in Ik−/− DCs compared with WT DCs (Figure 6B).5 A similar increase in Notch target genes was also observed in the Ik DN+/− DCs (data not shown). These data show that deficiency of Ikaros in DCs is associated with a constitutive increase in the Notch signaling pathway in a cell-autonomous manner.
Ik−/− DCs show enhanced Notch signaling. (A) DCs were harvested from Ik−/− or WT B6 animals (n = 3-5 mice/group) and analyzed by Q-PCR for constitutive expression of Notch receptors (Notch1-3) and their ligands (Jagged-1 and Jagged-2). (B) Notch target genes, Hes1, Deltex1, and pTa, in BMDCs and splenic DCs. Data are from 1 of 3 different experiments. (C) DCs from B6 WT, Ik−/−, and IkDN+/− animals were pretreated with DAPT for 12 hours and then used as stimulators in an MLR with T cells from syngeneic B6 or allogeneic BALB/c animals. Proliferation was determined by 3H-thymidine incorporation at 96 hours. Data are means ± SEM of quadruplicate cultures. Results are from 1 of 3 similar experiments. (D) T cells from CD4-Cre DNMAMAL animals were used as responders with BALB/c BMDCs that were pretreated with DAPT or control. Proliferation was determined by 3H-thymidine incorporation at 96 hours. Data are means ± SEM of quadruplicate cultures. Results are from 1 of 2 similar experiments.
Ik−/− DCs show enhanced Notch signaling. (A) DCs were harvested from Ik−/− or WT B6 animals (n = 3-5 mice/group) and analyzed by Q-PCR for constitutive expression of Notch receptors (Notch1-3) and their ligands (Jagged-1 and Jagged-2). (B) Notch target genes, Hes1, Deltex1, and pTa, in BMDCs and splenic DCs. Data are from 1 of 3 different experiments. (C) DCs from B6 WT, Ik−/−, and IkDN+/− animals were pretreated with DAPT for 12 hours and then used as stimulators in an MLR with T cells from syngeneic B6 or allogeneic BALB/c animals. Proliferation was determined by 3H-thymidine incorporation at 96 hours. Data are means ± SEM of quadruplicate cultures. Results are from 1 of 3 similar experiments. (D) T cells from CD4-Cre DNMAMAL animals were used as responders with BALB/c BMDCs that were pretreated with DAPT or control. Proliferation was determined by 3H-thymidine incorporation at 96 hours. Data are means ± SEM of quadruplicate cultures. Results are from 1 of 2 similar experiments.
Enhanced Notch signaling is critical for Ikaros-mediated modulation of DCs
To address the functional relevance of enhanced Notch signaling in causing the increased allostimulatory effects of the DCs deficient in Ikaros, we chemically inhibited the Notch pathway by pretreating DCs from Ik−/− and IkDN+/− animals with the γ-secretase inhibitor DAPT (5uM) or the diluent control, and then used them as stimulators of allogeneic T cells in an MLR. Pretreatment with DAPT mitigated the increased expansion of allogeneic T cells compared with the control diluent–treated Ik−/− and IkDN+/− DCs (Figure 6C). DAPT treatment of Ik−/− and IkDN+/− DCs did not completely abrogate the expansion of allogeneic T cells, but instead brought it to levels comparable to those caused by the control WT DCs (Figure 6C).
It was still possible that this reduction of expansion by the γ-secretase inhibitor DAPT was not directly due to blockade of Notch signaling just in the Ikaros-deficient DCs, but may have also been due to potential effects on Notch signaling in the allogeneic T cells in the MLR. To demonstrate the direct and specific effect of Notch inhibition only in the Ik−/− DCs, we used T cells from a CD4-Cre × ROSA26DNMAML mouse as responders in an MLR with Ik−/− DCs that had been treated with either the DAPT or a diluent control. The mature CD4+ T cells from this mouse show almost no canonical Notch signaling, so the DAPT did not have any effect on these T cells.16,18 As shown in Figure 6D, compared with diluent-treated Ik−/− DCs, treatment with DAPT reduced the expansion of the DNMAML T cells caused by the Ik−/− DCs.
We next determined whether enhanced Notch signaling in Ikaros-deficient DCs is also critical for the greater in vivo allogeneic donor T-cell expansion and the resultant aggravation of GVHD. We used the same allogeneic BMT model (C3H.SW→B6), in which activation and expansion of donor T cells by host APCs are critical for the induction of acute GVHD.31 The [Ik−/− → B6Ly5.2] and the [B6 WT → B6Ly5.2] chimeras were lethally irradiated with 9 Gy and transplanted with 2 × 106 CD90+ T cells and 5 × 106 BM cells from either the syngeneic B6 or the allogeneic C3H.SW donors. The allogeneic animals also received either 5μg /kg of DAPT or the diluent control IP on day −1, +1, and +3. This schedule was chosen to minimize the toxicity of DAPT on the gastrointestinal tract38,39 and to maximize the exposure and effect of DAPT on host APCs, most of which cannot be detected in the spleen at later time points.35 Ten days after BMT, these mice were examined for body weight loss (one of the clinical signs of GVHD) and the spleens were harvested for analyses of donor T-cell expansion. As expected, the control treated allogeneic [Ik−/− → B6Ly5.2] chimeras demonstrated severe body weight loss (mean −16.6% ± 1.88%, n = 8) at day 10. In contrast, the allogeneic [Ik−/− → B6Ly5.2] chimeras treated with DAPT showed significantly diminished weight loss compared with control allogeneic [Ik−/− → B6Ly5.2] chimeras (Figure 7A). The allogeneic [B6 WT → B6Ly5.2] animals (n = 8 per group) did not demonstrate a significant difference in weight loss regardless of whether they received DAPT or the diluent control (Figure 7A).
Notch signaling blockade with a γ-secretase inhibitor mitigates T-cell expansion in chimeras with Ik−/− host APCs. WT B6 Ly5.2 animals were lethally irradiated with 11 Gy and infused with 5 × 106 BM cells and 5 × 106 splenocytes from syngeneic Ly5.1 WT B6 or Ik−/− B6 donors. Four months later, these chimeras [B6 → B6Ly5.2] or [Ik−/− → B6Ly5.2] chimeras were re-irradiated with 9 Gy and transplanted with 2 × 106 CD90+ T cells along with 5 × 106 BM cells from either syngeneic B6 (n = 4/group) or allogeneic MHC-matched, multiple miHA-mismatched C3H.sw donors (n = 8/group). They were also injected IP with the γ-secretase inhibitor DAPT (5 μg/kg) on days −1, +1, and +3 after BMT. (A) The animals were analyzed for GVHD with as shown by body weight loss at day 10. Shown are C3H.sw → [Ik−/− → B6Ly5.2] (○) versus C3H.sw → [Ik−/− → B6 Ly5.2] + DAPT (□) (n = 8 mice/group, P < .05) and C3H.sw → [B6 → B6Ly5.2] (●) versus C3H.sw → [B6 → B6 Ly5.2] + DAPT (■) (n = 8 mice/group, P = not significant). (B) Donor CD4+ and CD8+ T-cell expansion at day 10. Shown are C3H.sw → [Ik−/− → B6Ly5.2] (○) versus C3H.sw → [Ik−/− → B6 Ly5.2] + DAPT (□) (P < .05) and C3H.sw → [B6 → B6Ly5.2] (●) versus C3H.sw → [B6 → B6 Ly5.2] + DAPT (■) (n = 8 mice/group, P = not significant).
Notch signaling blockade with a γ-secretase inhibitor mitigates T-cell expansion in chimeras with Ik−/− host APCs. WT B6 Ly5.2 animals were lethally irradiated with 11 Gy and infused with 5 × 106 BM cells and 5 × 106 splenocytes from syngeneic Ly5.1 WT B6 or Ik−/− B6 donors. Four months later, these chimeras [B6 → B6Ly5.2] or [Ik−/− → B6Ly5.2] chimeras were re-irradiated with 9 Gy and transplanted with 2 × 106 CD90+ T cells along with 5 × 106 BM cells from either syngeneic B6 (n = 4/group) or allogeneic MHC-matched, multiple miHA-mismatched C3H.sw donors (n = 8/group). They were also injected IP with the γ-secretase inhibitor DAPT (5 μg/kg) on days −1, +1, and +3 after BMT. (A) The animals were analyzed for GVHD with as shown by body weight loss at day 10. Shown are C3H.sw → [Ik−/− → B6Ly5.2] (○) versus C3H.sw → [Ik−/− → B6 Ly5.2] + DAPT (□) (n = 8 mice/group, P < .05) and C3H.sw → [B6 → B6Ly5.2] (●) versus C3H.sw → [B6 → B6 Ly5.2] + DAPT (■) (n = 8 mice/group, P = not significant). (B) Donor CD4+ and CD8+ T-cell expansion at day 10. Shown are C3H.sw → [Ik−/− → B6Ly5.2] (○) versus C3H.sw → [Ik−/− → B6 Ly5.2] + DAPT (□) (P < .05) and C3H.sw → [B6 → B6Ly5.2] (●) versus C3H.sw → [B6 → B6 Ly5.2] + DAPT (■) (n = 8 mice/group, P = not significant).
Consistent with the in vitro data, allogeneic donor T-cell expansion was significantly attenuated in the allogeneic [Ik−/− → B6Ly5.2] chimeras that received DAPT compared with control animals (Figure 7B). To rule out the possibility that this may have been due to a direct effect on donor T cells or on the function of normal WT DCs by DAPT, we also treated allogeneic [B6 → B6Ly5.2] animals with or without DAPT. Notch inhibition with DAPT only modestly reduced T-cell expansion in these WT chimeras (Figure 7B). Although neither the weight loss nor the donor T-cell expansion were significantly different in the WT chimeras regardless of their treatment with DAPT or diluent, there was a trend toward blunting weight loss and T-cell expansion. These observations suggest that inhibition of Notch in cells/tissues other than host hematopoietically derived cells might also have a direct or indirect impact on GVH responses. Nonetheless, the short course of DAPT treatment did not prolong the overall long-term survival in these animals (data not shown), whereas the toxicity from prolonged administration of DAPT in the context of lethal radiation prevented the analyses of long-term Notch inhibition on overall survival in these models.17 Nonetheless, our data, in conjunction with recent observations that a deficiency of Notch signaling in donor T cells alone does not abrogate their expansion after allogeneic BMT,17 suggest that the effect observed in the Ik-deficient chimeras was at least in part due to the mitigation of excess Notch signaling observed in the Ik−/− host APCs. The data demonstrate that enhanced Notch signaling in Ik−/− host APCs is critical for regulating donor T-cell expansion and GVHD severity.
Discussion
Host DCs and other APCs are critical for the activation of alloreactive donor T cells and therefore the induction of GVHD.1,2 However, the host APC autonomous molecular mechanisms that can regulate GVHD remain poorly understood. In the present study, we showed that deficiency of Ikaros in host APCs aggravated the severity of GVHD. We addressed the role of Ikaros deficiency specifically in host APCs by making BM chimeras into WT hosts with 2 genetically distinct but complementary models: knock-out (Ik−/−) and dominant-negative (IkDN+/−) chimeras. It is possible that the BM chimeras had a few radio-resistant host hematopoietic APCs and/or that they reconstituted not just the donor hematopoietically derived APCs, but also other cells such as vascular endothelial or BM stromal cells from donor cells. However, these models have been useful and critical for analyses of the role of hematopoiesis-derived APCs in modulating GVHD and graft-vs-leukemia (GVL).1,2,21,31,32 Although a reduction in total DCs was observed, deficiency of Ikaros did not lead to an increase in their activation phenotype. Functional studies demonstrated comparable innate TLR-4 responses but a greater allostimulatory function. Ikaros deficiency was associated with constitutively enhanced Notch signaling.
The role of APC subsets and their intrinsic molecular mechanisms in the severity of GVHD remain poorly understood. Nonetheless, host DCs alone have been shown to be sufficient for the induction of GVHD in the absence of other types of APC subsets, and one recent study demonstrated a critical role for the NF-kB/Rel family member RelB in host APCs in causing GVHD.21 We have now extended those observations and demonstrated that the Ikaros-Notch axis is required in a host APC–autonomous fashion for the regulation of GVHD.
Ikaros is a critical transcription factor for the development of the lymphoid lineage,40 but the function of Ikaros in differentiated and mature DCs is not clear. We found significantly fewer conventional CD8+ and CD8−CD11c+ DCs and no pDCs in Ik−/− animals compared with WT animals, which is consistent with previous studies.12,13 Whereas it is possible that the exacerbation of GVHD by the Ik−/− host APCs might have been due to the lack of immunoregulation by the pDC subsets,13,41 a recent study demonstrated that host pDCs alone were sufficient to prime alloreactive T cells and cause GVHD, suggesting that a lack of pDCs in the absence of Ikaros does not explain the intensification of GVHD.35
In contrast to the observations by Wu et al, we also found CD8α− DC subsets in the spleens of Ik−/−animals.12 Both CD8α+ and CD8α− DCs were also found among the DCs generated from ex vivo BM cultures and after chimera generation, suggesting that deficiency of Ikaros did not significantly affect their generation and differentiation. This difference may have been because, in contrast to the study by Wu et al, in which the analyses were performed on Ik−/− in C57BL/6 × 129 mice, we analyzed the DC phenotype from Ik−/− animals that had been back-crossed into the C57BL/6 background for 8 generations. In addition, Wu et al evaluated DC subsets after enrichment of splenocytes for DCs and then gating for CD11c+ cells only among the MHC II+ cells, and we analyzed for CD11c+DC subsets in freshly harvested splenocytes by gating on all cells and without the potential artifact or loss of cells from enrichment.12 Nevertheless, our data indicate that the intensification of GVHD that occurs when host APCs are deficient in Ikaros was unlikely to be due to differences in the DC subsets compared with WT controls. Furthermore, the phenotypic analyses of the DCs did not demonstrate a pattern of expression in the costimulatory or coinhibitory molecules to consistently explain the aggravation of GVHD in the absence of Ikaros in host APCs. Our data are also consistent with the observation that the maturity of DCs as determined only by the expression of costimulatory molecules is not always correlated with enhanced immunogenicity of the DCs.42,43 Nonetheless, the exact correlation between phenotype and T-cell stimulation will need to be further clarified in future studies. Although Ik−/− DCs showed similar responses to innate TLR-4 stimulation with LPS, they consistently demonstrated significantly greater stimulation of allogeneic T cells. The greater T-cell expansion was associated with increased amounts of IFN-γ and IL-17, all of which exacerbate GVHD. This effect was observed in an in vitro mixing experiment demonstrating that the increased proliferation by Ik−/− DCs was due to an intrinsic cellular effect. Our data demonstrating increased allogeneic T-cell proliferation by DCs, macrophages, and total splenocytes suggest that the absence of Ik enhances the T-cell–stimulatory function of all hematopoietically derived professional APCs. However, our data did not directly address the impact of Ik deficiency on different APC subsets.
Consistent with previous observations in other cells, we found enhanced Notch signaling, as determined by the expression levels of several Notch-specific target genes such as Hes-1, pTa, and Deltex1, in the absence of Ikaros in DCs.23,24,28,29,44 These data suggest that even in DCs, Ikaros and Notch antagonistically regulate target genes. Blockade of Notch signaling by a γ-secretase inhibitor reduced the in vitro and in vivo allostimulatory effects of Ik−/− DCs. Furthermore, our in vivo observations, together with the recent observations that complete absence of Notch signaling in only donor T cells did not alter their in vivo proliferative response to alloantigens,17 demonstrate a cell-intrinsic functional role for enhanced Notch signaling in Ik−/− DCs. These data are consistent with previous studies demonstrating a role for Notch signaling in enhancing DC functions.19,45 Moreover, both Ik−/− and IkDN+/− DCs showed an increase in the expression of both the Notch2 and Notch3 receptors and the Jagged-1 and Jagged-2 ligands at steady state. Therefore, it is possible that the mechanism for constitutively enhanced Notch signaling in Ik−/− DCs might be a consequence of the de-repression of the expression of the some of the Notch receptor and ligands in the absence of transcriptional repression by Ikaros. Nonetheless, the specific Notch receptor-ligand interactions that are directly repressed by Ikaros are not known.20,46-48
Host hematopoietically derived APCs are also pivotal for the induction of GVL responses.31 Ikaros has been shown to play a key role in the pathogenesis of several types of leukemia.48-50 Deficiency of Ikaros is known to occur frequently in ALL49,50 and is associated with a high risk of relapse and poor outcomes.51 Although these patients are often treated with allogeneic BMT, the relapse rates are higher, suggesting a reduced GVL effect despite not having any significant reduction in GVHD. Future studies will determine whether, despite the increase in GVHD, there is a lack of similar intensification or even a reduction of GVL in the absence of Ikaros in host hematopoietically derived APCs.
In conclusion, we demonstrate for the first time to our knowledge a role for a novel molecular pathway, the Ikaros-Notch axis, in DC biology and as a critical pathway in host hematopoietically derived APCs for modulating GVH responses. Combined with recent data demonstrating a role for T-cell–intrinsic Notch in modulating GVHD,17 and because multiple pharmacologic methods are available to inhibit the Notch pathway (some of which are entering human clinical trials), these data suggest that targeting the Ikaros-Notch axis in host hematopoietically derived APCs may be a represent a novel strategy for immunotherapy against GVHD.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This study was supported by National Institutes of Health grants AI-075284 and HL-090775 (both to P.R.).
National Institutes of Health
Authorship
Contribution: T.T. designed and performed experiments, analyzed data, and wrote the manuscript; Y.S. and I.T. designed and performed experiments and analyzed data; A.F., R.E., E.N., C.M., and P.C. performed experiments; I.M. and S.W. analyzed data, contributed reagents, and helped write the manuscript; and P.R. designed experiments, analyzed data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Pavan Reddy, Department of Internal Medicine, Division of Hematology and Oncology, Blood and Marrow Transplantation Program, University of Michigan Comprehensive Cancer Center, 3312 CCGC, 1500 E Medical Center Dr, Ann Arbor, MI 48105-1942; e-mail: reddypr@med.umich.edu.

![Figure 1. Ikaros deficiency in host APCs exacerbates GVHD. (A) WT B6 Ly5.2 animals were lethally irradiated with 11 Gy and infused with 5 × 106 BM cells and 5 × 106 splenocytes from syngeneic Ly5.1 WT B6 or Ik−/− B6 donors. Four months later, these animals [B6 → B6Ly5.2] or [Ik−/− → B6Ly5.2] chimeras received 9 Gy and 2 × 106 CD90+ T cells along with 5 × 106 BM cells from either syngeneic B6 or allogeneic MHC-matched, multiple miHA-mismatched C3H.sw donors and analyzed for survival. Shown are B6 → [B6 → B6Ly5.2] (n = 7) (■), B6 → [Ik−/− → B6Ly5.2] (n = 6) (♦), C3H.sw → [B6 → B6 Ly5.2] (n = 12) (▴), and C3H.sw → [Ik−/− → B6Ly5.2] (n = 11) (●). ***P < .001 for C3H.sw → [B6 → B6Ly5.2] compared with C3H.sw → [Ik−/− → B6Ly5.2]. Data are representative of 2 independent experiments. (B) Representative hematoxylin and eosin–stained images and histopathologic score of gastrointestinal tracts (small and large bowels), liver, and skin on days +14 and +21 after allogeneic BMT. Each group includes 5-9 mice. (C) Serum IFN-γ and IL-17A levels at day 7. Each group includes 5-9 mice. (D) Donor T-effector and Treg expansion and ex vivo suppression. Donor CD8+ T-cell expansion was analyzed in splenocytes harvested on day +14 from allogeneic [B6 → Ly5.2] or [Ik−/− → B6Ly5.2] chimeras (n = 5 animals/group) as shown by the black bars versus the gray bars, respectively (P < .05). Donor CD4+CD25+Foxp3+ Treg expansion was analyzed in the spleens harvested on days +14 and +21 and in the liver harvested on day +21 from the allogeneic [B6 → Ly5.2] or [Ik−/− → B6Ly5.2] animals (n = 5 animals/group) as shown by the black bars versus the gray bars, respectively (P = not significant at both day +14 and day +21). The donor CD4+CD25+ and the CD4+CD25− T cells were isolated from the livers (pooled from 5 mice/group) harvested on day +21 from the allogeneic [B6 → Ly5.2] or [Ik−/− → B6Ly5.2] animals. The suppressive function of CD4+CD25+ T cells was analyzed by coculturing the isolated 2.5 × 103 CD4+CD25+ T cells with 104 CD4+CD25− T cells in the presence of 5 × 104 irradiated WT B6 spleen cells for 120 hours. Incorporation of 3H-thymidine (1 μCi/well) by proliferating cells was measured during the last 6 hours of culture. Data show means ± SEM. P = not significant. Data are from 1 of 2 similar experiments. (E) WT B6 Ly5.2 animals were lethally irradiated with 11 Gy and infused with 5 × 106 BM cells and 5 × 106 splenocytes from syngeneic Ly5.1 WT B6 or Ik−/− B6 donors. Four months later, [B6 → B6Ly5.2] or [Ik−/− → B6Ly5.2]) animals were irradiated with 9 Gy and transplanted with 2 × 106 CD90+ T cells along with 5 × 106 BM cells from either syngeneic B6 or allogeneic MHC-mismatched BALB/c donors and analyzed for survival. Shown are B6 → [B6 → B6Ly5.2] (n = 7) (■), BALB/c → [B6 → B6Ly5.2] (n = 10) (▴), and BALB/c → [Ik−/− → B6Ly5.2] (n = 13) (●) animals. Data are representative of 2 independent experiments. (F) WT B6Ly5.2 animals were lethally irradiated with 11 Gy and infused with 5 × 106 BM cells and 5 × 106 splenocytes from syngeneic Ly5.1 WT B6 or IkDN+/− B6 donors. Four months later, [B6 → B6Ly5.2] or [IkDN+/− → B6Ly5.2]) animals were used as recipients, irradiated with 9 Gy, and transplanted with 2 × 106 CD90+ T cells along with 5 × 106 BM cells from either syngeneic B6 or allogeneic MHC-matched, miHA-mismatched C3H.sw donors, and the animals were evaluated for survival. Shown are B6 → [B6 → B6Ly5.2] (n = 6) (■), C3H.sw → [B6 → B6 Ly5.2] (n = 9) (▴), and C3H.sw → [IkDN+/− → B6Ly5.2] (n = 11) ●. Data are representative of 2 independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/118/1/10.1182_blood-2010-12-324616/4/m_zh89991171940001.jpeg?Expires=1769890975&Signature=ews8odwlJ9kPhFfF9r2Ye~ojPH-OVabTiBvIV-l64zGORhAilt~TfiHkTXuo7LlpGZx6SR3J3G9dJZBqBVqGxtLDdmv4sr-34k~hWcTERvibQAoq18dp0vPmbSimBRNXbBVfl70FIJTPQOFmeNMdbRkASKr0OwpRYd-SGfe~BSVJU8BwYQO0wziUeEt4SdGDn16qfntYvSuhp4m~RELdKhmdSWUDygQikjWgfbanTwBhyv8W3ynb9MORLtCjHE85mK-0VHZqkHyD71V6dwZk73kSMztQCeJagIubuXfIgt3tgWyY95rbb167~dgIz~wxB~pVFkPonJ7Tf4Lad1ErwQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 3. Reconstitution of DCs, numbers, and phenotype in the [B6 → B6Ly5.2] and [Ik−/− → B6 Ly5.2] chimeras. WT B6 Ly5.2 animals were lethally irradiated with 11 Gy and infused with 5 × 106 BM cells and 5 × 106 splenocytes from syngeneic Ly5.1 WT B6 or Ik−/− B6 donors. Four months later, these animals (n = 4-5 animals/group) were killed and the splenocytes were harvested and analyzed for total splenocyte count (A), frequency and number of CD11c+ DCs (B), and frequency and absolute numbers of CD8+CD11c+ DCs and CD8−CD11c+ DCs (C). The CD8+CD11c+ and CD8−CD11c+ DCs were analyzed for expression of CD80 (D), CD86 (E), CD40 (F), CD83 (G), and B7H1 (PD-L1) (I) I-Ab. *P < .05, **P < .01.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/118/1/10.1182_blood-2010-12-324616/4/m_zh89991171940003.jpeg?Expires=1769890975&Signature=f0bCVBuxV62qyzpJ2bpSppxN0L-SNQGe8p-zY050pvDeLUdtw8RiRSeC2YZSM8CxFIqWGpDh-hii7joubdesTN-MdnyN2JYH0atnexXInQgdkxrL3Sz6g8koyCBPKOePHeur5t9VkO3~~zowQEr3ZdTfgrUQx3MFgWV2h5DUjEmFXVg-FGikR6hb7BVoYKrCAMyR4RUWakDMCDHphC22XCCLMvYOs0zTEugK74V1FNosc0wD1CSJC6zBTMiNUQuN8M0Ac9rq5ZFaJgJHGEl7h2q9ms63IPFeTk9DEXdkaS1duHOjiwKNIj9cucpf7dJUAEB2n9048MfhPK2xhUYHXQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 7. Notch signaling blockade with a γ-secretase inhibitor mitigates T-cell expansion in chimeras with Ik−/− host APCs. WT B6 Ly5.2 animals were lethally irradiated with 11 Gy and infused with 5 × 106 BM cells and 5 × 106 splenocytes from syngeneic Ly5.1 WT B6 or Ik−/− B6 donors. Four months later, these chimeras [B6 → B6Ly5.2] or [Ik−/− → B6Ly5.2] chimeras were re-irradiated with 9 Gy and transplanted with 2 × 106 CD90+ T cells along with 5 × 106 BM cells from either syngeneic B6 (n = 4/group) or allogeneic MHC-matched, multiple miHA-mismatched C3H.sw donors (n = 8/group). They were also injected IP with the γ-secretase inhibitor DAPT (5 μg/kg) on days −1, +1, and +3 after BMT. (A) The animals were analyzed for GVHD with as shown by body weight loss at day 10. Shown are C3H.sw → [Ik−/− → B6Ly5.2] (○) versus C3H.sw → [Ik−/− → B6 Ly5.2] + DAPT (□) (n = 8 mice/group, P < .05) and C3H.sw → [B6 → B6Ly5.2] (●) versus C3H.sw → [B6 → B6 Ly5.2] + DAPT (■) (n = 8 mice/group, P = not significant). (B) Donor CD4+ and CD8+ T-cell expansion at day 10. Shown are C3H.sw → [Ik−/− → B6Ly5.2] (○) versus C3H.sw → [Ik−/− → B6 Ly5.2] + DAPT (□) (P < .05) and C3H.sw → [B6 → B6Ly5.2] (●) versus C3H.sw → [B6 → B6 Ly5.2] + DAPT (■) (n = 8 mice/group, P = not significant).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/118/1/10.1182_blood-2010-12-324616/4/m_zh89991171940007.jpeg?Expires=1769890975&Signature=N9hi7mjWA6CHsCQDtLR11SXIY9fh6os9c76IZSjPKBQ2SkFH~sw4tvIyXmqClNrVcuBlPazyOnYfoE5pWgdlq6HPZE4AJS9WlwEYtIAUoQRMdntkwMXrM69bVpCYpni6U7TbxPD7pqmAynfT~sRlg~MsFkE5YF8o2ajP71ELZaie7MeItP5hNjDLEOCS1GJX2JYYiIJ~djkawHaOZQ~PX3erFLX5foionX64nSUyZ1mhA9sj047BbhGwLAF9kU0M5u5EJr6OLbJ8PmOn-QP7oCKyYH01lSQUJFNqlhhzn7UxEO7Lw~mQNorsL3-DGIhl6Ftkw2RjVI9-WBVgKhgzBQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal