Haploidentical hematopoietic stem cell transplantation (haplo-HSCT) is an alternative treatment to patients with high-risk acute leukemia lacking a human leukocyte antigen-matched donor. We analyzed 173 adults with acute myeloid leukemia (AML) and 93 with acute lymphoblastic leukemia (ALL) who received a haplo-HSCT in Europe. All grafts were T cell–depleted peripheral blood progenitor cells from a direct family or other related donor. At transplantation, there were 25 patients with AML in CR1 (complete remission 1), 61 in more than or equal to CR2, and 87 in nonremission, and 24 with ALL in CR1, 37 in more than or equal to CR2, and 32 in nonremission. Median follow-up was 47 months in AML and 29 months in the ALL groups. Engraftment was observed in 91% of the patients. Leukemia-free survival at 2 years was 48% plus or minus 10%, 21% plus or minus 5%, and 1% for patients with AML undergoing transplantation in CR1, more than or equal to CR2, and nonremission, and 13% plus or minus 7%, 30% plus or minus 8%, and 7% plus or minus 5% in ALL patients, respectively. In conclusion, haplo-HSCT can be an alternative option for the treatment of high-risk acute leukemia patients in remission, lacking a human leukocyte antigen-matched donor.
Introduction
The great interest in transplantation from haploidentical donors arises from the immediate availability of a suitable one-haplotype mismatched donor for virtually all patients, particularly for those who urgently need a transplantation. Indeed, most adults with acute lymphoblastic leukemia (ALL) or acute myeloid leukemia (AML) relapse when they have unfavorable cytogenetics at diagnosis, when they do not achieve complete remission (CR) after the first induction cycle and when they are in second or later remission. Under these circumstances, an allogeneic hematopoietic stem cell transplantation (HSCT) is preferred as post-remission therapy.1 In the absence of a human leukocyte antigen (HLA) full-matched donor, alternative donors such as cord blood or haploidentical HSCT have been investigated.2,–4 T cell–depleted grafts with a high cell dose of CD34+ cells coming from family haploidentical donors have been proposed as a source of hematopoietic stem cells for allogeneic transplantation.5 Since this initial report, progress has been made in the optimization of conditioning regimens and graft selection to allow a stable hematopoietic engraftment across major HLA barriers, with promising leukemia-free survival (LFS) in adults with acute leukemias.6,7 However, only heterogeneous series of patients, with a variety of diagnoses and results from single-center studies, are reported.8 For this reason, we conducted an analysis of outcomes on 266 adults with de novo acute leukemia who received a transplant from a family haploidentical HSCT, reported to the Acute Leukemia Working Party (ALWP) registry of European Blood and Marrow Transplant (EBMT) Group. A risk factor analysis was performed only for patients in hematologic remission at transplantation.
Methods
Collection of data and inclusion criteria
The EBMT Group registry provided data on adults with de novo acute leukemia who underwent transplantation from “family HLA mismatched related donor” in accordance with the Declaration of Helsinki. Because this database encompasses all kinds of HLA mismatches, centers were asked to indicate true fully haplotype mismatched transplantation according to the definition of 2 or more antigen mismatch in HLA-A, HLA-B, HLA-DRB1 loci. Between January 1995 and December 2004, 266 adults with de novo acute leukemia met the eligibility criteria. Additional inclusion criteria were: (1) use of peripheral blood as source of stem cells and (2) use of ex vivo T-cell depletion by commercially available devices. Information on these patients was collected through a specific clinical form, recording data addressing questions about the disease, the origin of HSCT, the number of cells infused, HLA typing, and transplantation outcome. Transplantations were performed in 49 different centers, which reported up to 96 cases (see the Appendix). In particular, 161 patients were given transplantations in 6 centers and 105 patients in 43 centers. All patients reported in this study were consecutive, and no patient was excluded from the analysis by the participating institutions according to selection criteria. Patients and donors were typed for the currently recognized HLA antigens using mono-oligospecific antisera or by low-resolution DNA typing, whereas high-resolution molecular typing was used for DRB1 antigens in all donor/recipient pairs.
End points
Myeloid engraftment was defined as the first of 3 consecutive days when the absolute neutrophil count was 0.5 × 109/L or higher with evidence of donor hematopoiesis. Engraftment that occurred after day 60 was considered as nonengraftment. The end point of acute graft-versus-host disease (GVHD) was diagnosed and graded according to published criteria.9 Chronic GVHD was diagnosed according to standard criteria.10 Transplantation-related mortality (TRM) was defined as any death not related to relapse. Relapse was defined on the basis of morphologic evidence of leukemia in bone marrow or other sites. Survival was calculated from transplantation to death from any cause, and LFS was defined as the time from transplantation to either first relapse or death in CR. Detailed infections/complications after haplo-HSCT were not collected because definitions of infections, necessary tools of diagnosis and treatment, have varied among centers and time.
Statistical analysis
The duration of follow-up was the time to the last assessment for survivors. Variables considered in risk factor analysis for engraftment, TRM, relapse, and LFS were: recipient age and sex; disease characteristics (type of leukemia, cytogenetic, French-American-British classification for AML and phenotype for ALL, white blood count at time of diagnosis, cytomegalovirus (CMV) serology), donor characteristics (age, sex, CMV serology), disease status at transplantation (CR1, CR2, or more advanced disease); and transplantation characteristics (number of antigen incompatibilities (2 vs > 2); receipt of a previous autologous transplantation, dose of nucleated cells infused, conditioning regimen, including total body irradiation or not, GVHD prophylaxis, and year of transplantation). Data on KIR compatibility (yes or no) were available only in patients with AML in remission at time of transplantation, and its impact on outcomes was studied only in this population. Cumulative incidence curves were used in a competing risks setting, with death being treated as a competing event to calculate probabilities of acute and chronic GVHD, neutrophil recovery, TRM, and relapse.11 Probabilities of survival and LFS were calculated using the Kaplan-Meier estimate; the log-rank test was used for univariate comparisons. The multivariate analyses were performed using Cox proportional-hazards regression model for LFS and overall survival and Fine and Gray's proportional-hazards model for subdistribution for other outcomes.12 Because 36% of the patients were transplanted at the University of Perugia, Italy, all multivariate analyses were adjusted for the site of transplantation (Perugia vs other centers). All P values are 2-sided, with a type I error rate fixed at .05. Statistical analyses were performed with SPSS and S-Plus (MathSoft) software.
Results
Total population
Patient, disease, and donor characteristics.
Table 1 summarizes the main characteristics of all haplo-HSCT recipients. The median time from diagnosis to transplantation for patients who received transplantations in primary refractory and relapsed leukemia were 195 days (range, 73-925 days) and 454 days (range, 89-3820 days), respectively. The median period of follow-up was 47 months (range, 14-84 months) and 29 months (range, 12-103 months) for the entire population of AML and ALL, respectively. A significant proportion of AML (51%) and ALL (34%) patients received transplants in advanced chemoresistant phase. Twenty-five patients with AML underwent transplantation in first CR for the following reasons: in 14 patients, the time from diagnosis to obtain remission was more than 50 days associated or not to bad cytogenetics group, 2 had poor cytogenetic (abnormalities of chromosomes 5 and 7), and 6 patients had white blood cells at diagnosis more than 30 000 and or/associated to FAB classification (M0, M5, and M6). For 3 patients, data on those factors were missing. Twenty-four patients with ALL underwent transplantation in first CR: 16 had poor cytogenetic (most of them t(9;22)) and 6 had high cell counts more than 35 000 and/or time to obtain first remission was more than 45 days. Potential donor-versus-recipient NK cell alloreactivity was analyzed according to missing expression of HLA-C groups 1 or 2 and of HLA-Bw4 group alleles. Data were available in 58 AML patients transplanted in remission; in 39 patients, the donor was NK-alloreactive, and in 19 they were not.
Patient, disease, donor, and transplantation characteristics of 266 haplo-HSCT recipients with acute leukemia according to diagnosis and disease status
| Characteristics . | Patients in remission at transplantation . | Patients in relapse at transplantation . | ||
|---|---|---|---|---|
| AML (n = 86) . | ALL (n = 61) . | AML (n = 87) . | ALL (n = 32) . | |
| Patient-related | ||||
| Median age at transplantation, y (range) | 37 (17-66) | 21 (16-51) | 36 (16-63) | 22 (16-46) |
| Female sex | 36 (42%) | 19 (31%) | 50 (57%) | 13 (41%) |
| Positive CMV serologic status at transplantation | 70 (88%) | 35 (63%) | 59 (76%) | 18 (75%) |
| Disease-related | ||||
| Status at transplantation | ||||
| CR1 | 25 (29%) | 24 (39%) | ||
| CR2 | 46 (53%) | 26 (42%) | ||
| CR3 | 15 (17%) | 11 (18%) | ||
| PIF | 36 (41%) | 8 (25%) | ||
| 1st relapse | 29 (33%) | 12 (37%) | ||
| 2nd relapse | 22 (25%) | 12 (37%) | ||
| Median WBC at diagnosis × 109/L | 8.8 (0.5-140) | 16.4 (0.6-289) | 26 (1.2-850) | 27.6 (2.7-400) |
| AML cytogenetics | ||||
| Good | 13 | 5 | ||
| Intermediate | 51 | 55 | ||
| Poor | 8 | 10 | ||
| ALL cytogenetics | ||||
| t(9;22) or t(4;11) | 27 | 13 | ||
| Normal or other | 21 | 7 | ||
| Previous autologous transplantation | 24 (28%) | 6 (10%) | 17 (20%) | 3 (9%) |
| Donor-related | ||||
| Age, y (range) | 38 (14-63) | 42 (14-75) | 36 (12-70) | 42 (17-61) |
| Female sex | 33 (39%) | 27 (44%) | 30 (35%) | 8 (25%) |
| Female to male | 16 (19%) | 19 (31%) | 10 (12%) | 4 (13%) |
| Positive CMV serologic status | 62 (79%) | 35 (66%) | 51 (67%) | 15 (63%) |
| Donor type | ||||
| Mother | 7 (9%) | 15 (31%) | 9 (12%) | 2 (8%) |
| Father | 7 (9%) | 10 (20%) | 14 (19%) | 10 (39%) |
| Brother | 20 (26%) | 10 (20%) | 19 (26%) | 9 (35%) |
| Sister | 11 (14%) | 9 (18%) | 5 (7%) | 3 (11%) |
| Others | 31 (41%) | 5 (10%) | 27 (37%) | 2 (8%) |
| Cousin | 9 | 2 | 5 | |
| Daughter | 9 | 1 | 11 | |
| Nephew | 1 | 0 | 1 | |
| Son | 8 | 2 | 11 | |
| Uncle | 1 | 0 | ||
| Unknown | 10 | 12 | ||
| HLA compatibility/total | ||||
| 2/6 | 18 (26%) | 23 (47%) | 23 (32%) | 7 (30%) |
| 3/6 | 49 (71%) | 23 (47%) | 49 (67%) | 16 (67%) |
| 4/6 | 2 (3%) | 3 (6%) | 1 (1%) | 1 (4%) |
| Transplantation-related | ||||
| Year of transplantation [median (range)] | 2002 (95-04) | 2002 (96-04) | 2001 (95-04) | 2000 (95-04) |
| Conditioning regimen based on TBI | 62 (74%) | 56 (92%) | 61 (71%) | 28 (87%) |
| ATG-based in vivo T-cell depletion | 74 (93%) | 51 (91%) | 80 (95%) | 27 (96%) |
| CD34+ ex vivo selection by Clinimacs | 55 (77%) | 42 (86%) | 63 (84%) | 17 (63%) |
| Cell dose | ||||
| No. of CD34+ × 106/kg [median (range)] | 10 (1.43-25) | 11.9 (3.69-30) | 9.4 (0-51) | 10.5 (2-22) |
| No. of CD3+ × 104/kg [median (range)] | 1 (0-6.4) | 1 (0-2.3) | 0.2 (0-3.6) | 0.2 (0.01-4) |
| Characteristics . | Patients in remission at transplantation . | Patients in relapse at transplantation . | ||
|---|---|---|---|---|
| AML (n = 86) . | ALL (n = 61) . | AML (n = 87) . | ALL (n = 32) . | |
| Patient-related | ||||
| Median age at transplantation, y (range) | 37 (17-66) | 21 (16-51) | 36 (16-63) | 22 (16-46) |
| Female sex | 36 (42%) | 19 (31%) | 50 (57%) | 13 (41%) |
| Positive CMV serologic status at transplantation | 70 (88%) | 35 (63%) | 59 (76%) | 18 (75%) |
| Disease-related | ||||
| Status at transplantation | ||||
| CR1 | 25 (29%) | 24 (39%) | ||
| CR2 | 46 (53%) | 26 (42%) | ||
| CR3 | 15 (17%) | 11 (18%) | ||
| PIF | 36 (41%) | 8 (25%) | ||
| 1st relapse | 29 (33%) | 12 (37%) | ||
| 2nd relapse | 22 (25%) | 12 (37%) | ||
| Median WBC at diagnosis × 109/L | 8.8 (0.5-140) | 16.4 (0.6-289) | 26 (1.2-850) | 27.6 (2.7-400) |
| AML cytogenetics | ||||
| Good | 13 | 5 | ||
| Intermediate | 51 | 55 | ||
| Poor | 8 | 10 | ||
| ALL cytogenetics | ||||
| t(9;22) or t(4;11) | 27 | 13 | ||
| Normal or other | 21 | 7 | ||
| Previous autologous transplantation | 24 (28%) | 6 (10%) | 17 (20%) | 3 (9%) |
| Donor-related | ||||
| Age, y (range) | 38 (14-63) | 42 (14-75) | 36 (12-70) | 42 (17-61) |
| Female sex | 33 (39%) | 27 (44%) | 30 (35%) | 8 (25%) |
| Female to male | 16 (19%) | 19 (31%) | 10 (12%) | 4 (13%) |
| Positive CMV serologic status | 62 (79%) | 35 (66%) | 51 (67%) | 15 (63%) |
| Donor type | ||||
| Mother | 7 (9%) | 15 (31%) | 9 (12%) | 2 (8%) |
| Father | 7 (9%) | 10 (20%) | 14 (19%) | 10 (39%) |
| Brother | 20 (26%) | 10 (20%) | 19 (26%) | 9 (35%) |
| Sister | 11 (14%) | 9 (18%) | 5 (7%) | 3 (11%) |
| Others | 31 (41%) | 5 (10%) | 27 (37%) | 2 (8%) |
| Cousin | 9 | 2 | 5 | |
| Daughter | 9 | 1 | 11 | |
| Nephew | 1 | 0 | 1 | |
| Son | 8 | 2 | 11 | |
| Uncle | 1 | 0 | ||
| Unknown | 10 | 12 | ||
| HLA compatibility/total | ||||
| 2/6 | 18 (26%) | 23 (47%) | 23 (32%) | 7 (30%) |
| 3/6 | 49 (71%) | 23 (47%) | 49 (67%) | 16 (67%) |
| 4/6 | 2 (3%) | 3 (6%) | 1 (1%) | 1 (4%) |
| Transplantation-related | ||||
| Year of transplantation [median (range)] | 2002 (95-04) | 2002 (96-04) | 2001 (95-04) | 2000 (95-04) |
| Conditioning regimen based on TBI | 62 (74%) | 56 (92%) | 61 (71%) | 28 (87%) |
| ATG-based in vivo T-cell depletion | 74 (93%) | 51 (91%) | 80 (95%) | 27 (96%) |
| CD34+ ex vivo selection by Clinimacs | 55 (77%) | 42 (86%) | 63 (84%) | 17 (63%) |
| Cell dose | ||||
| No. of CD34+ × 106/kg [median (range)] | 10 (1.43-25) | 11.9 (3.69-30) | 9.4 (0-51) | 10.5 (2-22) |
| No. of CD3+ × 104/kg [median (range)] | 1 (0-6.4) | 1 (0-2.3) | 0.2 (0-3.6) | 0.2 (0.01-4) |
AML indicates acute myelogenous leukemia; ALL, acute lymphoblastic leukemia; CR1, first complete remission; CR ≥ 2, second or subsequent complete remissions; and PIF, primary induction failure.
Transplantation and graft cell selection.
Hematopoietic stem cells were collected from local blood banks; Clinimacs (Miltenyi Biotec, Bergisch Gladbach, Germany) devices were the leading method of processing donor's blood cells collected from peripheral blood after G-CSF stimulation. Cellpro (Bothell, WA) and Isolex (Baxter Biotech, Deerfield, IL) devices were reported in 23 of 147 transplantations. The median number of CD34+ cells infused in recipients was 10 × 106/kg (range, 1.43-25) and 11.9 × 106/kg (range, 3.69-30) for AML and ALL patients, respectively. The median number of CD3+ cells infused in recipients was 104/kg (range, 0-6.4) and 104/kg (range, 0-2.3) for AML and ALL patients, respectively. Using these cell selection procedures, median T-cell depletion was more than 4 log. In vivo T-cell depletion by antithymocyte globulin (ATG) was used in a majority of reported transplantations. All patients received a myeloblative conditioning regimen; total body irradiation was reported in 74% of AML and in 92% of ALL patients.
Outcomes for all patients
Table 2 shows the outcomes for patients with AML and ALL according to disease status at transplantation.
Two-year cumulative incidence of transplantation-related mortality (TRM), relapse (RI), and probability of leukemia-free survival (LFS) in 266 patients with AML and ALL receiving a haplo-HSCT according to disease status
| . | TRM (%) . | RI (%) . | LFS (%) . | |||
|---|---|---|---|---|---|---|
| AML . | ALL . | AML . | ALL . | AML . | ALL . | |
| CR1 | 36 ± 10 | 61 ± 10 | 16 ± 10 | 26 ± 9 | 48 ± 10 | 13 ± 7 |
| CR2 | 54 ± 5 | 44 ± 9 | 23 ± 5 | 27 ± 8 | 21 ± 5 | 30 ± 8 |
| Advanced | 66 ± 4 | 44 ± 9 | 32 ± 1 | 49 ± 9 | 1 ± 1 | 7 ± 5 |
| . | TRM (%) . | RI (%) . | LFS (%) . | |||
|---|---|---|---|---|---|---|
| AML . | ALL . | AML . | ALL . | AML . | ALL . | |
| CR1 | 36 ± 10 | 61 ± 10 | 16 ± 10 | 26 ± 9 | 48 ± 10 | 13 ± 7 |
| CR2 | 54 ± 5 | 44 ± 9 | 23 ± 5 | 27 ± 8 | 21 ± 5 | 30 ± 8 |
| Advanced | 66 ± 4 | 44 ± 9 | 32 ± 1 | 49 ± 9 | 1 ± 1 | 7 ± 5 |
In the 119 patients who were transplanted in advanced-stage disease, the cumulative incidence of TRM was 66% for AML and 44% for ALL; the incidence of leukemia relapse was 32% and 49% in AML and ALL, respectively. LFS in this poor-risk population was very low, 1% and 7% in AML and ALL, respectively. At last follow-up, 5 of 119 patients are alive between 5 and 56 months after transplantation.
Risk factors analysis for outcomes in patients with AML or ALL in remission at transplantation
Because outcomes of patients transplanted in advanced phase of the disease were poor, a risk factor analysis was conducted only for patients transplanted in remission. Therefore, 86 AML patients and 61 ALL patients receiving a haplo-HSCT were analyzed, and their disease, donor, and transplantation characteristics are listed in Table 1.
Neutrophil engraftment and chimerism analysis
Neutrophil recovery was documented in 134 (91%) recipients at a median of 12 days (range, 7-72 days). Among 13 patients who did not show evidence of neutrophil recovery, 4 died earlier than 14 days and cannot be considered for engraftment, 2 patients lost their grafts and died in aplasia, and of the other 7 patients who have received a second transplantation, only 1 is alive at 6 months. Conditioning for second transplantation was fludarabine-cyclophosphamide-ATG in 5 patients, fludarabine-melphalan-ATG in 1 patient, and cyclophosphamide-melphalan-ATG in 1 patient; graft was CD34+ selected from the same donor in 3 patients and from a different family haploidentical donor in 4 patients. Of the 7 patients who underwent a second transplantation, 5 engrafted, 2 died before day 14 of the second transplantation, and only one of the engrafted patients is alive. The diagnosis, CD34+ cell dose, and the number of HLA A, B, DR mismatched antigens had no significant impact on neutrophil engraftment. Chimerism analysis at day +100 is available for 101 patients of 134 who engrafted: 89 (88%) had full donor chimerism, 12 (12%) mixed chimerism.
Acute and chronic GVHD
Grades II through IV acute GVHD was observed in 4 (5%) patients (2 grade II, 1 grade III, and 1 grade IV) and 11 (18%) patients (8 grade II, 3 grade III, and 4 grade IV) of AML and ALL recipients, respectively. Among patients who survived more than 100 days, chronic GVHD was observed in 6 of 56 (10%) and 7 of 39 (19%) of AML and ALL patients, respectively.
Transplantation-related mortality and nonrelapse causes of death
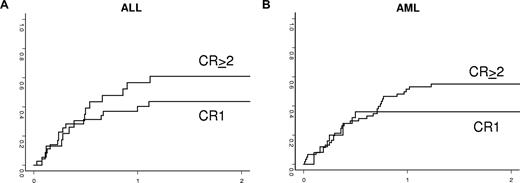
The cumulative incidence of TRM according to disease status at transplantation is shown in Table 2 and in Figure 1.
Incidence of transplantation-related death in adults with acute lymphoblastic leukemia (A) and acute myeloid leukemia (B) according to disease status at transplantation.
Incidence of transplantation-related death in adults with acute lymphoblastic leukemia (A) and acute myeloid leukemia (B) according to disease status at transplantation.
In univariate analysis for the AML group, TRM was decreased when the donor was a parent or a sibling compared with other relatives (35% ± 7% vs 65% ± 9%, P = .03). There was no statistical association of NK alloreactivity with TRM (4%5 ± 8% in patients with KIR incompatibilities vs 47% ± 10% in the remaining patients, P = .77). Therefore, in the multivariate analyses, only type of donor was associated with TRM (relative risk, 0.44; 95% confidence interval [CI], 0.22-0.87; P = .018).
Whereas for ALL, TRM was 25% plus or minus 13% when both recipient and donor were CMV-seronegative compared with 59% plus or minus 8% for other combinations (P = .01), only this factor was selected in the multivariate model (relative risk, 0.3; 95% CI, 0.08-0.84; P = .002). Other patient-, disease-, and transplantation-related factors were not associated with TRM in the ALL group.
Nonleukemic deaths show a pattern of linear distribution in the first year after transplantation, with the last event at 24 months. Reported causes of nonrelapse mortality in patients with AML or ALL, respectively, were idiopathic interstitial pneumonitis in 15% and 8%, GVHD in 8% and 10%, and infections in 64% and 59% (Table 3). Two patients died of veno-occlusive disease. No death is reported by Epstein-Barr virus–induced lymphoproliferative disease.
Causes of death after haploidentical transplantation for patients with high-risk acute leukemia in remission at transplantation
| Cause of death . | AML (N = 86) . | ALL (N = 61) . |
|---|---|---|
| Related to relapse | 13 | 15 |
| Related to transplantation | ||
| Infection | 26 | 18 |
| Interstitial pneumonia | 9 | 3 |
| GVHD | 5 | 4 |
| Others or unknown | 5 | 4 |
| Total | 58 | 44 |
| Cause of death . | AML (N = 86) . | ALL (N = 61) . |
|---|---|---|
| Related to relapse | 13 | 15 |
| Related to transplantation | ||
| Infection | 26 | 18 |
| Interstitial pneumonia | 9 | 3 |
| GVHD | 5 | 4 |
| Others or unknown | 5 | 4 |
| Total | 58 | 44 |
GVHD indicates graft-versus-host disease.
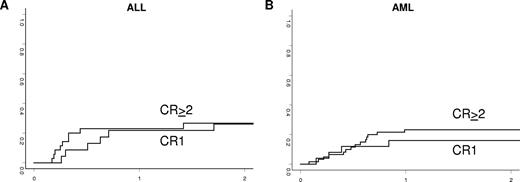
Relapse incidence
The cumulative incidence of relapse according to diagnosis and status of the disease is shown in Table 2 and Figure 2. In patients transplanted in remission, the 2-year cumulative incidence of relapse was 21% plus or minus 4% for AML and 27% plus or minus 5% for ALL. In the AML group, there was no statistical association of NK alloreactivity with relapse (21% ± 6% in patients with KIR incompatibilities vs 21% ± 10% in the remaining patients, P = .99). No other disease-, patient-, and transplantation-related factors were associated with relapse incidence in the ALL or AML groups transplanted in remission.
Incidence of leukemia relapse in adults with ALL (A) and AML (B) according to disease status at transplantation.
Incidence of leukemia relapse in adults with ALL (A) and AML (B) according to disease status at transplantation.
Donor lymphocyte infusion
Donor lymphocyte infusion (DLI) was reported in 36 of 78 (46%) AML recipients and in 21 of 61 (34%) ALL patients. Reasons for DLI in AML and ALL, respectively, were relapse in 2 and 3 patients, graft failure in 0 and 1, preemptive in 26 and 12, and persistent disease in 1 and 0. No DLI has been reported for mixed chimerism. The delay from transplantation to DLI was 61 days (range, 15-315 days) in AML and 55 days (range, 16-578 days) in ALL patients. GVHD occurred after DLI in 12 of 36 and in 8 of 21 AML and ALL patients, respectively.
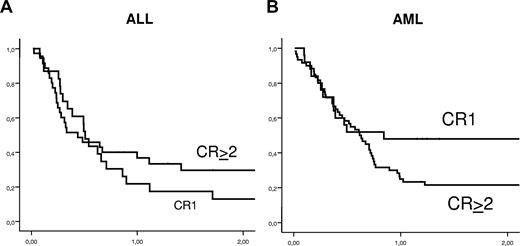
Leukemia-free survival
Kaplan-Meier estimates of 2-year LFS according to the type of leukemia and the disease status at the time of the transplantation are shown in Figure 3 and listed in Table 2. LFS was 29% plus or minus 5% and 23% plus or minus 6% in AML or ALL, respectively.
Kaplan-Meier estimates of leukemia-free survival in adults with ALL (A) and AML (B) according to disease status at time of transplantation.
Kaplan-Meier estimates of leukemia-free survival in adults with ALL (A) and AML (B) according to disease status at time of transplantation.
In univariate analysis, patients with AML who received a transplant from parents and siblings had a significantly higher probability of LFS compared with those who received a transplant from other family members (44% vs 14%, respectively, P = .04). LFS was 33% plus or minus 7% in 39 patients whose donor was NK-alloreactive and 32% plus or minus 10% in 19 whose donor was not (P = .78). There was a trend for improved LFS for patients who received a transplant in Perugia (n = 37) compared with other sites (n = 48; 38% ± 8% vs 23% ± 6%, P = .09). In a multivariate analysis, adjusting for other factors, only the use of a direct family member as the donor (sibling or parents) was associated with improved LFS (relative risk, 0.55; 95% CI, 0.31-0.99; P = .045).
In univariate analysis for ALL patients, 2-year LFS was 35% plus or minus 9% for patients who received a transplant after 2002 compared with 10% plus or minus 6% for those who received a transplant earlier (P = .01). In addition, CMV seronegativity of both recipient and donor was associated with improved LFS. Indeed, LFS at 2 years was 30% plus or minus 11% for negative CMV serology patients and 37% plus or minus 12% when the donors were CMV negative compared with 18% plus or minus 6% and 17% plus or minus 7% positive CMV recipients and donors, respectively (P = .05 and .04, respectively). Moreover, LFS was 45% plus or minus 14% when donors and recipients were CMV negative compared with 16% plus or minus 6% (P = .01) for other combinations. LFS in patients undergoing HSCT for Ph+ ALL and ALL with other cytogenetics was 22% and 23%, respectively (P = .99). Other patient-, disease-, and transplantation-related factors were not statistically associated with LFS. In multivariate analysis (Table 4), only CMV−/− patient/donor status was a significant covariate associated with higher LFS in patients with ALL (relative risk, 2.6; 95% CI, 1.14-6.07; P = .02). No statistical association of number of CD34+ cells and CD3+ cells infused and the outcomes was documented.
Multivariate analysis of outcomes after haploidentical transplantation for patients with high-risk acute leukemia in remission at transplantation
| Diagnosis . | Outcomes . | ||
|---|---|---|---|
| Transplantation-related mortality . | RI . | Leukemia-free survival . | |
| AML | Type of donor (parents or siblings):* RR = 0.44; 95% CI, 0.22-0.87; P = .018 | No risk factor | Type of donor (parents or siblings):† RR = 1.81; 95% CI, 1.01-3.22; P = .045 |
| ALL | Donor's negative CMV serology to a patient's negative CMV serology:‡ RR = 0.3; 95% CI, 0.08-0.84; P = .002 | No risk factor | Donor's negative CMV serology to a patient's negative CMV serology:§ RR = 2.6; 95% CI, 1.14-6.07; P = .02 |
| Diagnosis . | Outcomes . | ||
|---|---|---|---|
| Transplantation-related mortality . | RI . | Leukemia-free survival . | |
| AML | Type of donor (parents or siblings):* RR = 0.44; 95% CI, 0.22-0.87; P = .018 | No risk factor | Type of donor (parents or siblings):† RR = 1.81; 95% CI, 1.01-3.22; P = .045 |
| ALL | Donor's negative CMV serology to a patient's negative CMV serology:‡ RR = 0.3; 95% CI, 0.08-0.84; P = .002 | No risk factor | Donor's negative CMV serology to a patient's negative CMV serology:§ RR = 2.6; 95% CI, 1.14-6.07; P = .02 |
Other variables included in the model if P < .20.
RI indicates relapse; CMV, cytomegalovirus; RR, relative risk; and 95% CI, 95% confidence interval.
Type of donor, site of transplantation, status of the disease at transplantation, and number of previous transplantations.
Patient CMV serology, female donor to male recipient, type of donor, site of transplantation, and status of the disease at transplantation.
Recipient's age, year of transplantation, status of the disease at transplantation, and donor CMV/patient CMV.
Recipient's age, year of transplantation (RR = 1.9; 95% CI, 0.98-3.8; P = .06), status of the disease at transplantation, and donor CMV/patient CMV.
Discussion
In this registry-based retrospective study, we analyzed clinical outcome of allogeneic transplantation from a haploidentical donor in 266 adult patients with de novo acute leukemia. This is the largest series of patients with acute leukemia given a haplo-HSCT reported in the literature. Because almost 50% of these patients received transplants in advanced phase of the disease, it is evident that this procedure was the last therapeutic option that contributed to the very poor outcomes. Therefore, a risk factor analysis was conducted only in the 147 patients who were in any remission at the transplantation.
This large multicenter registry-based study confirms that a high engraftment rate and minimal GVHD in the absence of any posttransplantation immunosuppressive therapy after HSCT from haploidentical donors can be achieved by infusing a large number of immunoselected CD34+ cells. Neutrophil engraftment was fast and reproducible, with a median time of engraftment in the range of results obtained with unmanipulated peripheral blood stem cells in an HLA-identical setting.13 Primary graft failure, which occurred in 9% of patients in remission at transplantation, was largely rescued by a second haploidentical transplantation accounting for a total final engraftment rate of 96%. The threshold dose of 104 CD3+ cell/kg prevented severe GVHD as long as it was associated with ATG in the conditioning. The automated graft processing system, which provided reliable and reproducible yields, has contributed to make extensively T cell–depleted haploidentical transplantation feasible in any transplantation center.
Transplantation-related deaths were observed in a significant proportion of recipients transplanted both in advanced and in CR of disease. Leading causes of deaths reported were infections and interstitial pneumonia; any further reduction in TRM will only be achieved by hastening posttransplantation immune recovery. The group in Perugia has recently developed a strategy for transferring donor pathogen-specific immune responses safely across the HLA barrier.14 Although the strategy proved safe, highly effective in preventing and controlling the specific pathogens while preventing GVHD, it is still affected by a low feasibility profile, mainly because of a long and complex in vitro manipulation procedure, and it is not effective in providing a wide immune-reconstitution. Several additional strategies are currently exploring different cell therapy approaches aimed at improving posttransplantation immune reconstitution while controlling GVHD: T cells depleted of alloreactive lymphocytes,15 regulatory T cells,16 CD3/CD19 depletion,17 mesenchymal stem cells,18 and the add-back infusions of donor lymphocytes genetically engineered with the herpes simplex virus-thymidine kinase suicide gene.19
LFS was poor in patients with advanced disease at transplantation, with a median survival time of 3 months for both AML and ALL and a 2-year Kaplan-Meier estimate of survival less than 10%. According to the results obtained in this large cohort of patients, the transplantation of CD34+ selected cells from haploidentical donors should not be considered as an appropriate option for patients with advanced chemorefractory acute leukemia. Despite the ability of full myeloablative regimens in inducing a transient control of leukemia burden, the lack of a fast donor T-cell recovery secondary to profound graft T-cell depletion is limiting the overall antileukemia potential of this transplantation modality, compared with alternative T cell–replete options recently reported in HLA-matched settings.20
In this multicenter registry-based study, relevant LFS was reported for patients in CR at transplantation. Data collected from more than 40 centers confirmed LFS at 2 years more than 20% to 30% for AML and ALL in CR more than or equal to 2. The expected reduction of TRM provided by the developing cell-based tools for posttransplantation immune intervention may further improve the outcome of this procedure for patients in urgent need of a transplantation.
The multivariate analysis for factors influencing LFS failed to demonstrate a correlation with covariates related to demography, disease characteristics, donors, use of total body irradiation, numbers of HLA antigen mismatches, cell doses, centers, and year of transplantation. In particular, no clear center-effect was documented despite the unbalanced distribution of activity of haplotransplantations, and no statistically difference was documented for transplantations performed before and after 1999, the year of widespread diffusion of cell-selection devices. In addition, selection criteria excluded from the analysis patients with myelodysplastic syndromes or secondary acute myeloid leukemia. The dramatic impact of CMV seronegativity of both donor and recipient on nonrelapse mortality in ALL reemphasizes the importance of CMV infection as a major determinant of mortality and as an indicator of poor immune recovery after T cell–depleted autologous and allogeneic HSCT,21 although we were not able to find any explanation for the association of CMV serology status only in patients with ALL. Another factor associated with improved LFS was the donor as a direct family member (sibling or parent), with no direct association with cell dose or any other unbalanced known factors. Noninherited maternal and paternal antigens have been associated with outcomes in HLA mismatched family donors.22 Unfortunately, because of the absence of HLA data of donors and/or recipients' parents, we were not able to study the association of inherited maternal and paternal antigens in our series or because of relative small number of patients receiving a graft from a mother (n = 22) or father donor (n = 17).
Potential donor-versus-recipient NK cell alloreactivity was analyzed according to missing expression of HLA-C groups 1 or 2 and of HLA-Bw4 group alleles. According to local standard policies of HLA typing in family pairs, data were available in only 58 of 86 AML patients in remission at transplantation; in 39 patients, the donors were NK alloreactive; and in 19 patients, they were not. In the AML group, there was no statistical association of NK alloreactivity with relapse (21% ± 6% in patients with KIR incompatibilities vs 21% ± 10% in the remaining patients, P = .99). Probably, we have not found any association between KIR incompatibilities in the GVHD direction and outcomes because of the small number of patients analyzed or other differences that can be present in our analysis compared with the Perugia study,7 such as the inclusion of myelodysplastic syndrome and secondary AML, homogeneous conditioning regimen, GVHD, and infections prophylaxis. Therefore, any conclusion based on these results should be taken with caution.
In conclusion, LFS obtained in patients transplanted from a full-haplotype mismatched donor were relevant, both in high-risk AML and ALL when in CR. Cell-based strategies aimed at improving posttransplantation immune recovery are in current development and will beneficially impact on high infectious mortality reported. Because virtually all patients in need of an HSCT have a family haploidentical donor who is immediately available, we think that a T cell–depleted mismatched transplantation should be included in the treatment algorithm as a viable option to adults with high-risk acute leukemia lacking a matched donor.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank all data managers and the ALWP for collecting data from the EBMT Group centers.
A list of the other members of the ALWP of the EBMT Group appears in the Appendix.
Authorship
Contribution: F.C. and F.A. designed research and wrote the paper; M.L. designed research and analyzed data; J.M.R., M.T.L.S., P.L., A.N., P.D., and J.F.L. provided data; D.B. provided data and wrote the paper; E.P. collected and checked the data and contacted transplantation centers; F.F. and M.F.M. designed research; and V.R. analyzed data and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Fabio Ciceri, San Raffaele Scientific Institute, Via Olgettina 60, 20132, Milano, Italy; e-mail: ciceri.fabio@hsr.it.
Appendix
The authors are members of the Acute Leukemia Working Party of the European Blood and Marrow Transplant Group. Additional members who participated in this study are as follows:
Centre Identification Code (CIC) 160: Hôpital Necker, Service Hematologie Adulte, Paris, France; CIC 203: Leiden University Hospital, BMT Centre Leiden, Leiden, The Netherlands; CIC 204: Klinik fuer Innere Medzin III, Universitätsklinikum Ulm, Ulm, Germany; CIC 209: University Hospital Gasthuisberg, Department of Hematology, Leuven, Belgium; CIC 214: Hospital Clinic, Institute of Hematology & Oncology, Barcelona, Spain; CIC 215: Institut Jules Bordet, Experimental Hematology, Brussels, Belgium; CIC 216: Royal Free Hospital and School of Medicine, Department of Hematology, London, United Kingdom; CIC 223: Universität Tübingen, Medizinische Klinik, Tübingen, Germany; CIC 230: Unité de Transplantation et de Thérapie Cellulaire, Inserm Unité Mixte de Recherche (UMR) 599, Marseille, France; CIC 232: University La Sapienza, Dipartimento Biotecnologie Cellulari ed Ematologia, Rome, Italy; CIC 238: Hospital Reina Sofia, Córdoba Hospital, Córdoba, Spain; CIC 245: University of Parma, Cattedra di Ematologia, Parma, Italy; CIC 248: Ospedale Civile, Department of Hematology, Pescara, Italy; CIC 252: Hôpital Henri Mondor, Sve d' Hematologie, Creteil, France; CIC 256: Universitätsklinikum Schleswig-Holstein (UKSH), Campus Kiel, Division of Stem Cell and Immunotherapy, Kiel, Germany; CIC 258: Hadassah University Hospital, Department of Bone Marrow Transplantation, Jerusalem, Israel; CIC 261: Hopitaux Universitaires de Geneve, Departement Medecine Interne, Geneva, Switzerland; CIC 262: Groupe Hospitalier Pitié-Salpêtrière, Paris, France; CIC 267: Centre Hospitalier Universitaire (CHU) Bordeaux, Hôpital Haut-Leveque, Pessac, France; CIC 272: Hopital Bretonneau, Service d'Oncologie Médicale, Tours, France; CIC 279: Ospedale San Gerardo, Clinica Pediatrica dell'Universita, Monza, Italy; CIC 282: Hospital Clínico Universitario, Servicio de Hematologia y Oncologia, Valencia, Spain; CIC 295: Hannover Medical University, Department of Transfusion Medicine, Hannover, Germany; CIC 299: Hospital San Maurizio, Department of Hematology-BMT Unit, Bolzano, Italy; CIC 304: Ospedale di Careggi, BMT Unit Department of Hematology, Firenze, Italy; CIC 305: Onco-Ematologia Pediatrica, Centro Trapianti Cellule Staminali, Ospedale Infantile Regina Margherita, Torino, Italy; CIC 308: Fondacion Jimenes Diaz, Madrid, Spain; CIC 311: Deutsche Klinik für Diagnostik, Knochenmarktransplantations-zentrum (KMT Zentrum), Wiesbaden, Germany; CIC 339: AZ Stuivenberg, Lange Beeldekensstraat 267, Antwerp, Belgium; CIC 379: Ospedale Santa Maria Goretti, Ematologia, Latina, Italy; CIC 386: Bristol Royal Hospital for Children, Department of Paediatric Oncology/BMT, Bristol, United Kingdom; CIC 392: Ospedale V. Cervello, Division di Ematologia e Unità Trapianti, Palermo, Italy; CIC 525: Istituto per l'Infanzia Burlo Garofolo, Centro Trapianti Clinica Pediatrica, Trieste, Italy; CIC 528: St Anna Kinderspital, BMT Unit, Vienna, Austria; CIC 535: University Hospital, Department of Pediatrics, Tübingen, Germany; CIC 544: University Di Milano-Bicocca, Dipartimento Medicina Clinica Prevenzione, Monza, Italy; CIC 557: Istituti di Ricovero e Cura a Carattere Scientifico (IRCCS) Policlinico San Matteo, Pediatric Hematology-Oncology, Pavia, Italy; CIC 572: Chaim Sheba Medical Center, Department of Pediatric Hemato-Oncology, Tel-Hashomer, Israel; CIC 587: Azienda Ospedaliera, Centro Unico Regionale Trapianti, Reggio Calabria, Italy; CIC 622: Evangelismos Hospital, Division of Hematology, BMT Unit, Athens, Greece; CIC 628: Ege University Medical School, Department of Hematology, Bornova-Izmir, Turkey; CIC 636: Hospital de Santa Maria, BMT Unit, Lisboa, Portugal; CIC 649: Universita degli Studi di Bari, Cattedra e Servizio di Ematologia, Bari, Italy; CIC 651: Universitaetsklinikum, Klinik für Kinder-Onkologie, Hämatologie, Düsseldorf, Germany; CIC 656: Institute of Hematology and Blood Transfusion, Prague, Czech Republic; CIC 658: Ospedale Bergamo, Divisione di Ematologia, Bergamo, Italy; CIC 663: Hospital Universitario La Fe, Servicio de Hematologia, Valencia, Spain; CIC 710: RP Group Royal Perth Hospital, Department of Haematology, Perth, Australia; CIC 750: University of Jena, Department of Pediatrics, Jena, Germany; CIC 754: Chaim Sheba Medical Center, Tel-Hashomer, Israel; CIC 756: Universita di Roma Tor Vergata, Policlinico Universitario Tor Vergata, Divisione di Onco-Ematologia e Trapianto, Rome, Italy; CIC 786: Johannes-Gutenberg-University, 3rd Department of Medicine, Mainz, Germany; CIC 794: University of Perugia, Department of Hematology, Perugia, Italy; CIC 808: Universitaetsklinikum Dresden, Medizinische Klinik und Poliklinik I, Dresden, Germany; CIC 810: University of Freiburg, Department of Medicine-Hematology, Oncology, Freiburg, Germany; CIC 813: Istituto Scientifico H.S. Raffaele, Hematology and BMT, Milano, Italy; CIC 819: Hospital Gregorio Marañón, Sección de Trasplante de Medula Osea, Madrid, Spain.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal