Abstract
Band 3, the major anion transport protein of human erythrocytes, forms the core of a multiprotein complex in the erythrocyte membrane. Here we studied the spatiotemporal mechanisms of band 3 multiprotein complex assembly during erythropoiesis. Significant pools of intracellular band 3 and Rh-associated glycoprotein (RhAG) were found in the basophilic erythroblast. These intracellular pools decreased in the polychromatic erythroblast, whereas surface expression increased and were lowest in the orthochromatic erythroblast and reticulocytes. Protease treatment of intact cells to remove extracellular epitopes recognized by antibodies to band 3 and RhAG was used to study surface delivery kinetics and intracellular complex composition from the proerythroblast stage to the enucleated reticulocyte. Newly synthesized band 3 and protein 4.2 interact initially in the early stages of the secretory pathway and are found associated at the plasma membrane from the basophilic stage of erythropoiesis. Although we could successfully coimmunoprecipitate Rh with RhAG from plasma membrane pools at a similar stage, no intracellular interaction between these proteins was detectable. Knockdown of RhAG during early erythropoiesis was accompanied by a concomitant drop in membrane expression of Rh polypeptides. These data are consistent with assembly of major components of the band 3 macrocomplex at an early stage during erythropoiesis.
Introduction
During erythropoiesis erythroblasts mature and exhibit a progressive decrease in cell volume, an increase in hemoglobin synthesis, and a loss of intracellular organelles culminating in the extrusion of the nucleus from the cell to form an enucleated reticulocyte. The reticulocyte exits the bone marrow niche and enters the circulation where additional membrane protein and skeletal remodeling occurs to form the recognizable biconcave erythrocyte.1 While undergoing this unique process, each progenitor cell must synthesize, deliver, and assemble a variety of membrane protein complexes into the plasma membrane. A proportion of these membrane complexes become attached to the cell cytoskeleton where they contribute to the specialized structure-function properties of the erythrocyte membrane. Although a variety of studies in a range of model systems have broadly established the temporal expression of individual membrane complex components,2-9 there remains a paucity of information regarding the timing and subcellular localization at which the key interactions and associations within multiprotein membrane complexes establish. One of the highest density multiprotein complexes in the erythrocyte membrane is the band 3 macrocomplex.10,11 This complex is itself composed of 2 well-described subcomplexes, the band 3 complex and the Rh complex (for a review see van den Akker et al12 ).
The band 3 complex consists of a tetramer of band 3 associated with glycophorin A and is connected to the erythrocyte spectrin skeleton via ankyrin and protein 4.2. The absence of band 3 results in the complete secondary absence of protein 4.2,10,13,14 while severe band 3 deficiency results in a proportional reduction in protein 4.2, highlighting the dependency of protein 4.2 expression on band 3.11 We recently showed that protein 4.2 associates with band 3 at the basophilic stage of erythropoiesis and that the characteristic membrane alterations associated with protein 4.2 deficiency are evident from this point, suggesting that assembly of proteins into complexes begins at this early stage.9 The location within the cell at which this initial association occurs is unknown.
The Rh complex comprises a group of associated membrane proteins that are missing or severely reduced in erythrocytes of individuals with the Rh-null phenotype. These are the Rh polypeptides (RhCe and RhD), Rh-associated glycoprotein (RhAG), CD47, LW, and glycophorin B.15 Forming the core of this complex are the Rh polypeptides and RhAG, which were originally thought to form a tetramer16,17 but now seem more likely to be heterotrimeric.18 RhAG can transport NH3 and CO2,19-21 but the contribution of Rh polypeptides to erythrocyte function is unclear.22 The majority of reported Rh-null phenotypes are the result of mutations in RhAG,23-26 and because these erythrocytes also lack Rh polypeptides, a role for RhAG in regulating Rh expression is likely. A similar dependence was observed on expression of RhD with RhAG in K562 and human embryonic kidney (HEK) cells leading to the suggestion that the presence of RhAG posttranslationally regulates expression of Rh.27 The expression of RhAG is reported to occur before Rh during in vitro differentiation,6,7 although we recently showed in a different in vitro culture system that RhAG and Rh follow parallel expression patterns.9 Of interest, although expressed at low levels earlier during erythropoiesis, the bulk of Rh/RhAG surface expression appeared delayed relative to that of band 3, raising the possibility that these proteins may form a complex at a later stage. Establishing when and where RhAG and Rh associate during erythropoiesis and determining the stage of differentiation at which the dependency of Rh on RhAG actually arises will provide insight into complex formation in both normal and aberrant erythropoiesis.
In this study we have focused on the establishment of 2 critical associations within the band 3 macrocomplex: the interaction between band 3 and protein 4.2 and that between RhAG and Rh. We use an in vitro culture system to expand and differentiate erythroblasts alongside confocal imaging and biochemical techniques to provide a better understanding of the trafficking dynamics and interactions of band 3, protein 4.2, RhAG, and Rh throughout normal terminal differentiation. This has allowed us to dissect both the temporal and spatial organization of this developing multiprotein complex and of its key interactions as it assembles during erythropoiesis.
Methods
Erythroblast expansion and differentiation
PBMCs were isolated by density purification (ρ = 1.077; Percoll; GE Healthcare) from healthy donors with informed consent given in accordance with the Declaration of Helsinki. The use of human donor peripheral blood progenitors was approved by the Southmead Research Ethics Committee. Erythroblasts were expanded and differentiated as described previously.28 To remove free nuclei and nucleated precursors, cells were filtered after 10 days in differentiation medium, using a PALL RCM3 leukocyte filter as described by Douay and Giarratana.29
Antibodies
Monoclonal antibodies (International Blood Group Reference Laboratory) were BRIC6, BRAC66, and BRIC170 (band 3); LA1818 (RhAG); BRIC69; R6A; and BRIC207 (Rh). Rabbit polyclonal antibodies to protein 4.2, band 3, RhAG, and Rh were described previously,9-11,30 and β-actin was purchased from Santa Cruz Biotechnology Inc. Secondary antibodies used were PE-conjugated rabbit anti–mouse, rabbit anti–mouse IgG1, HRP-conjugated rabbit-anti–mouse and swine anti–rabbit (Dako), and goat anti–mouse-Alexa 488 (Invitrogen).
Staining of cytospins
Cells (5 × 105) were cytospun onto glass slides, fixed in methanol, and stained with May-Grünwald-Giemsa stains according to the manufacturer's protocol. Images were taken with a Leica DM750 microscope coupled to a Pixera Penguin 600CL camera using a 40× lens and processed using Adobe Photoshop 9.0 (Adobe Systems).
Immunofluorescence
Cells (6 × 105) were fixed in suspension in 0.5% acrolein in PBS (Sigma-Aldrich) for 5 minutes at room temperature and washed 3 times in PBS-0.1M glycine at 400g for 5 minutes before being cytospun onto coverslips coated with Cell-Tak (BD Biosciences) according to the manufacturer's instructions and permeabilized with 0.05% Triton X-100 for 5 minutes at room temperature. Cells were blocked in PBS-4% BSA for 45 minutes, incubated with primary antibodies in PBS-4% BSA for 1 hour, washed with PBS, and incubated for 1 hour with goat anti–mouse Alexa 488–conjugated (Invitrogen) secondary antibodies and 4′,6-diamidino-2-phenylindole (Invitrogen). Coverslips were washed and mounted on microscope slides using Mowiol (Calbiochem) containing 2.5% (wt/vol) Dabco antifade reagent (Sigma-Aldrich). Confocal images were taken using a Leica AOBS SP2 confocal microscope (63×/1.4 NA oil-immersion lens), with settings maintained throughout (PMT gain was set at 500) and processed using Adobe Photoshop 9.0.
Immunoprecipitations and total cell lysates
Immunoprecipitations were performed on erythrocytes (3 × 107 cells) and differentiating erythroblasts (1.5 × 107 or 3 × 107 cells). For total cell immunoprecipitations, cells were lysed, and immunoprecipitation of band 3, Rh, or RhAG was performed as described previously.9 For plasma membrane complex immunoprecipitations, antibodies recognizing extracellular epitopes (BRIC6, BRIC69, and LA1818) were incubated with intact cells at 4°C for 1 hour in the presence of PBS-4% BSA with constant mixing by rotation. Cells were washed twice in ice-cold PBS, lysed (as described previously9 ) and incubated for 1 hour at 4°C while rotating with protein G beads preblocked with 4% BSA. Beads were washed 5 times in lysis buffer, and bound material was eluted using Laemmli sample buffer at 95°C. For internal compartment immunoprecipitations, cells were treated with pronase (500 μg/mL; Sigma-Aldrich) in PBS at 37°C for 30 minutes. If required, cells were preincubated at 37°C for 3 hours in media containing 2.5 μg/mL brefeldin A (BFA; Sigma-Aldrich) or 100 ng/mL cycloheximide (CHX; Sigma-Aldrich). The presence of BFA or CHX was maintained during pronase treatment and subsequent washes. After pronase treatment, cells were washed at least 3 times (ice-cold PBS) and lysed, and immunoprecipitations were performed as described for total cell immunoprecipitations using antibodies recognizing extracellular epitopes of band 3, RhAG, and Rh. Immunoprecipitates were separated by SDS-PAGE and immunoblotted as described previously.9
Deglycosylation of immunoprecipitates
For deglycosylation reactions, 2 volumes of 2.5% NP40 (wt/vol) in 125mM sodium phosphate buffer (pH 7.5) were added to immunoprecipitates eluted in sample buffer or cell lysates. Equal aliquots of the sample were not digested or were digested with 500 U of endoglycosidase H (EndoH) or N-glycanase F (PNGase F; New England Biolabs).
Flow cytometry
Cells (3 × 105) were washed twice in PBS and then either treated or not treated with pronase (500 μg/mL) in PBS at 37°C for 30 minutes followed by 3 washes in ice-cold PBS. Cells were incubated with antibodies recognizing extracellular epitopes for 1 hour on ice, washed in ice-cold PBS, and incubated with PE-conjugated anti–mouse secondary antibodies. Fluorescent signals were measured using a FACS machine (Canto II-F60; BD Biosciences). Data were analyzed using FlowJo 7.2.5 software (TreeStar Inc). For reappearance experiments, pronase-treated cells (30 minutes, 37°C, in PBS) were incubated for 0, 30, 60, or 120 minutes at 37°C in prewarmed differentiation medium. At the indicated times, cells were taken, washed in ice-cold PBS-1% BSA and incubated with antibodies for flow cytometry as above. If required, cells were pretreated for 30 minutes with 100 ng/mL CHX or 3 hours with 2.5 μg/mL BFA, which were maintained during successive steps.
Small interfering RNA knockdown
Erythroblasts (1.5 × 106) were transfected with a 1μM pool of 3 siRNAs (sequences: GGGCAUAUUCUUUGAGUUA, GCACUAUUGUACAGGGAAU, and CCAUUUGGUUCUAUGAUUA) directed against RhAG mRNA or 1μM control nontargeting siRNA (Santa Cruz Biotechnology Inc) using the Amaxa Nucleofector System (setting X-001, end volume 100 μL; Lonza Biologics) according to the manufacturer's protocol. Transfected cells were transferred to differentiation medium.
HEK293T cell culture and transfection
HEK293T cells were cultured in DMEM supplemented with 10% FBS, 100 U/mL penicillin, and 100 μg/mL streptomycin under 5% CO2 at 37°C. Cells were transfected with 5 μg of pBUDCE4.1RhAGV5-His together with 30 μg of pBUDCE4.1-RhCemyc-His or pBUDCE4.1-RhDmyc-His or control vector by the calcium phosphate precipitation method and allowed to express for 48 hours before use for immunoprecipitations.
Results
Intracellular pools of band 3 and RhAG are observed early in erythropoiesis.
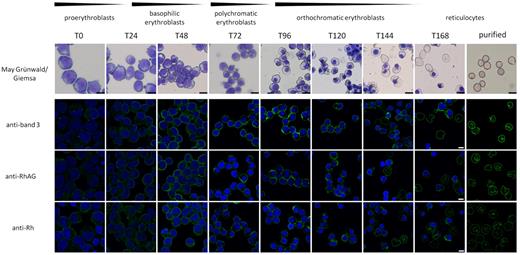
We investigated the localization of proteins of the band 3 multiprotein complex and their assembly during erythropoiesis using human erythroblasts expanded and differentiated from PBMCs according to van den Akker et al.28 Using confocal microscopy significant amounts of intracellular band 3, RhAG, and Rh were observed at the basophilic stage of erythropoiesis (T24-T72 in differentiation; Figure 1 and supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). These proteins appear at the plasma membrane within 48 hours of differentiation, and the intracellular protein pools are markedly reduced at later stages of terminal differentiation (T96-T168). This observation suggested to us the possibility that interactions within the band 3 multiprotein complex may establish initially within intracellular compartments before being delivered to the plasma membrane.
Intracellular pools of band 3, RhAG, and Rh are visible early in erythropoiesis. The top panel represents cells removed from cultures at the indicated time points during differentiation and cytospin and stained with May-Grünwald-Giemsa. Scale bar represents 10 μm. For immunofluorescence, cells were fixed in 0.5% acrolein, cytospun onto Cell-Tak–coated coverslips, and permeabilized using 0.05% Triton X-100. Cells were stained with antibodies against band 3 (BRIC170), RhAG (LA1818), and Rh (BRIC69) as indicated and visualized using goat anti–mouse-Alexa 488. Nuclei were stained with 4′,6-diamidino-2-phenylindole. Scale bar represents 5 μm. T = time in hours.
Intracellular pools of band 3, RhAG, and Rh are visible early in erythropoiesis. The top panel represents cells removed from cultures at the indicated time points during differentiation and cytospin and stained with May-Grünwald-Giemsa. Scale bar represents 10 μm. For immunofluorescence, cells were fixed in 0.5% acrolein, cytospun onto Cell-Tak–coated coverslips, and permeabilized using 0.05% Triton X-100. Cells were stained with antibodies against band 3 (BRIC170), RhAG (LA1818), and Rh (BRIC69) as indicated and visualized using goat anti–mouse-Alexa 488. Nuclei were stained with 4′,6-diamidino-2-phenylindole. Scale bar represents 5 μm. T = time in hours.
Interactions of band 3 with protein 4.2 and RhAG with Rh are protease resistant
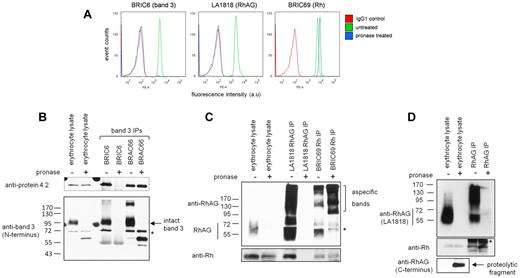
To study the subcellular localization of band 3 and RhAG complex assembly during erythropoiesis, we used proteolytic cleavage of extracellular epitopes and subsequent immunoprecipitation of intracellular protein pools. Erythrocytes were first used to evaluate the efficiency of protease epitope disruption because no intracellular reservoirs of band 3 or RhAG exist in these cells, and expression of the majority of erythroid membrane proteins is at its highest level. As expected, pronase treatment of intact erythrocytes completely removed the extracellular epitopes of specific antibodies against band 3 (BRIC6) and RhAG (LA1818) but not for Rh (BRIC69; Figure 2A), R6A, or BRIC207 (data not shown). Pronase treatment increased the fluorescence intensity with BRIC69 (Rh), probably because of increased epitope accessibility caused by removal of extracellular domains from proteins proximate to Rh (eg, the extracellular domains of RhAG). Other proteases tested (trypsin, bromelain, papain, subtilisin, or combinations of proteases) also did not result in Rh epitope destruction for the antibodies tested (data not shown).
Band 3 and RhAG extracellular epitopes are disrupted by cell-impermeable pronase treatment. (A) Flow cytometry histograms show the destruction of BRIC6 (band 3) and LA1818 (RhAG) but not BRIC69 (Rh) epitopes on treatment with 500 μg/mL pronase. a.u., arbitrary units. (B) 3 × 107 untreated erythrocytes or erythrocytes treated with 500 μg/mL pronase were lysed and used for each immunoprecipitation with anti–band 3 antibodies BRIC6 and BRAC66. Immunoprecipitates (IPs) were subjected to SDS-PAGE and Western blotting and were immunoblotted with anti–protein 4.2 and anti–band 3 N-terminal-specific antibodies sequentially without stripping. Lane 1 and 2 represent of the immunoprecipitation input. * indicates original protein 4.2 staining. (C) RhAG (LA1818) and Rh (BRIC69) immunoprecipitations from pronase-treated or untreated intact erythrocytes. Lanes 1 and 2 represent of the immunoprecipitation input. Proteins were detected with antibodies raised against the C termini of Rh and RhAG. (D) RhAG immunoprecipitation using a rabbit polyclonal antibody raised against the C terminus of RhAG. Proteins were detected with a carboxyl-terminal–specific Rh antibody or with LA1818 for RhAG. * indicates cross-reactivity with IgG. Erythrocyte lysates represent of the immunoprecipitation input.
Band 3 and RhAG extracellular epitopes are disrupted by cell-impermeable pronase treatment. (A) Flow cytometry histograms show the destruction of BRIC6 (band 3) and LA1818 (RhAG) but not BRIC69 (Rh) epitopes on treatment with 500 μg/mL pronase. a.u., arbitrary units. (B) 3 × 107 untreated erythrocytes or erythrocytes treated with 500 μg/mL pronase were lysed and used for each immunoprecipitation with anti–band 3 antibodies BRIC6 and BRAC66. Immunoprecipitates (IPs) were subjected to SDS-PAGE and Western blotting and were immunoblotted with anti–protein 4.2 and anti–band 3 N-terminal-specific antibodies sequentially without stripping. Lane 1 and 2 represent of the immunoprecipitation input. * indicates original protein 4.2 staining. (C) RhAG (LA1818) and Rh (BRIC69) immunoprecipitations from pronase-treated or untreated intact erythrocytes. Lanes 1 and 2 represent of the immunoprecipitation input. Proteins were detected with antibodies raised against the C termini of Rh and RhAG. (D) RhAG immunoprecipitation using a rabbit polyclonal antibody raised against the C terminus of RhAG. Proteins were detected with a carboxyl-terminal–specific Rh antibody or with LA1818 for RhAG. * indicates cross-reactivity with IgG. Erythrocyte lysates represent of the immunoprecipitation input.
Lysates from pronase-treated erythrocytes immunoblotted with BRIC170 (band 3, intracellular N-terminal epitope) or polyclonal anti-RhAG (carboxyl-terminal intracellular epitope) demonstrate the complete absence of full-length band 3 and RhAG and the presence of multiple cleavage products (Figure 2B-C). There was no cleavage of protein 4.2 (Figure 2B) or other intracellular proteins (protein 4.1, actin; data not shown), indicating that pronase is strictly cell impermeable. Immunoprecipitates from intact erythrocytes also confirmed the inability of BRIC6 (band 3) and LA1818 (RhAG) to bind to pronase-treated cells (Figure 2B-D). However, immunoprecipitation of band 3 using the intracellular N-terminal–specific antibody BRAC66 showed that protein 4.2 remained associated with band 3 even after cleavage of extracellular loops.
The fact that the epitope of the Rh extracellular-specific antibody BRIC69 was unaffected by pronase treatment (Figure 2C) allowed us to assess the interaction between Rh and RhAG in the absence of the intact extracellular domains of RhAG. Immunoprecipitation using BRIC69 demonstrated an interaction between Rh and the ∼ 28 kDa cleavage product of RhAG detectable with an antibody raised against the intracellular C terminus of RhAG as reported previously16 (Figure 2C-D). Likewise, Rh was also coimmunoprecipitated using this carboxyl-terminal RhAG antibody from both untreated and pronase-treated cells (Figure 2D).
Bulk of band 3 and RhAG is delivered to the plasma membrane early during differentiation
To gain a more detailed picture of band 3 and RhAG delivery to the plasma membrane during erythropoiesis, all detectable epitopes of LA1818 (RhAG) and BRIC6 (band 3) were cleared from the cell surface by pronase treatment, and the reappearance of BRIC6 and LA1818 epitopes as a measurement of band 3 and RhAG plasma membrane delivery at 37°C was monitored using flow cytometry. This approach, when used in conjunction with the protein synthesis inhibitor CHX, allows us to discriminate between de novo synthesis and delivery of already synthesized proteins from intracellular compartments to the plasma membrane at different times during erythropoiesis. Figure 3 shows that during the first 72 hours of differentiation, band 3 (A) and RhAG (B) extracellular epitopes steadily reappear, consistent with continuous delivery of both proteins to the plasma membrane. This delivery is severely attenuated at the later stages of erythropoiesis (96 and 120 hours) and was not detectable in the late erythroblast and enucleated reticulocyte stage (144-168 hours). CHX treatment of differentiating erythroblasts at these early stages before pronase recovery experiments attenuates but does not negate the reappearance of both band 3 and RhAG epitopes consistent with the existence of a significant mobile intracellular pool of band 3 and RhAG early during differentiation (Figure 3C). Thus, the gross reappearance shown in Figure 3A and B is composed both of newly synthesized proteins and of proteins trafficked from intracellular compartments and occurs mainly between 0 and 96 hours. Supplemental Figure 2 shows that 100 ng/mL CHX treatment halted all band 3 synthesis, indicating that protein synthesis is blocked.
Reappearance of BRIC6 (band 3) and LA1818 (RhAG) epitopes after pronase treatment occurs most rapidly at the early stages of erythropoiesis. (A-B) Assessment of recovery of BRIC6 (band 3; A) and LA1818 (RhAG; B) epitopes on cells pretreated with 500 μg/mL pronase for 0, 60, 120, and 180 minutes, as measured by flow cytometry (mean fluorescent intensity [a.u., arbitrary units] in differentiation medium at 37°C at indicated periods of time during differentiation [hours]). Means and SDs represent 3 experiments. (C) Cells were pretreated with 100 ng/mL CHX (□, ▵, ♢, ○) or not (■, ▴, ♦, ●) before pronase treatment. Recovery of BRIC6 (band 3) and LA1818 (RhAG) epitopes was monitored at 0, 60, 120, and 180 minutes after pronase treatment at specific time points during differentiation. Data are expressed as mean fluorescence intensity (a.u.) as a function of recovery time from 2 independent experiments.
Reappearance of BRIC6 (band 3) and LA1818 (RhAG) epitopes after pronase treatment occurs most rapidly at the early stages of erythropoiesis. (A-B) Assessment of recovery of BRIC6 (band 3; A) and LA1818 (RhAG; B) epitopes on cells pretreated with 500 μg/mL pronase for 0, 60, 120, and 180 minutes, as measured by flow cytometry (mean fluorescent intensity [a.u., arbitrary units] in differentiation medium at 37°C at indicated periods of time during differentiation [hours]). Means and SDs represent 3 experiments. (C) Cells were pretreated with 100 ng/mL CHX (□, ▵, ♢, ○) or not (■, ▴, ♦, ●) before pronase treatment. Recovery of BRIC6 (band 3) and LA1818 (RhAG) epitopes was monitored at 0, 60, 120, and 180 minutes after pronase treatment at specific time points during differentiation. Data are expressed as mean fluorescence intensity (a.u.) as a function of recovery time from 2 independent experiments.
Protein 4.2 associates with band 3 in an intracellular compartment
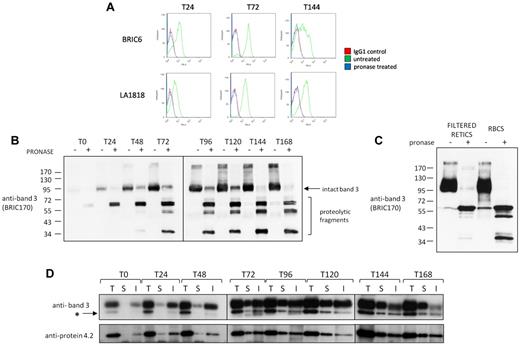
To dissect the subcellular location and timing of key band 3 and RhAG interactions during erythropoiesis we used pronase treatment in combination with immunoprecipitation. Aliquots of differentiating cells at 24-hour intervals were taken from the culture. Figure 4A confirms that pronase treatment of progenitors results in the complete removal of extracellular epitopes of BRIC6 (band 3) and LA1818 (RhAG). Total cell lysates from pronase treated or untreated cells were immunoblotted with BRIC170 and revealed cleavage of band 3 and the existence of an intact pronase-resistant pool of band 3 (Figure 4B). Taken together with the fact that all BRIC6 epitopes were removed from the plasma membrane (Figure 4A), these results lead to the conclusion that the pronase-resistant pool in Figure 4B represents intracellular band 3.
Band 3 and protein 4.2 interact initially in an intracellular compartment. (A) Example of flow cytometry histograms confirming destruction of BRIC6 (band 3) and LA1818 (RhAG) epitopes on differentiating erythroblasts at 24, 72, and 144 hours of differentiation. (B) 1 × 106 cells from indicated stages of differentiation were either treated (+) or not (−) with 500 μg/mL pronase before lysis (T = time in hours). Proteins were immunoblotted with the N-terminal intracellular epitope antibody BRIC170 (band 3). Intact uncleaved band 3 and N-terminal cleavage products are indicated. (C) Total cell lysate from equal numbers (1 × 106) of a purified reticulocyte population (RETICS) or erythrocytes (RBCS) treated or not with 500 μg/mL pronase were immunoblotted with anti–band 3 antibody BRIC170. Note the existence of a pool of internal band 3 (∼ 1% by densitometry) in pronase-treated reticulocytes that is absent in pronase-treated erythrocytes. (D) 1.5 × 107 cells at the indicated stages of differentiation were used for total (T), internal (I), or surface (S) immunoprecipitations, respectively, using the band 3 antibody BRIC6 as described in “Immunoprecipitations and total cell lysates.” Proteins were detected by immunoblotting with polyclonal antibodies raised against protein 4.2 and the C terminus of band 3 sequentially without stripping. * indicates the original protein 4.2 staining before detection of band 3.
Band 3 and protein 4.2 interact initially in an intracellular compartment. (A) Example of flow cytometry histograms confirming destruction of BRIC6 (band 3) and LA1818 (RhAG) epitopes on differentiating erythroblasts at 24, 72, and 144 hours of differentiation. (B) 1 × 106 cells from indicated stages of differentiation were either treated (+) or not (−) with 500 μg/mL pronase before lysis (T = time in hours). Proteins were immunoblotted with the N-terminal intracellular epitope antibody BRIC170 (band 3). Intact uncleaved band 3 and N-terminal cleavage products are indicated. (C) Total cell lysate from equal numbers (1 × 106) of a purified reticulocyte population (RETICS) or erythrocytes (RBCS) treated or not with 500 μg/mL pronase were immunoblotted with anti–band 3 antibody BRIC170. Note the existence of a pool of internal band 3 (∼ 1% by densitometry) in pronase-treated reticulocytes that is absent in pronase-treated erythrocytes. (D) 1.5 × 107 cells at the indicated stages of differentiation were used for total (T), internal (I), or surface (S) immunoprecipitations, respectively, using the band 3 antibody BRIC6 as described in “Immunoprecipitations and total cell lysates.” Proteins were detected by immunoblotting with polyclonal antibodies raised against protein 4.2 and the C terminus of band 3 sequentially without stripping. * indicates the original protein 4.2 staining before detection of band 3.
Densitometry analysis on BRIC170 untreated and treated lysates shows that the internal pool of band 3 in basophilic erythroblasts after 48 hours of differentiation is 32.4% ± 1.71% (n = 3). This is reduced to < 5% in late orthochromatic erythroblasts. It is notable that at the orthochromatic and reticulocyte stage of differentiation, an internal pool of band 3 remains detectable by Western blot despite the negligible reappearance of BRIC6 epitopes in our reappearance assay and absence of staining by immunofluorescence. To exclude the possibility that a minority of remaining nucleated cells were contributing to this internal pool at T168, we used pronase-treated cells matured for a further 48 hours that were passed through a leukofilter to remove any remaining nucleated cells. This more mature, purified reticulocyte population showed a reduced but still detectable internal pool of band 3 representing approximately < 1% of total band 3 (Figure 4C), indicating that a pool of internal band 3 does exist in reticulocytes but not in erythrocytes. This could be slowly incorporated during further membrane remodeling to form the biconcave erythrocyte or degraded during the maturation process via unknown mechanisms, similar to the degradation of cytosolic actin and tubulin at this stage as reported previously.31
BRIC6, which recognizes an extracellular epitope, was used to immunoprecipitate band 3 from total cell lysates, from the cell plasma membrane, and from the internal pool (ie, after treatment of cells with pronase to remove extracellular epitopes). A comparison of total cell lysates and cell surface immunoprecipitates shows that at the proerythroblast stage (T0) band 3 is undetectable at the surface, consistent with confocal imaging (Figure 1) and our previously published flow cytometry data.6,9 As differentiation progresses, the surface expression of band 3 increases, but the internal band 3 pool, which initially increases, decreases at the latest stages of differentiation (T120-T168, particularly after enucleation). Immunoblotting of the BRIC6 immunoprecipitates from total cell lysates demonstrates that protein 4.2 was coimmunoprecipitated with the intracellular pool of band 3 before the appearance of band 3 at the cell surface (Figure 4D). Protein 4.2 was found to be associated with intracellular band 3 throughout erythropoiesis. This finding demonstrates for the first time that the association between band 3 and protein 4.2 occurs in an intracellular compartment, before delivery to the membrane. Levels of coimmunoprecipitated protein 4.2 mimic band 3 levels throughout erythropoiesis at both the surface and internal pools, further confirming the dependence of protein 4.2 expression on band 3.
Band 3 and protein 4.2 interact in the early Golgi or endoplasmic reticulum
To identify the intracellular compartment where band 3 and protein 4.2 interact, immunoprecipitations were performed on pronase-treated erythroblasts differentiated for 48 hours that had been pretreated for 3 hours with BFA (a secretory pathway inhibitor) or the protein synthesis inhibitor CHX. BFA treatment results in Golgi collapse, preventing trafficking from the endoplasmic reticulum (ER) to the Golgi and from the Golgi to the plasma membrane and is thus predicted to result in increased ER-localized band 3. As expected, a slight increase in band 3 (6.62 ± 1.1%, n = 3) is observed because of the BFA block in anterograde trafficking (compare lanes 2 and 3; Figure 5A), which was more pronounced in the immunoprecipitations (compare lanes 6 and 7, 16.1% ± 4.7%, n = 3; Figure 5A). In these experiments, there was no discernible alteration in the amount of protein 4.2 coimmunoprecipitated with band 3, indicating that the initial association does not occur after exit from the Golgi (ie, between the Golgi and plasma membrane) because trafficking out of the Golgi is blocked by BFA (Figure 5A-B). Importantly, flow cytometry experiments showed that the concentration of BFA used in Figure 5A is sufficient to block trafficking of band 3 to the plasma membrane (Figure 5B) and also caused Golgi collapse in erythroblasts (supplemental Figure 3).
The pronase-resistant internal pool of band 3 in basophilic erythroblasts is EndoH sensitive. (A) 1.5 × 107 cells differentiated for 48 hours were either left untreated (lanes 1 and 5) or were treated with pronase only (lanes 2 and 6), BFA + pronase (lanes 3 and 7), or CHX + pronase (lanes 4 and 8) as described in “Deglycosylation of immunoprecipitates.” Cells were then lysed and used to immunoprecipitate band 3 using BRIC6 (lanes 5-8). Immunoprecipitated proteins were detected by immunoblotting with polyclonal antibodies raised against protein 4.2 and the C terminus of band 3 sequentially without stripping. Lanes 1-4 represent the total cell lysate of of the immunoprecipitation input. (B) Cells differentiated for 48 hours were left untreated or were pretreated with 100 ng/mL CHX or the indicated concentrations of BFA for 3 hours followed by 500 μg/mL pronase treatment. Reappearance of the BRIC6 (band 3) epitope at the cell surface was monitored at the indicated time points by flow cytometry. Data are expressed as mean fluorescence intensity (arbitrary units [a.u.]); n = 2 for each treatment. (C-D) Cells removed from culture after 48 hours of differentiation were treated or not with 500 μg/mL pronase and lysed. (C) BRIC6 was used to immunoprecipitate the internal pool of band 3. Immunoprecipitates were treated with EndoH, PNGase F, or water as a control as detailed in “Immunoprecipitates and total cell lysates,” and proteins were separated on 6% gels. Blots were stained with anti–band 3 carboxyl-terminal antibody. Note that the pronase-insensitive BRIC6 immunoprecipitated band 3 is sensitive to both EndoH and PNGase F. (D) Total cell lysates from the pronase-untreated sample were treated with EndoH, PNGase F, or water as a control and proteins were separated by SDS-PAGE. Blots were stained with anti–band 3 carboxyl-terminal antibody. Note that the majority of band 3 is sensitive to PNGase F but not to EndoH.
The pronase-resistant internal pool of band 3 in basophilic erythroblasts is EndoH sensitive. (A) 1.5 × 107 cells differentiated for 48 hours were either left untreated (lanes 1 and 5) or were treated with pronase only (lanes 2 and 6), BFA + pronase (lanes 3 and 7), or CHX + pronase (lanes 4 and 8) as described in “Deglycosylation of immunoprecipitates.” Cells were then lysed and used to immunoprecipitate band 3 using BRIC6 (lanes 5-8). Immunoprecipitated proteins were detected by immunoblotting with polyclonal antibodies raised against protein 4.2 and the C terminus of band 3 sequentially without stripping. Lanes 1-4 represent the total cell lysate of of the immunoprecipitation input. (B) Cells differentiated for 48 hours were left untreated or were pretreated with 100 ng/mL CHX or the indicated concentrations of BFA for 3 hours followed by 500 μg/mL pronase treatment. Reappearance of the BRIC6 (band 3) epitope at the cell surface was monitored at the indicated time points by flow cytometry. Data are expressed as mean fluorescence intensity (arbitrary units [a.u.]); n = 2 for each treatment. (C-D) Cells removed from culture after 48 hours of differentiation were treated or not with 500 μg/mL pronase and lysed. (C) BRIC6 was used to immunoprecipitate the internal pool of band 3. Immunoprecipitates were treated with EndoH, PNGase F, or water as a control as detailed in “Immunoprecipitates and total cell lysates,” and proteins were separated on 6% gels. Blots were stained with anti–band 3 carboxyl-terminal antibody. Note that the pronase-insensitive BRIC6 immunoprecipitated band 3 is sensitive to both EndoH and PNGase F. (D) Total cell lysates from the pronase-untreated sample were treated with EndoH, PNGase F, or water as a control and proteins were separated by SDS-PAGE. Blots were stained with anti–band 3 carboxyl-terminal antibody. Note that the majority of band 3 is sensitive to PNGase F but not to EndoH.
As shown previously, incubation with CHX prevents de novo protein synthesis of band 3 (Figure 3C) but not trafficking of band 3 through the secretory pathway to the plasma membrane and thus is predicted to result in a time-dependent decrease in the intracellular band 3 pool. Cells incubated with CHX for 3 hours and then treated with pronase showed a reduced level of protein 4.2 coimmunoprecipitating with band 3 (Figure 5A), accompanied by a concomitant drop in intracellular band 3 expression. Taken together, the experiments in Figures 4D and 5A suggest that the intracellular compartment in which newly synthesized band 3 and protein 4.2 interact is most likely to be the ER or an early Golgi compartment. This conclusion is further supported by our observation that the pronase-inaccessible intracellular band 3 pool is sensitive to EndoH and so is the high-mannose (or core glycosylated) form. This finding indicates that this pool of band 3 has not reached the medial Golgi compartment where complex glycosylation occurs (Figure 5C) unlike the majority of band 3 visible in the pronase-untreated cells, which is PNGase F sensitive but largely EndoH insensitive (Figure 5D).
Rh and RhAG interact at the plasma membrane
Experiments similar to those conducted on band 3 were performed using the RhAG extracellular antibody LA1818 to determine when and where RhAG associates with Rh polypeptides. The significant intracellular staining of RhAG at the proerythroblast and basophilic erythroblast stage observed by confocal immunofluorescence was confirmed by the presence of uncleaved RhAG detectable with LA1818 in pronase-treated erythroblasts (Figure 6A-B). As differentiation progresses, the internal pool of RhAG is depleted almost entirely, with very little intact protein detectable between 120 and 168 hours. Importantly, an increasing amount of Rh polypeptide is coimmunoprecipitated with RhAG from the plasma membrane surface as differentiation progresses (Figure 6B). There was consistently more Rh polypeptide present when RhAG was immunoprecipitated from the cell surface than from total cell lysates. This result suggests that the association between Rh and RhAG at the cell surface is more stable than that in cell lysates. This stabilization may be the result of the presence of antibodies used for immunoprecipitation. This is the first demonstration that RhAG and Rh establish their association at the plasma membrane early during erythroid terminal differentiation and that this interaction is maintained throughout the whole differentiation process as expression levels increase.
RhAG binds Rh at the plasma membrane in basophilic erythroblasts. (A) 1 × 106 cells from the indicated time points in differentiation were either treated (internal) or not (total) with 500 μg/mL pronase before lysis (T = time in hours). Proteins were immunoblotted with LA1818. Uncleaved pronase-resistant intracellular RhAG is detectable at the early stages of erythropoiesis. (B) 1.5 × 107 cells at the indicated time points in differentiation were used for total (T), internal (I), or surface (S) immunoprecipitations, respectively, using the RhAG antibody LA1818 as described in “Immunoprecipitations and total cell lysates.” Proteins were detected by immunoblotting with polyclonal antibodies raised against the C-termini of Rh and RhAG. (C) Lysate from 3 × 107 erythroblasts differentiated for 48 hours and treated or not with 500 μg/mL pronase were used for RhAG (LA1818) immunoprecipitations. Rh is coimmunoprecipitated with RhAG from total cell lysates but not from pronase-treated cell lysates (internal pool immunoprecipitate). (D) Coimmunoprecipitation of RhD (lane 4) and RhCe (lane 6) with RhAG using LA1818 (RhAG, surface immunoprecipitate [IP]; see “Immunoprecipitations and total cell lysates”) but not with the IgG1 control immunoprecipitate (lane 5) from the plasma membrane of HEK293T cells transfected with the indicated combinations of expression constructs encoding human RhD, RhCe, and RhAG. Lanes 1-3 represent of the total cell lysates used in the immunoprecipitations.
RhAG binds Rh at the plasma membrane in basophilic erythroblasts. (A) 1 × 106 cells from the indicated time points in differentiation were either treated (internal) or not (total) with 500 μg/mL pronase before lysis (T = time in hours). Proteins were immunoblotted with LA1818. Uncleaved pronase-resistant intracellular RhAG is detectable at the early stages of erythropoiesis. (B) 1.5 × 107 cells at the indicated time points in differentiation were used for total (T), internal (I), or surface (S) immunoprecipitations, respectively, using the RhAG antibody LA1818 as described in “Immunoprecipitations and total cell lysates.” Proteins were detected by immunoblotting with polyclonal antibodies raised against the C-termini of Rh and RhAG. (C) Lysate from 3 × 107 erythroblasts differentiated for 48 hours and treated or not with 500 μg/mL pronase were used for RhAG (LA1818) immunoprecipitations. Rh is coimmunoprecipitated with RhAG from total cell lysates but not from pronase-treated cell lysates (internal pool immunoprecipitate). (D) Coimmunoprecipitation of RhD (lane 4) and RhCe (lane 6) with RhAG using LA1818 (RhAG, surface immunoprecipitate [IP]; see “Immunoprecipitations and total cell lysates”) but not with the IgG1 control immunoprecipitate (lane 5) from the plasma membrane of HEK293T cells transfected with the indicated combinations of expression constructs encoding human RhD, RhCe, and RhAG. Lanes 1-3 represent of the total cell lysates used in the immunoprecipitations.
It is notable that despite our ability to efficiently immunoprecipitate intracellular RhAG in the first 0 to 72 hours of differentiation, no detectable Rh protein was coimmunoprecipitated with the intracellular pool (Figure 6B). This result could indicate that unlike the observed association of protein 4.2 and band 3 in an intracellular compartment, Rh and RhAG may first traffic to the plasma membrane independently, before association is initiated (surface immunoprecipitation; Figure 6B). However, because only low levels of Rh polypeptides were coimmunoprecipitated with RhAG from total cell lysates using LA1818 during erythropoiesis, the amount of Rh polypeptide immunoprecipitated from internal pools may be too low to detect. Doubling the number of cells used for immunoprecipitation, which significantly increased the amount of Rh coimmunoprecipitated from total cell lysates using LA1818, did not result in coimmunoprecipitation of Rh with RhAG from intracellular pools (Figure 6C). Of interest, RhAG plasma membrane surface immunoprecipitation from HEK293T cells transiently transfected with plasmids encoding RhD or RhCe together with RhAG showed that both RhD and RhCe were coimmunoprecipitated with RhAG from the plasma membrane (Figure 6D). This finding demonstrates that the interaction between Rh and RhAG at the plasma membrane is not critically dependent on other erythroid proteins (Figure 6D).
Dependence of Rh surface expression on RhAG is established early in erythropoiesis
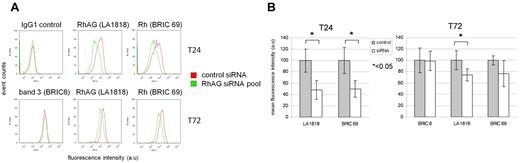
The dependence of Rh expression on RhAG expression is well documented in erythrocytes,23-26 but the point at which this dependency is established during erythropoiesis has never been addressed. We demonstrate here that RhAG is delivered to the cell surface predominantly during the first 72 hours of differentiation and becomes associated with Rh polypeptides as judged by coimmunoprecipitation at this early stage. To investigate whether Rh expression is dependent on the presence of RhAG from the onset of erythropoiesis, RhAG was depleted in erythroblasts using siRNA. Transient transfection with a pool of 3 siRNAs against RhAG resulted in confirmed depletion of RhAG by Western blot (supplemental Figure 4) and a ∼ 55% decrease in RhAG at the cell surface after 24 hours, as assessed by flow cytometry, which was ∼ 30% after 72 hours (Figure 7A-B). Surface staining with anti-Rh antibody BRIC69 showed a similar reduction in Rh surface levels, which was evident at the earliest basophilic stage. Band 3 surface levels at 72 hours were unaffected by knockdown of RhAG (Figure 7A-B), demonstrating both that band 3 surface expression is unaffected by a 50% reduction in RhAG during erythropoiesis and that this level of knockdown of RhAG causes no delay in erythroid differentiation. These data show for the first time that the dependency of Rh on RhAG is established early during erythropoiesis at a point where these 2 proteins associate in the plasma membrane.
Rh dependency on RhAG is established early in erythropoiesis. (A) Flow cytometry histograms showing knockdown of RhAG and effect on band 3 and Rh. As described in “Small interfering RNA knockdown,” 1.5 × 106 expanding erythroblasts were transfected with control siRNA or pooled siRNAs directed against RhAG. Cells were reseeded in differentiation medium RhAG (LA1818), and Rh (BRIC69) surface expression levels were monitored after 24 and 72 hours of differentiation and band 3 after 72 hours. (B) Quantification of RhAG and Rh knockdown at 24 and 72 hours in differentiation as shown in panel A, normalized against cells transfected with nontargeting siRNA. ★ indicates statistical significance (Student t test).
Rh dependency on RhAG is established early in erythropoiesis. (A) Flow cytometry histograms showing knockdown of RhAG and effect on band 3 and Rh. As described in “Small interfering RNA knockdown,” 1.5 × 106 expanding erythroblasts were transfected with control siRNA or pooled siRNAs directed against RhAG. Cells were reseeded in differentiation medium RhAG (LA1818), and Rh (BRIC69) surface expression levels were monitored after 24 and 72 hours of differentiation and band 3 after 72 hours. (B) Quantification of RhAG and Rh knockdown at 24 and 72 hours in differentiation as shown in panel A, normalized against cells transfected with nontargeting siRNA. ★ indicates statistical significance (Student t test).
Discussion
In this study we have used confocal imaging, flow cytometry, and biochemical techniques to define the dynamics of localization and associations of several key erythroid proteins throughout terminal erythroid differentiation. We have previously shown, using our in vitro culture system, that expression of erythroid-specific proteins increases dramatically during the initial stages of differentiation.9 We now show that this flood of newly synthesized protein results in a considerable intracellular pool of band 3 and RhAG at the early basophilic and polychromatic stages of erythropoiesis, which is depleted by the orthochromatic and reticulocyte stage. Our results suggest that an expression window exists within the first 0-72 hours of differentiation during which the bulk of band 3 and RhAG is synthesized and rapidly delivered to the plasma membrane. Delivery to the plasma membrane was attenuated at the orthochromatic stage and was negligible immediately before and after enucleation within the recovery period studied, suggesting that trafficking of band 3 and RhAG to the surface at later stages of differentiation occurs relatively slowly, hence the weak delivery within the 3-hour time frame. This fits with published results32 and our unpublished data (T.J.S., November 2009), suggesting that the secretory pathway is lost during these later stages of erythropoiesis, leaving only the endocytic pathways to explain any alterations in protein delivery. It seems likely that because the most active delivery of RhAG occurs during the first 72 hours, the increased Rh and RhAG surface expression we previously observed by flow cytometry during the later stages of differentiation9 reflects an increased epitope availability of Rh and RhAG, possibly because of increased stability of these proteins on incorporation into existing membrane protein complexes, further consolidation of membrane complexes, or perhaps a progressive increase in cytoskeletal attachment.33
Importantly, while proteins are being delivered to the plasma membrane, the assembly of specific plasma membrane–protein complexes composed of peripheral (cytosolic) proteins and membrane proteins is also occurring. Alongside our previous observation that band 3 and protein 4.2 associate early in erythroblast differentiation in parallel with their expression,9 our BFA and CHX experiments now show for the first time that protein 4.2 associates with band 3 initially in the ER or early Golgi compartment before delivery to the plasma membrane. This work now raises the possibility that in human erythroblasts a core complex of “band 3-protein 4.2–ankyrin” may be assembled in the ER before moving to the plasma membrane as a complex, because Gomez and Morgans34 used metabolic labeling to report that band 3 interacts with ankyrin in the ER or the first Golgi compartment in the mouse MEL cell line. However, metabolic labeling studies used in chick erythroblasts suggest that ankyrin binding to band 3 occurs after recycling to the Golgi apparatus from the plasma membrane.35 Unfortunately, we were unable to specifically coimmunoprecipitate ankyrin with band 3–protein 4.2 from human progenitors during terminal differentiation using the immunoprecipitation conditions and antibodies available to us (T.J.S., unpublished observations, October 2009).
We showed for the first time that an association between Rh and RhAG is detectable at the plasma membrane during erythropoiesis, coincident with the appearance of Rh and RhAG on the cell surface during erythroblast differentiation. The ability to coimmunoprecipitate Rh with RhAG at the plasma membrane increased proportionally with the expression levels of both proteins and the amount of these proteins present at the cell surface. Interestingly, unlike for band 3 and protein 4.2, we were unable to detect an intracellular association of Rh with RhAG. We observed that a plasma membrane immunoprecipitation (in which an extracellular epitope–specific antibody is incubated first with intact cells before lysis) was more efficient than an immunoprecipitation conducted after cells had been lysed. Although our data suggest that the interaction is established at the plasma membrane, we cannot exclude the possibility of a weak intracellular interaction existing below the level of detection or that is disrupted on lysis. Interestingly, the HEK293T cell experiments demonstrate that at least within the plasma membrane, Rh and RhAG can interact in a nonerythroid environment. This is the first report of an observable interaction between these proteins outside erythrocytes and indicates that the 2 proteins probably interact directly.
The total absence of Rh polypeptides in erythrocytes from patients with Rh-null syndrome of the regulator type (RhAG deficiency23-26 ) and in RhAG knockout mice36 shows the dependency of expression of Rh on RhAG. It has also been suggested that the stability of RhD is posttranslationally regulated by RhAG.27 In mice Rh appears to exist as part of the junctional complex, based on the observation that in protein 4.1 knockout mice, Rh but not RhAG is reduced, suggesting that Rh expression is not solely dependent on RhAG.37 The RhAG knockdown experiments we conducted show that the dependency of Rh on RhAG becomes established early in erythropoiesis at the basophilic stage, consistent with the observed interaction at the plasma membrane between these 2 proteins at this stage of differentiation. Therefore, we speculate that the deficiency in Rh induced by RhAG mutations in Rh-null patients probably arises during the early stages of erythropoiesis. This hypothesis needs to be addressed through expansion and differentiation of erythroblasts from an Rh-null patient in the future.
The observations made here are important for our general understanding of both erythrocyte membrane biogenesis and the pathophysiology behind hemolytic anemias. The numerous transitions that a proerythroblast must undergo during terminal differentiation to form a nascent reticulocyte and then the final biconcave erythrocyte mean that there are multiple stages at which the absence of a protein because of a specific genetic mutation can result in the secondary loss of other proteins within a particular protein complex, leading to specific membrane protein deficiencies observed in hereditary elliptocytosis and hereditary spherocytosis. Because key membrane protein associations and dependencies are beginning to be established from the onset of erythropoiesis, it is likely that characteristic secondary changes observed in patients (eg, band 3 deficiency, protein 4.2–null, and Rh-null) will also begin to manifest at these early stages. The fact that we recently observed characteristic changes evident in protein 4.2–null erythrocytes occurring early during erythropoiesis further supports this idea.9 Because further remodeling and connection to the cytoskeleton occurs at later stages, both during and after enucleation, missorting of proteins during enucleation or remodeling processes remains a further point at which these and other proteins can potentially be lost in such diseases.38
In summary, we have generated sufficient numbers of erythroblasts to begin to study the cellular localization of erythroid proteins during terminal differentiation and also determine at what stage specific protein-protein interactions and dependencies become established in erythropoiesis. Our data support a model of protein complex assembly during erythropoiesis in which the critical interactions that form the core of multiprotein complexes and the associated protein dependencies they impart begin at an early stage when the rate of synthesis and delivery to the plasma membrane are greatest. The presence of residual intracellular pools of proteins in late-stage enucleated reticulocytes but not erythrocytes suggests that further membrane remodeling and cytoskeletal binding may still be occurring at this penultimate stage.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Rosey Mushens for monoclonal BRIC/BRAC antibodies and Dr Marieke von Lindern for cell culture reagents.
This work was supported by a NHSBT Wellcome Trust Fellowship to A.M.T., a BBSRC DTA NHSBT Case Studentship for T.J.S. (A.M.T.), a Wellcome Trust PhD studentship for A.J.B., and NHSBT project grants for E.v.d.A. and S.P. (G.D.).
Authorship
Contribution: T.J.S. conceived and designed experiments, performed the majority of the experiments, and wrote and edited the manuscript; A.J.B. and S.P. produced the images in Figure 1; S.K. leukofiltered reticulocytes; K.R. provided Rh and RhAG mammalian expression vectors and edited the manuscript; G.D. and D.J.A. wrote and edited the manuscript; E.v.d.A. conceived and designed experiments, performed experiments, and wrote and edited the manuscript; and A.M.T. was the principal investigator, conceived and designed experiments, and wrote and edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Ashley Toye, PhD, School of Biochemistry, Medical Sciences Building, University Walk, Bristol, BS8 1TD, United Kingdom; e-mail: ash.m.toye@bristol.ac.uk.
References
Author notes
E.v.d.A. and A.M.T. contributed equally to this work.

![Figure 3. Reappearance of BRIC6 (band 3) and LA1818 (RhAG) epitopes after pronase treatment occurs most rapidly at the early stages of erythropoiesis. (A-B) Assessment of recovery of BRIC6 (band 3; A) and LA1818 (RhAG; B) epitopes on cells pretreated with 500 μg/mL pronase for 0, 60, 120, and 180 minutes, as measured by flow cytometry (mean fluorescent intensity [a.u., arbitrary units] in differentiation medium at 37°C at indicated periods of time during differentiation [hours]). Means and SDs represent 3 experiments. (C) Cells were pretreated with 100 ng/mL CHX (□, ▵, ♢, ○) or not (■, ▴, ♦, ●) before pronase treatment. Recovery of BRIC6 (band 3) and LA1818 (RhAG) epitopes was monitored at 0, 60, 120, and 180 minutes after pronase treatment at specific time points during differentiation. Data are expressed as mean fluorescence intensity (a.u.) as a function of recovery time from 2 independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/118/1/10.1182_blood-2010-10-314187/4/m_zh89991173180003.jpeg?Expires=1769186637&Signature=Qay0UTRB79OPFqVE37T2ICqU5FA~oySs8IHBSgVdhVpxqmG7Yjf8lvGFeIkuDRMwVw~ME5FQlf1nnJ21x8aqLtAaSaSYT5Js7s1u~ezJ0yqKhZjg0~IESvgFCDCtbdLHwB7wyRuZUnWYadZDb3FVUHLoO1O-C7BK8411bHjFi8NoPk7E4ungFEAaN4cTov0GPw0p2L0KGVBoEUIVt5uwn2inGIQu7aqMLHFI-SH9zMKu~i6n6jTVkW79XJtWERWzvQ1-k6iQgilKXd4r3NmiWUKM7Iwh9SLg~xWExXU2eULq1I7hV6IdvdH6LUxAgAHvznSC~qM2OTwv~eXBXQTXwQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 5. The pronase-resistant internal pool of band 3 in basophilic erythroblasts is EndoH sensitive. (A) 1.5 × 107 cells differentiated for 48 hours were either left untreated (lanes 1 and 5) or were treated with pronase only (lanes 2 and 6), BFA + pronase (lanes 3 and 7), or CHX + pronase (lanes 4 and 8) as described in “Deglycosylation of immunoprecipitates.” Cells were then lysed and used to immunoprecipitate band 3 using BRIC6 (lanes 5-8). Immunoprecipitated proteins were detected by immunoblotting with polyclonal antibodies raised against protein 4.2 and the C terminus of band 3 sequentially without stripping. Lanes 1-4 represent the total cell lysate of 115 of the immunoprecipitation input. (B) Cells differentiated for 48 hours were left untreated or were pretreated with 100 ng/mL CHX or the indicated concentrations of BFA for 3 hours followed by 500 μg/mL pronase treatment. Reappearance of the BRIC6 (band 3) epitope at the cell surface was monitored at the indicated time points by flow cytometry. Data are expressed as mean fluorescence intensity (arbitrary units [a.u.]); n = 2 for each treatment. (C-D) Cells removed from culture after 48 hours of differentiation were treated or not with 500 μg/mL pronase and lysed. (C) BRIC6 was used to immunoprecipitate the internal pool of band 3. Immunoprecipitates were treated with EndoH, PNGase F, or water as a control as detailed in “Immunoprecipitates and total cell lysates,” and proteins were separated on 6% gels. Blots were stained with anti–band 3 carboxyl-terminal antibody. Note that the pronase-insensitive BRIC6 immunoprecipitated band 3 is sensitive to both EndoH and PNGase F. (D) Total cell lysates from the pronase-untreated sample were treated with EndoH, PNGase F, or water as a control and proteins were separated by SDS-PAGE. Blots were stained with anti–band 3 carboxyl-terminal antibody. Note that the majority of band 3 is sensitive to PNGase F but not to EndoH.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/118/1/10.1182_blood-2010-10-314187/4/m_zh89991173180005.jpeg?Expires=1769186637&Signature=hprlW-ysgb3p-ZzjEO7AoyKHRxIxSHHK9FQgXLoRREYm~~ZGkF0SaFleROqg64eDpadTVF2rTVnaRvUW470-b6iely2mClwF~W5Ht2riUWgiNx~~ng-QZ10qjsYIiNyIWhKTkadMEFyGMtujtRCyrHRemCOe40m~ClZQpbhWFEKp0CgjecJazKbCIbNS7IAkXZ9AWux5buoVL50JlMGlkzRBED9Ax05FZvapB-IfKeqKfuIKIeG5R2Rhc-PygYtEKs5xHq~CRd9pfeH23~NbmGMKrHG3vu0a-L-aEVBUWqmaRwVt2ziVvmBmc83NVCxFiO0eeBSPSEDiJOhBW6uOmw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 6. RhAG binds Rh at the plasma membrane in basophilic erythroblasts. (A) 1 × 106 cells from the indicated time points in differentiation were either treated (internal) or not (total) with 500 μg/mL pronase before lysis (T = time in hours). Proteins were immunoblotted with LA1818. Uncleaved pronase-resistant intracellular RhAG is detectable at the early stages of erythropoiesis. (B) 1.5 × 107 cells at the indicated time points in differentiation were used for total (T), internal (I), or surface (S) immunoprecipitations, respectively, using the RhAG antibody LA1818 as described in “Immunoprecipitations and total cell lysates.” Proteins were detected by immunoblotting with polyclonal antibodies raised against the C-termini of Rh and RhAG. (C) Lysate from 3 × 107 erythroblasts differentiated for 48 hours and treated or not with 500 μg/mL pronase were used for RhAG (LA1818) immunoprecipitations. Rh is coimmunoprecipitated with RhAG from total cell lysates but not from pronase-treated cell lysates (internal pool immunoprecipitate). (D) Coimmunoprecipitation of RhD (lane 4) and RhCe (lane 6) with RhAG using LA1818 (RhAG, surface immunoprecipitate [IP]; see “Immunoprecipitations and total cell lysates”) but not with the IgG1 control immunoprecipitate (lane 5) from the plasma membrane of HEK293T cells transfected with the indicated combinations of expression constructs encoding human RhD, RhCe, and RhAG. Lanes 1-3 represent 130 of the total cell lysates used in the immunoprecipitations.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/118/1/10.1182_blood-2010-10-314187/4/m_zh89991173180006.jpeg?Expires=1769186637&Signature=ghXRyFUyPb06h9GPiRuvgbw059yQIcM239a0fER5~CMzrVAgTVmZnkpoHWEokG05WQ8X2JutNqfPTJ9iAYXqCDiW5ppVTYtCVHe43H-RPEOAPFZWzQcQyqVj3rR5fXcGSvlqG5IHXgyWi-8PPDeL2C9aZ~Ot9xBevlIgAl1oCQKmt-PB8RnIdU3Rv9dFw-CG~neu6chosU1Cuwl0ngsJf5~8Bw3CtM0S80vk4QvzQRYpXvHx2gMvWtIpoDKlOoScFyrF9VQb9cK0UeqYeNDHjrmbLfQb8nlFUDv2JArTMN0p3iNYnljc4W2Uhx6Vu7VhAp9Q8YBGFo5petyl~i1KRA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal