Abstract
Natural killer (NK) cells are key members of the innate immune system. In a self-environment, they sense and kill target cells lacking major histocompatibility complex class I molecules and release various cytokines on activation. The discovery of human leukocyte antigen (HLA) class I specific inhibitory receptors (including the allotype-specific killer immunoglobulin-like receptors), and of various activating receptors and their ligands, provided the basis for understanding the molecular mechanism of NK-cell activation and function, mainly resulting from the balance between activating and inhibitory signals. In an allogeneic setting, such as T cell–depleted haploidentical hematopoietic stem cell transplantation, NK cells may express inhibitory killer immunoglobulin-like receptors that are not engaged by any of the HLA class I alleles present on allogeneic cells. Such “alloreactive” NK cells greatly contribute both to eradication of leukemia blasts escaping the preparative regimen and to clearance of residual host dendritic cells and T lymphocytes (thus preventing graft-versus-host disease and graft rejection, respectively). Improved prevention of graft-versus-host disease might be achieved by redirecting to lymph nodes adoptively transferred, alloreactive NK cells by inducing CCR7-uptake in vitro. Recent studies suggested that, after immune-suppressive therapy, alloreactive NK cells from an HLA-haploidentical donor may prevent leukemia recurrence also in patients who have not received allogeneic hematopoietic stem cell transplantation.
Introduction
Natural killer (NK) cells represent an important component of the innate immunity. They are regulated by several receptors that finely tune potent effector functions, including cytolytic activity against different target cells and release of cytokines that play a major role in inflammation and immunoregulation.1-6
A group of inhibitory receptors interact specifically with major histocompatibility (MHC) class I molecules.7-9 These receptors prevent NK cell–mediated attack against normal (ie, MHC class I+) autologous cells. On the other hand, cells in which MHC class I expression is compromised (eg, by tumor transformation or viral infection) become susceptible to NK-mediated killing. In humans, there are 2 main types of inhibitory receptors for human leukocyte antigen (HLA) class I molecules: (1) killer immunoglobulin (Ig)–like receptors (KIRs) that belong to the Ig superfamily and are specific for determinants shared by groups of HLA-A, -B, or -C allotypes, and (2) CD94/NKG2A, a heterodimer related to C-type lectins that recognizes HLA-E, an HLA class Ib molecule.7-11 In addition, NK cells are equipped with various triggering receptors responsible for NK-cell activation in the process of natural cytotoxicity.12,13 An important role in tumor cell killing is exerted by NKp46,14,15 NKp30,16 and NKp44,17,18 a group of activating receptors (collectively referred to as natural cytotoxicity receptors) that are mostly restricted to NK cells. In particular, NKp46 is present in NK cells both in humans and in mice and represents the most reliable marker for NK-cell identification.14,19 Recent reports have also identified NKp46 expression on murine lymphoid-tissue inducer-like cells producing interleukin-22 in the gut and tonsils.20 The cellular ligands recognized by these receptors are still elusive, with the exception of B7-H6, a ligand for NKp30.21 Another receptor that plays a major role in NK cell–mediated recognition and killing of some tumors is NKG2D, a type II membrane protein characterized by a lectin-like domain.22 NKG2D recognizes the stress-inducible MICA/B or ULBP proteins.23,24 Other activating receptors include 2B425-27 (specific for CD48),28 NTB-A29 (mediating homotypic interactions),30 NKp8031 (specific for AICL1),32 and DNAM-133 (specific for poliovirus receptor, CD155, and Nectin-2, CD112)34 also involved in cell-to-cell adhesion and in leukocyte extravasation).35 Noteworthy, poliovirus receptor and Nectin-2 are frequently overexpressed on tumor cells and leukemia blasts.34 Recognition of self-ligands that are induced on viral infection, tumor transformation, and, in general, by cell stress may represent an important mechanism by which NK cells can identify and remove abnormal cells. Recent studies revealed that NK cells may exert other important functions. For example, they can sense different microbial products and release cytokines thanks to the expression of different Toll-like receptors.36 In addition, NK cells are particularly abundant in decidua37 where they produce a set of cytokines capable of inducing tissue remodeling and neoangiogenesis.38,39 Moreover, they are involved in the induction of regulatory T cells that are thought to play a major role in regulating the mother's immune response and in preventing fetus rejection.40
KIR repertoire and specificity for HLA class I alleles
That NK cells can sense allelic differences on hemopoietic target cells was first suggested by the hybrid resistance phenomenon in which NK cells could reject parental bone marrow (BM) grafts in F1 hybrid mice.41 Studies in both humans and mice allowed to clarify the general mechanisms underlying the NK-cell function and their capability of selectively killing tumor cells. In humans, 2 surface molecules expressed by subsets of NK cells that were capable of modulating NK-cell function were identified.42,43 They were shown to function as inhibitory receptors specific for distinct HLA-C alleles.44 Molecular cloning revealed novel members of the Ig superfamily characterized by 2 extracellular Ig-like domains (KIR2D) and by a cytoplasmic tail containing 2 immunoreceptor tyrosine-based inhibition motif.45,46 Three Ig-like domain KIRs (KIR3D) were also identified.47 They recognize a group of HLA-B alleles sharing the HLA-Bw4 supertypic specificity or certain HLA-A alleles.48,49 Remarkably, a novel KIR-associated function has recently been identified: KIR3DL2 can bind CpG ODN and shuttle them from the cell surface to the endosomal compartment where Toll-like receptor 9 is localized.50 This allows KIR3DL2-CpG ODN interaction, leading to NK-cell activation and cytokine production. Thus, such an unexpected finding supports the notion that KIR may directly sense also microbial products and further contribute to defense against pathogens.
Activating forms of KIRs were also identified and cloned.51-53 However, only for KIR2DS1 and KIR2DS4, the specificity for HLA class I molecules has been unequivocally documented.51,54-56 Table 1 summarizes the binding specificity of both inhibitory and activating KIRs. KIRs are clonally distributed in NK cells, and individual cells express different sets of inhibitory or activating KIRs. Notably, most (but not all) NK cells express at least one self-reacting inhibitory receptor, either a KIR or CD94/NKG2A.7 KIRs were also detected on a small subset of cytolytic T lymphocytes.57-60 These T cells display inhibitory KIR repertoires similar to those of NK cells from the same person. Because T cells are subjected to maturation constraints different from those of NK cells, this could further suggest that the expressed KIR repertoire is primarily determined by genetic rather than microenvironmental and/or selective factors. This interpretation is also supported by data obtained in vitro as well as from BM-transplanted patients in whom KIR repertoires of NK cells originating from donor hematopoietic precursors were consistently of donor type.61,62
Human KIRs
| KIR . | Identified ligand . |
|---|---|
| 2DL1 | HLA-CLys80 (C2 epitope) (eg, HLA-Cw4) |
| 2DL2* | HLA-CAsn80 (C1 epitope) (eg, HLA-Cw3) |
| 2DL3* | HLA-CAsn80 (C1 epitope) (eg, HLA-Cw3) |
| 2DL4 | HLA-G |
| 2DL5 | ? |
| 2DS1 | HLA-CLys80 (C2 epitope) |
| 2DS2 | ? |
| 2DS3 | ? |
| 2DS4 | HLA-A11 and subsets of C1+ C2+ HLA-C |
| 2DS5 | ? |
| 3DL1 | HLA-BBw4 (eg, HLA-B27), and HLA-ABw4 (eg, HLA-A32) |
| 3DL2 | HLA-A3, -A11 and CpG-ODN |
| 3DL3 | ? |
| 3DS1 | ? |
| KIR . | Identified ligand . |
|---|---|
| 2DL1 | HLA-CLys80 (C2 epitope) (eg, HLA-Cw4) |
| 2DL2* | HLA-CAsn80 (C1 epitope) (eg, HLA-Cw3) |
| 2DL3* | HLA-CAsn80 (C1 epitope) (eg, HLA-Cw3) |
| 2DL4 | HLA-G |
| 2DL5 | ? |
| 2DS1 | HLA-CLys80 (C2 epitope) |
| 2DS2 | ? |
| 2DS3 | ? |
| 2DS4 | HLA-A11 and subsets of C1+ C2+ HLA-C |
| 2DS5 | ? |
| 3DL1 | HLA-BBw4 (eg, HLA-B27), and HLA-ABw4 (eg, HLA-A32) |
| 3DL2 | HLA-A3, -A11 and CpG-ODN |
| 3DL3 | ? |
| 3DS1 | ? |
In general, KIRs with inhibitory activity are long-tailed, whereas short tails are present in the activating KIRs.
2D and 3D indicate the number of Ig-like domains; L (long) and S (short) indicate the length of the intracytoplasmic portion; and ? indicates that the ligand is still undefined.
2DL2 and 2DL3 bind also C2 alleles, although with low affinity. In addition, these 2 KIRs have been shown to recognize few HLA-B alleles that bear the C1 epitope (eg, HLA-B*4601 and HLA-B*7301).
While in an autologous setting, NK cells can kill only cells that do not express sufficient HLA class I molecules; in a non–self-environment, NK cells may kill allogeneic cells. “Alloreactive” human NK cells were originally discovered more than 20 years ago.43 Subsequently, it became evident that NK cells could kill allogeneic cells, both in vitro and in vivo, when they expressed inhibitory KIRs that did not recognize HLA class I alleles on target cells.44,63,64
Alloreactive NK cells
Because KIRs recognize allotypic determinants shared by groups of HLA class I alleles, in most instances, “alloreactive” NK cells express inhibitory KIR that are not engaged by any of the HLA class I alleles present on allogeneic target cells.64 In addition, these alloreactive NK cells should not express CD94/NKG2A+ because HLA-E molecules are present in all HLA class I+ cells.
Although the combined expression of inhibitory KIR with different HLA class I specificities and absence of CD94/NKG2A represents the main criterion for identifying alloreactive NK cells, other factors may greatly contribute to NK alloreactivity. In particular, killing of target cells may depend also on the surface density of certain activating receptors (such as natural cytotoxicity receptors) on NK cells and on the expression of their ligands on target cells.65-67 More importantly, activating KIRs (in particular KIR2DS1) were shown to play a substantial role in mediating alloreactivity.55,68 On the basis of the expression of inhibitory or activating KIR in NK cells and of the HLA class I alleles expressed on target cells (eg, leukemia blasts), it is now possible to identify different groups of alloreactive NK cells68 : (1) KIR2DL1+ NK cells. KIR2DL1 recognizes HLA-C alleles belonging to the C2 specificity (eg, Cw2, Cw4…), whereas it does not react with C1+ cells (eg, Cw1, Cw3…). Accordingly, KIR2DL1+ cells kill C1/C1 but not C2+ leukemias. (2) KIR2DL2/3+ NK cells. KIR2DL2/3 recognizes C1 alleles, whereas it can also bind with lower affinity to C2. Therefore, C1+ leukemias are resistant to KIR2DL2/3+ NK cells, whereas C2/C2 leukemias are lysed only partially. (3) KIR3DL1+ NK cells. This receptor recognizes HLA-B and HLA-A alleles carrying the Bw4 supertypic specificity. Thus, Bw4+ leukemic blasts are resistant to KIR3DL1+ NK cells, whereas Bw4− leukemias are susceptible. (4) KIR2DS1+ NK cells. KIR2DS1 activating receptor recognizes the C2 specificity. It should be mentioned that, in donors homozygous for C2, the cytolytic activity of KIR2DS1+ cells is tuned down.69 Importantly, however, in NK cells derived from C1/C2 or C1/C1 donors, activation via KIR2DS1 may overcome also the KIR2DL2/3-mediated inhibition, resulting in an efficient lysis of C2/C2 leukemias. In addition, KIR2DS1 can overcome the CD94/NKG2A-mediated inhibition, again resulting in killing of C2/C2 leukemias. Thus, the expression of KIR2DS1 may reveal NK cells endowed with alloreactivity and allow a more precise definition of the size of the alloreactive NK-cell subset.68
Haplo-HSCT
Over the past 4 decades, allogeneic hematopoietic stem cell transplantation (allo-HSCT) from an HLA-matched donor, either related or unrelated, has been increasingly used to treat patients affected by several malignant or nonmalignant disorders. Thanks to this procedure, thousands of subjects have been cured of their original disease.70 However, only 25% of patients who need an allograft have an HLA-identical sibling; and for less than 60% of the remaining patients, a suitable, HLA-compatible, unrelated volunteer can be found.71 In the absence of an HLA-matched donor, alternative donor/sources of HSCs, such as unrelated umbilical cord blood and HLA-haploidentical relatives, are being increasingly used.71-73 In particular, the majority of patients have a family member, identical for one HLA haplotype and fully mismatched for the other (ie, haploidentical), who could immediately serve as HSC donor.73,74 Thus, HSCT from an HLA-haploidentical relative (haplo-HSCT) offers an immediate transplantation treatment virtually to any patients lacking a matched donor or a suitable umbilical cord blood unit.
A milestone in the history of haplo-HSCT was the demonstration that an efficient T-cell depletion of the graft prevented both acute and chronic graft-versus-host disease (GVHD), even when using a related donor differing at the 3 major HLA loci.75 The benefits of T cell–depleted haplo-HSCT were first demonstrated in children with severe combined immunodeficiency,75 and it can now be estimated that hundreds of SCID patients have been transplanted worldwide using an HLA-haploidentical related donor, with a high rate of long-term, partial, or complete immune reconstitution.76 However, in the perspective of extending the use of haplo-HSCT to other much more common disorders, the infusion of BM cells obtained from an HLA-haploidentical relative was reported to be associated with a high incidence of graft failure in patients with acute leukemia.77,78 Indeed, because of the extensive T-cell depletion of the donor's graft, the balance between competing host and donor T cells shifted in favor of the unopposed host-versus-graft reaction.77,78 However, the use of “megadoses” of granulocyte colony-stimulating factor-mobilized peripheral blood-derived HSC was shown to overcome the barrier of HLA incompatibility in the donor/recipient pair and to elude the residual antidonor cytotoxic T-lymphocyte activity of the recipient.78,79 Indeed, in leukemia patients, the combination of high-intensity immune-suppressive/myeloablative conditioning regimens with the infusion of large numbers of highly purified peripheral blood CD34+ cells could guarantee: (1) the successful and sustained engraftment of donor hematopoiesis across the HLA barrier and (2) a very low incidence of grade II-IV acute GVHD, without the need for any posttransplantation immune suppression as prophylaxis.79-83 The importance of the cell dose infused in haplo-HSCT has recently been confirmed in children with acute lymphoblastic leukemia given a T cell–depleted, haploidentical allograft, where patients receiving numbers of CD34+ cells greater than 12 × 106 progenitors/kg have a better clinical outcome.83 However, the elimination of mature T cells from the graft leads to the consequence that recipients cannot benefit from the adoptive transfer of donor memory T lymphocytes, which are mainly responsible for protection from severe infections during the first months after transplantation. Indeed, a state of profound immune deficiency lasts in haplo-HSCT recipients for at least 4 to 6 months after transplantation.79-83 To overcome this relevant disadvantage, sophisticated strategies of adoptive infusions of T-cell lines or clones specific for the most common and life-threatening pathogens (ie, human cytomegalovirus, Epstein-Barr virus, aspergillus, and adenovirus) have been envisaged and successfully tested in a few pilot trials to protect the recipients in the early posttransplantation period.84-86
The absence of the T cell–mediated graft-versus leukemia (GVL) effect was also thought to render the recipients of a T cell–depleted allograft more susceptible to leukemia relapse.87 However, fundamental studies on haplo-HSCT showed that, in patients with acute myeloid leukemia (AML) transplanted in complete remission, a GVL effect could be mediated by donor NK cells.81,88 This NK-mediated GVL effect has been documented in patients transplanted from donors who had NK cells alloreactive toward recipient targets. These studies represented a true revolution in the field of allo-HSCT. In addition, they underlined, for the first time, that not only adaptive immunity, but also innate immunity, may be a crucial element for guaranteeing a patient's successful outcome.
Alloreactive NK cells have been first reported to positively affect the outcome of HSCT from an HLA-haploidentical relative in adults with AML and, more recently, in children with high-risk ALL.61,67,68,88 Indeed, in these patients, the probability of leukemia recurrence was particularly low, whereas the probability of leukemia-free survival was found to be at least as good as that of patients transplanted in a similar disease phase from an HLA-matched sibling or unrelated volunteer.61,68,88 Importantly, the peculiar GVL effect mediated by NK cells is completely separated by the occurrence of GVHD, this indicating that NK cells are able to kill leukemia targets while sparing normal tissues. In view of these findings, because virtually all patients in need of HSCT have a family haploidentical donor who is immediately available, a T cell–depleted haplo-HSCT has been proposed to be included in the treatment algorithm as a valuable option for patients with life-threatening hematologic disorders lacking a matched donor.71,82,83
Few studies have questioned the importance of donor NK alloreactivity in preventing leukemia relapse after T cell–depleted haplo-HSCT.89-91 In particular, 2 of these studies, one of which analyzed a very limited number of patients, have reported that, in the early posttransplantation period, donor NK cells derived by CD34+ HSCs are immature, as they are CD56bright and express high levels of NKG2A and low levels of activating receptors, all factors being associated with impaired functioning.89,90 Indeed, in our haplo-HSCT setting in pediatric patients, KIR+ (alloreactive) NK cells usually appear within 8 weeks, in agreement with data of the Perugia group.61 It is conceivable that the reported delay in the appearance of functionally effective NK cells could be responsible for the lack of a clinically relevant leukemia control, particularly in patients with a high leukemia burden at time of the allograft or transplanted in an unstable state of remission. Moreover, it cannot be excluded that the delay (or lack) of appearance of KIR+ alloreactive NK cells or their hypofunctional status may reflect significant differences in the conditioning regimen and/or be attributed to T-cell add-backs given to most patients in one of such studies.90 Another study suggested that the potency of the antileukemia effects increased with an increasing number of receptor-ligand mismatch pairs and that the accuracy of the prediction of relapse could be improved by a model taking into consideration the presence of inhibitory KIRs on the donor's NK cells and the absence of corresponding KIR ligand in the recipient's HLA repertoire (a receptor-ligand model) rather than being based on the ligand-ligand mismatch model discussed so far.91 This hypothesis, however, has not been further confirmed in studies conducted in centers with active program on haplo-HSCT in patients with acute leukemia.
Identification of alloreactive NK cells in haplo-HSCT: relevance for donor selection
In haplo-HSCT, information on the size of the “alloreactive” NK-cell population present in potential donors (eg, the parents) may be important for an optimal donor selection.67 In addition, given the key role played by the alloreactive NK-cell subset in eradicating leukemia, it is crucial to identify this population in the recipient after HSCT to assess whether and how it is generated in the allogeneic environment of the recipient and whether it persists over time.67,68
Phenotypic identification of the alloreactive NK-cell subset and evaluation of the NK cytolytic activity against leukemic cells represent important criteria in donor selection. Multicolor flow cytometric analysis, using appropriate combinations of monoclonal antibodies (mAbs), allows the identification and definition of the size of the alloreactive NK-cell population.67,68 Substantial progress has been made recently after the identification of mAbs discriminating between inhibitory and activating KIRs. Thanks to these mAbs, it is now possible to distinguish KIR3DL1 from KIR3DS1, KIR2DL1 from KIR2DS1, and KIR2DL3 (but not KIR2DL2) from KIR2DS2.68 In addition, the presence of activating KIRs can be assessed also by the analysis of the KIR genotype and by the use of appropriate redirected killing assays.67 Recently, the identification of the amino acid residues critical for staining of commonly used anti-KIR mAbs allowed substantial progress in the characterization of the NK-cell repertoire and an improved phenotypic/functional definition of given KIR+ subsets.92 Evaluation of the cytolytic activity of donor NK cells against leukemic blasts of the patient or, alternatively, against appropriate Epstein-Barr virus-induced B-cell lines could be performed to select the donor with the best alloreactive capacity. In general, the degree of cytolytic activity correlates with the size of phenotypically defined alloreactive NK-cell subsets.67,68
Alloreactive NK cells are generated and persist in the recipient of haplo-HSCT
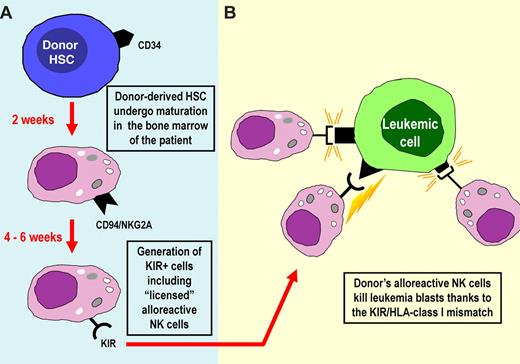
The generation of alloreactive NK cells in the recipient (Figure 1) was documented in the early studies published by Ruggeri et al.61 In these studies, alloreactive NK cells were detected for up to 1 year after transplantation. In some patients, the magnitude of the alloreactive NK-cell population was even larger in the recipient than in the donor, suggesting that they had been selected/expanded preferentially in vivo. More recent studies have confirmed and extended these findings. The donor's alloreactive NK-cell populations have been detected by both phenotypic and functional (cytolytic activity) criteria in several pediatric patients with high-risk leukemias even more than 4 years after transplantation.68,88 In these studies, a great variability in the size of the alloreactive NK-cell population was observed in different donors and in patients after transplantation. Notably, most patients characterized by high proportions of donor-derived alloreactive NK cells were disease-free after long time intervals.68 More importantly, a correlation was found between size of the alloreactive NK subset and clinical outcome. The alloreactive NK subset was detectable starting at 6 to 7 weeks after transplantation; and, in most instances, the pattern of expressed KIRs was similar to that originally found in the donor.68,88 A question arose regarding the interpretation of these data. During maturation, NK cells were shown to require recognition of self-MHC class I to acquire full function, a phenomenon referred to as “licensing” or “education.”93-95 Few NK cells lacking MHC-specific inhibitory receptors could also be generated, but they would remain virtually anergic or hyporesponsive.93 In view of these data, one may ask how donor NK-cell precursors undergoing maturation in the mismatched recipient can give rise to alloreactive NK cells (ie, effector cells capable of killing leukemia cells). A likely explanation is that, in haplo-HSCT, the infusion of “megadoses” of CD34+ cells may provide a BM microenvironment (ie, the main site where NK cells undergo maturation) predominantly of donor type. Under these conditions, the process of NK-cell education would be similar to that occurring in the donor and would allow the generation of “licensed” alloreactive NK cells (Figure 1).
Origin and differentiation of KIR+ alloreactive NK cells in the haplo-HSCT setting. (A) In haplo-HSCT, large numbers of CD34+ isolated from the donor are infused in the patient after the conditioning regimen. NK cells are the first lymphoid cells to appear in peripheral blood shortly after engraftment. The first wave of NK cells expresses the HLA-E-specific CD94-NKG2A inhibitory receptor. Appearance of KIR+ cells in peripheral blood requires 4 to 6 additional weeks. (B) KIR+ NK cells may contain alloreactive NK cells (ie, cells that express KIR specific for HLA ligands not expressed by the recipient). Alloreactive NK cells efficiently kill leukemia blasts. This would mean that they have been “licensed,” even in an allogeneic recipient. This may be explained by the fact that the “megadoses” of donor HSCs infused may build up a BM microenvironment that is largely of donor type.
Origin and differentiation of KIR+ alloreactive NK cells in the haplo-HSCT setting. (A) In haplo-HSCT, large numbers of CD34+ isolated from the donor are infused in the patient after the conditioning regimen. NK cells are the first lymphoid cells to appear in peripheral blood shortly after engraftment. The first wave of NK cells expresses the HLA-E-specific CD94-NKG2A inhibitory receptor. Appearance of KIR+ cells in peripheral blood requires 4 to 6 additional weeks. (B) KIR+ NK cells may contain alloreactive NK cells (ie, cells that express KIR specific for HLA ligands not expressed by the recipient). Alloreactive NK cells efficiently kill leukemia blasts. This would mean that they have been “licensed,” even in an allogeneic recipient. This may be explained by the fact that the “megadoses” of donor HSCs infused may build up a BM microenvironment that is largely of donor type.
Another puzzling question is why alloreactive NK cells do not mediate GVHD. Early experimental evidence suggested that NK cells predominantly attack the hematopoietic cells of the host while sparing tissues that are common targets of T cell–mediated GVHD. For example, in the hybrid resistance phenomenon in the mouse, NK cells rejected BM graft but did not attack other tissues. More recent studies in mice showed that allogeneic cells can mediate the GVL effect in the absence of GVHD.96 Ruggeri et al obtained direct evidence that murine alloreactive NK cells do not cause GVHD,61 whereas infusion of allogeneic T cells killed all the mice. In the same murine model, alloreactive NK cells were also shown to kill host antigen-presenting cells. It is of note that this effect can further reduce the risk of GVHD (see below). Another mechanism of NK cell–mediated GVHD reduction has been recently described in a murine model, whereby donor NK cells inhibited and lysed autologous donor T cells activated during the initiation of GVHD.97 The molecular basis of the resistance of recipient normal tissues other than the hematopoietic ones could be identified with the lack of ligands for activating NK receptors. These ligands become expressed or up-regulated by cells of different histotypes on cell stress, viral infection, or tumor transformation.12,66 Therefore, NK cells cannot “see” normal resting cells and do not become activated on interacting with them.
Future perspectives in the use of alloreactive NK cells
The intensive studies on NK-cell receptor function and specificity have provided a great opportunity for a rapid exploitation of these results in the treatment of high-risk leukemias in both adults and children. However, further relevant progress is expected by the use of donor alloreactive NK cells as a tool for improving the clinical outcome of severe malignancies and for preventing graft rejection or GVHD.
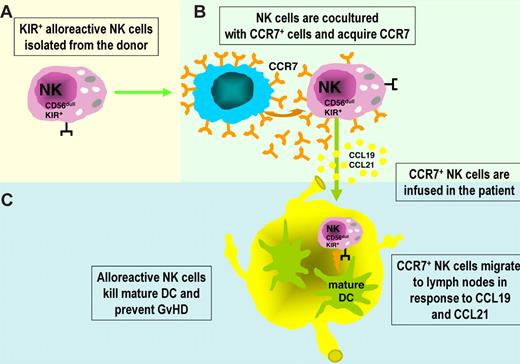
For example, the ability of alloreactive NK cells to kill host antigen-presenting cells, which are known to initiate T cell–mediated GVHD through presentation of host alloantigens to donor T cells, suggested a novel and interesting experimental approach in an animal model.96,98 Indeed, it was shown that the infusion of mature, donor-versus-recipient alloreactive NK cells in mice prevented GVHD to such an extent that mice that were given these cells as part of the conditioning regimen could receive mismatched BM grafts containing up to 30 times the lethal dose of allogeneic T cells, in the absence of clinical or histologic evidence of GVHD.96 Transfer of such an approach to humans is particularly promising, not only in HSCT to prevent or treat GVHD but also in the control of graft rejection. In humans, however, the adoptive transfer of KIR+ NK cells including the alloreactive subset to prevent GVHD, may be limited by the fact that KIR+ cells do not express CCR7 (ie, the chemokine receptor required for cell migration to lymph nodes in response to CCL19 and CCL21). Recent data provided a clue to overcome this limitation: KIR+ NK cells have been shown to take up CCR7 molecules on in vitro interaction with CCR7+ cells and acquire the ability to migrate to secondary lymphoid organs. In the course of inflammatory responses to infection, the encounter with CCR7+ dendritic cells (DCs) or virus-infected cells may redirect KIR+ NK cells to lymph nodes. Notably, the uptake of CCR7 molecules is negatively regulated by the interaction between KIR and HLA class I. Therefore, in the course of an infection in a self-environment, KIR+ NK cells can acquire CCR7 and be redirected to lymph nodes only when they interact with HLA class I–deficient CCR7+ cells (eg, virus-infected cells). In contrast, in an allogeneic setting characterized by KIR/HLA class I mismatch (as in haplo-HSCT), KIR+ alloreactive NK cells can acquire CCR7 on interaction with any CCR7+ cell, including those with high expression of HLA class I molecules (eg, DCs). Thus, the newly acquired migratory capability to lymph nodes of alloreactive NK cells may greatly enhance the likelihood of their interaction with DCs and T cells at these sites.99 NK-mediated killing of recipient DCs may prevent priming of alloreactive donor T cells and induction of GVHD. Similarly, alloreactive NK cells may play a relevant role in preventing host-versus-graft reactions by killing residual recipient T cells. Figure 2 shows a possible approach to improve the effectiveness of alloreactive NK cells to prevent/treat GVHD. We propose that KIR+ NK cells isolated from an alloreactive donor before their adoptive transfer be preincubated with CCR7+ HLA class I–deficient cells to allow CCR7 uptake and their immediate recruitment into lymph nodes.
Redirecting KIR+ alloreactive NK cells to lymph nodes to clear recipient DCs and prevent GVHD. (A) KIR+ alloreactive NK cells isolated from an allogeneic donor may be exploited in different adoptive immunotherapy protocols in leukemia patients because of their ability to kill host leukemia cells, DCs, and T cells. Remarkably, attempts to clear recipient DCs to prevent or treat GVHD may be hampered by the fact that KIR+ NK cells do not express CCR7, a chemokine receptor required for response to CCL19 and CCL21 and migration to lymph nodes (particularly rich of mature DCs). (B) Importantly, KIR+ NK cells have been shown to rapidly take up CCR7 on in vitro interaction with CCR7+ cells and acquire the capability of migrating to lymph nodes. (C) As shown in this figure, this property may be exploited to confer alloreactive NK cells the ability of targeting DCs present in secondary lymphoid organs, thus preventing the induction of allogeneic T-cell responses and GVHD.
Redirecting KIR+ alloreactive NK cells to lymph nodes to clear recipient DCs and prevent GVHD. (A) KIR+ alloreactive NK cells isolated from an allogeneic donor may be exploited in different adoptive immunotherapy protocols in leukemia patients because of their ability to kill host leukemia cells, DCs, and T cells. Remarkably, attempts to clear recipient DCs to prevent or treat GVHD may be hampered by the fact that KIR+ NK cells do not express CCR7, a chemokine receptor required for response to CCL19 and CCL21 and migration to lymph nodes (particularly rich of mature DCs). (B) Importantly, KIR+ NK cells have been shown to rapidly take up CCR7 on in vitro interaction with CCR7+ cells and acquire the capability of migrating to lymph nodes. (C) As shown in this figure, this property may be exploited to confer alloreactive NK cells the ability of targeting DCs present in secondary lymphoid organs, thus preventing the induction of allogeneic T-cell responses and GVHD.
In the haplo-HSCT setting, differentiation of KIR+ alloreactive NK cells from HSC precursors may require 6 to 8 weeks (Figure 1). Thus, their antileukemia effect may occur only after this time point. In case of high residual tumor burden and/or of rapidly proliferating leukemia blasts, this delay may represent a major limitation, resulting in leukemic relapses. To minimize this risk, mature alloreactive NK cells isolated from the haploidentical donor may be infused at short time intervals after HSCT. These mature donor NK cells could be properly activated with specifically active cytokines, such as interleukin-15, for further ameliorating the clinical results of haplo-HSCT, and the timing of infusion should be chosen also on the basis of the type of conditioning regimen used, paying particular attention to the persistence of antithymocyte globulin if this serotherapy has been administered to the patient during preparation of the allograft.
Regarding other possible settings in which alloreactive NK cells can be of relevant clinical interest, 2 recent studies reported on the infusion of purified NK cells in patients with various malignancies, including either relapsed or first complete remission AML, who had not received allogeneic HSCT.100,101 In both reports, patients were given immune-suppressive chemotherapy before and interleukin-2 after NK-cell infusion to prevent rejection and facilitate NK-cell functioning. These studies demonstrated that, in the majority of patients, adoptively transferred human NK cells derived from haploidentical donors transiently engrafted and expanded in vivo, without causing any relevant adverse event. The results in patients with AML treated with alloreactive NK cells were particularly encouraging.100,101 These data indicate that preparation consisting of cyclophosphamide and fludarabine followed by donor-recipient inhibitory KIR-HLA-mismatched NK cells is well tolerated by patients, results in successful engraftment, and is a promising novel therapy for reducing the risk of relapse in patients with myeloid malignancies treated with conventional chemotherapy. It is worth mentioning that another promising approach to control leukemia progression resides in the NK-cell manipulation using anti-KIR mAb. This reagent, which is undergoing phase II clinical trials, confers specific, stable blockade of KIR, thus boosting NK-mediated killing of HLA-matched AML blasts in vitro and in vivo.102
In conclusion, the discovery of NK-cell alloreactivity has represented a sort of revolution in the field of allo-HSCT, underlining, for the first time, that not only adaptive immunity, but also innate immunity, is a crucial element for guaranteeing a patient's successful outcome. Moreover, it has significantly changed the criteria for choosing the HSCT donor in the context of T cell–depleted allograft from an HLA-disparate relative. Is the mechanism of NK alloreactivity in leukemia completely understood and the role of NK cells in transplantation fully defined? The answer is no, as suggested by findings deriving from studies addressing the role of activating NK receptors68 ; and, clearly, there is still much to be learned about NK-cell function, interactions with other cell types, and migratory capability to different sites. The identification of MHC class I–specific NK receptors and their molecular and functional characterization represent a clear example of how studies in basic science may provide fundamental knowledge for important and successful applications in the clinic. In view of the role played by NK cells in HSCT recipients, studies that in the future will further contribute to elucidate the most sophisticated mechanisms of their function may be particularly relevant. Indeed, an even closer synergy between translational researchers and clinicians should provide the ideal environment for optimizing strategies able to render the exploitation of NK-cell alloreactivity even more successful.
Acknowledgments
This work was supported by Associazione Italiana per la Ricerca sul Cancro (L.M., F.L., A.M.), Ministero dell'Istruzione, dell'Università e della Ricerca (MIUR-FIRB 2003 project RBLA039LSF-001/003; L.M., A.M.), MIUR-PRIN 2008 (project prot 2008PTB3HC_005; L.M.), Ministero della Salute (RF2006 Ricerca Oncologica, Project of Integrated Program 2006-08, agreements RO strategici 3/07, L.M., A.M.; and RF2007 agreement 59; D.P.), Progetto di Ricerca Corrente from Ospedale Bambino Gesù (F.L.), Progetto di Ricerca di Ateneo 2008 dell'Università di Genova, project (E.M.), and Special Project 5 × 1000 from the Associazione Italiana per la Ricerca sul Cancro (Associazione Italiana per la Ricerca sul Cancro) projects (L.M., F.L., A.M.).
Authorship
Contribution: L.M. wrote the review and prepared the table and explanatory figures; F.L. and A.M. largely contributed to paper writing, revised the figures and the table, and had performed some of the research reported; and D.P., E.M., and M.C.M. had performed some of the research reported and revised the manuscript and figures.
Conflict-of-interest disclosure: A.M. is founder and shareholder of Innate-Pharma. The remaining authors declare no competing financial interests.
Correspondence: Lorenzo Moretta, Istituto Giannina Gaslini, L.go G. Gaslini n.5, 16147 Genova-Quarto, Italy; e-mail: lorenzomoretta@ospedale-gaslini.ge.it.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal