Patients with multiple myeloma (MM) have an increased risk of deep venous thrombosis (DVT), particularly when treated with immunomodulatory drugs. Recently, 2 small hospital-based studies observed persons with the MM precursor condition, monoclonal gammopathy of undetermined significance (MGUS), to be at increased risk of developing DVT. Among 4 196 197 veterans hospitalized at least once at US Veterans Affairs hospitals, we identified a total of 2374 cases of MGUS, and 39 272 persons were diagnosed with DVT (crude incidence 0.9 per 1000 person-years). A total of 31 and 151 DVTs occurred among MGUS and MM patients, respectively (crude incidence 3.1 and 8.7 per 1000 person-years, respectively; P < .01). Compared with the entire study population, the relative risk (RR) of DVT after a diagnosis of MGUS and MM was 3.3 (95% confidence interval [CI], 2.3-4.7) and 9.2 (95% CI, 7.9-10.8), respectively. The most prominent excess risk of DVT was found during the first year after diagnosis of MGUS (RR = 8.4; 95% CI, 5.7-12.2) and MM (RR = 11.6; 95% CI, 9.2-14.5). Among 229 MGUS cases (9.5%) that progressed to MM, only one person had a DVT diagnosis before transformation. Our findings suggest the operation of shared underlying mechanisms causing coagulation abnormalities among patients with MGUS and MM.
Introduction
Venous thromboembolism is a frequent complication of malignant disease and is associated with substantial mortality.1,–3 Deep vein thrombosis (DVT) in the pelvic and deep veins of the lower extremities give rise to approximately 70% of all pulmonary embolisms, the potentially lethal complication of DVT.4 Reducing the incidence of DVT is therefore critical to decrease the rates of morbidity and mortality, particularly among high-risk groups such as persons with certain cancers.1
Compared with the general population, persons affected with multiple myeloma have been reported to be at higher risk of developing DVT.2,5,,,,,,,,,,,,–18 Furthermore, with the recent introduction of the antimyeloma therapy class called immunomodulatory drugs (IMiDs), a substantially increased risk of DVT has been observed among patients with multiple myeloma.2,3,5,,,,,,,,–14 The elevated risk of thromboembolic events associated with modern multiple myeloma therapy has stimulated multiple research groups to initiate efforts to uncover causative underpinnings of DVT in plasma cell disorders.15,,,–19
Interestingly, 2 small hospital-based studies have found an association between the multiple myeloma precursor condition monoclonal gammopathy of undetermined significance (MGUS) and subsequent risk of DVT.20,21 Although MGUS is a clinically benign and typically asymptomatic state, whereas multiple myeloma usually is associated with bone pain, infections, cytopenias, and renal failure, both MGUS and multiple myeloma have been found to share similar molecular abnormalities.3,22 Thus, if the observed elevated risk of DVT in MGUS cases is true, the underlying pathogenetic mechanisms causing DVT among patients with multiple myeloma might be detectable at a much earlier stage (ie, at MGUS diagnosis).23 This could be of potential clinical value in that it could help facilitate the development of more tailored DVT prophylaxis in patients with multiple myeloma receiving modern therapy. Further, there are data to suggest an association between hypercoagulation and progressive neoplastic activity,2,24,25 suggesting that DVT potentially could be a marker of multiple myeloma progression among persons with a diagnosis of MGUS.
We were motivated by prior investigations to conduct a large hospital-based study of DVT risk among persons with plasma cell disorders. Our investigation included more than 4 million adult male military veterans admitted to US Veterans Affairs (VA) hospitals with up to 17 years of follow-up. Aims of the study were to define the risk of DVT after MGUS and multiple myeloma, respectively, and to test whether MGUS cases with a subsequent DVT diagnosis have an elevated risk of developing multiple myeloma.
Methods
Hospitals and patients
Patients were selected from computerized discharge records for inpatient visits captured by the Patient Treatment File26 from October 1, 1980, to September 30, 1996, at US Veterans Affairs (VA) hospitals. The target population included all African American (N = 781 137) and white (N = 3 415 060) veterans hospitalized at least once, at age 18 or older. Other ethnic-racial groups were not included in this study because of small numbers. MGUS cases were defined as persons from the eligible population, with an International Classification of Diseases (ICD9-CM) discharge diagnosis of 273.1, and multiple myeloma cases were persons with an ICD-9-CM discharge diagnosis of 203 (Table 1). In accord with the International Myeloma Working Group criteria,27 we searched the database for MGUS cases with a preceding reported lymphoproliferative tumor diagnosis and set those subjects as not having MGUS. We used ICD-9-CM codes 451.1, 453.4, and 453.8 to assign persons as having a discharge diagnosis of DVT.
Characteristics of patients with multiple myeloma and MGUS, respectively, and veterans without a MGUS/multiple myeloma diagnosis
| Variable . | Multiple myeloma . | MGUS . | Veterans without a multiple myeloma/MGUS diagnosis . |
|---|---|---|---|
| Total no. of patients, n (%) | 6192 (100.0) | 2374 (100.0) | 4 187 631 (100.0) |
| Median age at entry, y (range)* | 67 (23-98) | 68 (30-99) | 56 (19-100) |
| African American race, n (%) | 1978 (32) | 834 (35) | 778 325 (19) |
| Patients with a DVT diagnosis, n (%)† | 151(2.4) | 31(1.3) | 39 272(0.9) |
| Median age at DVT, y (range) | 67 (34-88) | 73 (53-87) | 65 (21-100) |
| Incidence of DVT‡ | 8.7 | 3.1 | 0.9 |
| Variable . | Multiple myeloma . | MGUS . | Veterans without a multiple myeloma/MGUS diagnosis . |
|---|---|---|---|
| Total no. of patients, n (%) | 6192 (100.0) | 2374 (100.0) | 4 187 631 (100.0) |
| Median age at entry, y (range)* | 67 (23-98) | 68 (30-99) | 56 (19-100) |
| African American race, n (%) | 1978 (32) | 834 (35) | 778 325 (19) |
| Patients with a DVT diagnosis, n (%)† | 151(2.4) | 31(1.3) | 39 272(0.9) |
| Median age at DVT, y (range) | 67 (34-88) | 73 (53-87) | 65 (21-100) |
| Incidence of DVT‡ | 8.7 | 3.1 | 0.9 |
DVT indicates deep vein thrombosis; MGUS, monoclonal gammopathy of undetermined significance.
Multiple myeloma diagnosis date for patients with multiple myeloma, MGUS diagnosis date for patients with MGUS, and enrollment for the veterans without a multiple myeloma/MGUS diagnosis.
For patients with multiple myeloma and MGUS, only DVTs diagnosed after multiple myeloma/MGUS diagnosis were included.
Per 1000 person-years.
An exemption from the institutional review board was obtained from the National Institutes of Health Office of Human Subjects Research because we analyzed existing data without personal identifiers. Informed consent was waived because there was no contact with study subjects.
Statistical methods
Cumulative risk estimates were based on survival distribution estimates that were a function of estimated cumulative hazard functions. The confidence intervals used standard errors obtained using the delta method. Estimates of the overall cumulative risk of DVT used the entire VA cohort and took age as the time variable so entry was the age at the start of follow-up. For patients with multiple myeloma, the estimated cumulative risk was defined as the time from multiple myeloma diagnosis to DVT event, death, or the end of follow-up (September 30, 1996), whichever came first. For patients with MGUS, the estimated cumulative risk was defined as the time from MGUS diagnosis to DVT event, transformation to multiple myeloma, death, or the end of follow-up, whichever came first. For non-MGUS/multiple myeloma study subjects, follow-up began with first hospitalization in a VA hospital and for DVT cases, ended as an event with DVT. For those who were not DVT cases, follow-up ended as death or the end of the follow-up period whichever came first. Crude incidence was estimated simply as the number of events per person-year of follow-up, and crude incidences were compared with the use of a Poisson assumption. On the basis of an adjusted product limit estimate for survival without DVT, cumulative risk of DVT was estimated, starting at age 20 in the entire VA cohort (Figure 1).
Cumulative risk (and 95% confidence interval) for DVT in relation to age among 4 196 197 US veterans.
Cumulative risk (and 95% confidence interval) for DVT in relation to age among 4 196 197 US veterans.
We also fit Cox proportional hazards models to calculate relative risk (RR) and 95% confidence intervals (CIs) as measures of risk for DVT among MGUS and multiple myeloma patients (compared with the entire VA cohort). To test the adequacy of our models, we conducted both unadjusted as well as age-adjusted analyses. Because the models yielded similar estimates (data not shown), we present results from the unadjusted models only.
All P values and confidence intervals were 2-sided. P values less than .05 were considered statistically significant. All calculations were performed with SAS 9.1 (SAS Institute, Cary, NC).
Results
We identified a total of 2374 (0.06%) MGUS cases and 6192 (0.15%) patients with multiple myeloma. The proportion of African Americans among the MGUS and multiple myeloma cases was 35.1% and 31.9%, respectively (Table 1). Patients with MGUS and multiple myeloma were followed for up to 16.0 and 15.4 years, respectively, with a median of 3.0 and 1.6 years, respectively.
Incidence of DVT
The entire VA population of 4 196 197 men was hospitalized between October 1, 1980, and September 30, 1996 (a total of 41 720 791 person-years of follow-up), with 39 272 DVT diagnoses. This corresponded with a crude DVT incidence of 0.9 per 1000 person-years. There were 2374 MGUS cases with 31 DVT diagnoses during 9910 years of follow-up reflected in a crude DVT incidence of 3.1 per 1000 person-years. There were 6192 multiple myeloma cases with 151 DVT diagnoses during 17 393 years of follow-up (crude DVT incidence, 8.7 per 1000 person-years) which was significantly greater than that for the MGUS cases (P < .01; Table 1). The risk of DVT after MGUS and multiple myeloma was 3.3-fold (95% CI, 2.3-4.7) and 9.2-fold (95% CI, 7.9-10.8) increased, respectively.
Cumulative and relative risks of DVT
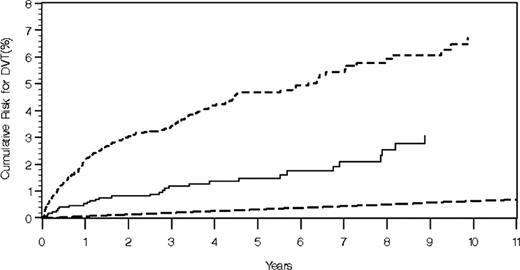
In the entire VA population the cumulative risk of developing DVT at ages 40, 60, and 80 years was 0.6% (95% CI, 0.5%-0.7%), 2.0% (95% CI, 1.9%-2.0%), and 4.7% (95% CI, 4.6%-4.8%), respectively (Figure 1). Among MGUS and multiple myeloma cases, the cumulative risk of developing DVT 4 years after diagnosis was 1.4% (95% CI, 0.8%-2.0%) and 4.2% (95% CI, 3.5%-4.9%), respectively (Figure 2). At 8 years of follow-up, the corresponding numbers were 2.5% (95% CI, 1.5%-3.6%) and 5.9% (95% CI, 4.9%-7.0%).
Cumulative risk of DVT among 2374 MGUS cases (solid line), 6192 patients with multiple myeloma (short dashed line), and 4 187 631 persons without a diagnosis of MGUS/multiple myeloma (long dashed line).
Cumulative risk of DVT among 2374 MGUS cases (solid line), 6192 patients with multiple myeloma (short dashed line), and 4 187 631 persons without a diagnosis of MGUS/multiple myeloma (long dashed line).
As shown in Figure 2, a very high DVT risk was observed 1 year after a diagnosis of multiple myeloma. After adjustment for age, compared with the entire VA cohort, the relative risk (RR) of DVT during the first year after diagnosis of MGUS (RR = 8.4; 95% CI, 5.7-12.2) and multiple myeloma (RR = 11.6; 95% CI, 9.2-14.5) was significantly elevated.
When we assessed the risk of DVT among white and African American patients with MGUS and multiple myeloma, respectively, the estimates were similar (data not shown).
MGUS, DVT, and subsequent multiple myeloma
Among persons with MGUS, there were 229 (9.5%) who progressed to multiple myeloma. Only one of them had a diagnosis of DVT before development of multiple myeloma.
Discussion
In this large study that included more than 4 million US veterans, compared with all other subjects in the database, we observed a 3-fold and 9-fold increased risk of DVT in 2374 MGUS and 6192 multiple myeloma cases, respectively. Among patients with multiple myeloma, the most prominent risk was observed during the first year after diagnosis. In contrast, among MGUS cases, we found a very stable increased risk of DVT over time compared with the entire VA population. Almost 10% of the MGUS cases progressed to multiple myeloma during the study period. Only one MGUS case had a diagnosis of DVT before multiple myeloma transformation. These results indicate an elevated risk of developing DVT among MGUS cases, and they are of importance for future studies designed to explore underlying molecular mechanisms and of potential effect for the development of upcoming DVT prophylaxis strategies for patients with multiple myeloma.
On the basis of more than 6000 patients with multiple myeloma, we observed the cumulative incidence of DVT to be approximately 6% at 8 years of follow-up. According to the literature, the incidence of DVT among patients with multiple myeloma varies greatly; however, available information is sparse, and prior investigations have typically been single-institution studies and often restricted by small sample size.2,3,5,,,,,,,,–14 The past few years, studies focusing on IMiD therapy and DVT risk in multiple myeloma have reported the cumulative incidence of DVT (with both single-agent IMiD therapy as well as combinations with dexamethasone and chemotherapy) to vary between approximately 2% and 75%, with greatest risk in previously untreated patients receiving combination therapy.7,14 An important consideration when interpreting our results is the fact that our study provides estimates of DVT incidence among patients with multiple myeloma in the pre-IMiD era. Thus, our study shows that patients with multiple myeloma who were treated in the pre-IMiD era had a 9-fold higher risk of DVT compared with the background population. The reason for the observed increased risk of DVT is not completely understood. Immobilization, surgery, infections, indwelling central venous catheters, acquired and inherited hypercoagulable states are known risk factors for DVT and have probably contributed to the excess risk.23,28,29 However, because the highest risk of DVT was observed in the first year after diagnosis, it seems reasonable to suggest that the hypercoagulable state, at least in part, could also reflect accelerated neoplastic activity and tumor burden, perhaps in combination with unknown influences caused by treatment.14
On the basis of 2374 MGUS cases, we found MGUS to be associated with a 3-fold increased risk of DVT. To our knowledge, only 2 previous smaller MGUS studies by Sallah et al20 (n = 310) and Srkalovic et al21 (n = 174) with less than 5 years of follow-up reported patients with MGUS to have an increased occurrence of DVT of approximately 6% to 7.5%. In our study, during the up to 17-year follow-up period, we found patients with MGUS to have a stable excess DVT risk over time. This pattern is different from what we found among patients with multiple myeloma. Indeed, we observed patients with multiple myeloma to have a highly increased risk of DVT in the first year after diagnosis and then it leveled off. Furthermore, to follow-up on the findings by Sallah et al20 who reported that patients with MGUS with DVT might be at increased risk of multiple myeloma progression, we assessed the relation between DVT and multiple myeloma among our MGUS cases. In contrast to their study, we found that only 1 of the 229 persons with MGUS who later transformed to multiple myeloma had a diagnosis of DVT preceding the development of malignant disease. However, because of the rarity of the conditions; it imposes severe limitations on statistical power; therefore, we cannot rule out an association.
Our study adds significantly to the restricted literature on DVT and plasma cell disorders by providing a quantitative measure of DVT risk among patients with MGUS and multiple myeloma using a large US patient population with a long follow-up. On the basis of the observed stable increase of DVT over time among patients with MGUS, in parallel with the lack of a statistical association between DVT and multiple myeloma transformation, we have speculated that the elevated risk of DVT among patients with MGUS is less likely caused by accelerating neoplastic activity, but rather manifestations because of ongoing clonal plasma cell activities as has been suggested by other authors.23 A recent hospital-based study assessed coagulation abnormalities in various types of plasma cell disorders, including MGUS, multiple myeloma, Waldenström macroglobulinemia, and systemic amyloidosis.23 Interestingly, factor VIII and von Willebrand factor levels were found to be increased among MGUS cases. Furthermore, in good accord with our findings, the increase in factor VIII and von Willebrand factor among patients with MGUS was similar to that of patients with untreated multiple myeloma.23 Future investigations are needed to clarify underlying mechanisms of our observations.
Given that DVT is a common public health problem and little is known about the epidemiology and the natural history of venous thromboembolism, we were also interested in defining the cumulative risk of DVT in US veterans, independent of underlying disease. On the basis of more than 4 million veterans, we defined the cumulative incidence of DVT to be 0.6%, 2.0%, and 4.7% at 40, 60, and 80 years of age, respectively (Figure 1). Our findings are in accord with a prior Swedish study based on 855 men who were followed-up prospectively from the age of 50 years to the age of 80 years.30 Although it has been suggested that changes in peripheral vein vascular biology may promote an increased risk of local thrombosis, thrombus growth and embolization, the underlying biologic mechanisms for the observed increase of DVT by age, remain largely unclear.31
The strengths of the current study include its large size in a patient population with relatively stable and standardized access to medical care that is provided to US veterans independent of socioeconomic status. In addition, study subjects were older than age 18 at enrollment and were followed for intervals as long as 16 years. We have included 2 different types of plasma cell disorder to further clarify the risks.
Limitations include the lack of demographic, clinical, and laboratory data for individual patients. The identification of the cohort from hospital discharge diagnoses is likely to have led to under-ascertainment of multiple myeloma, MGUS, and DVT. The results may therefore only apply to hospitalized patients with MGUS and multiple myeloma, thus decreasing the generalizability of our study. The study extends to 1996, ie, before low-molecular-weight heparins were in routine use in ambulatory treatment of DVT32 ; therefore, only a minority of DVT cases may have been missed in the few patients not admitted to hospital for treatment. The use of a retrospective cohort might potentially have resulted in under-ascertainment of DVT cases. We are not able to address the effect of the novel agents, mainly the IMiDs, on the risk of DVT in patients with multiple myeloma. The restriction to male sex might limit the generalizability of our results. The lack of independent validation of DVT diagnosis is another limitation in this study; however, the ascertainment is probably similar among multiple myeloma/MGUS cases and non–multiple myeloma/MGUS patients, so the chance of significant bias is reduced. In addition, we previously found more than 98% validity for cancer diagnoses in VA discharge records.33
In summary, compared with the background population, we found patients with multiple myeloma to have a 9-fold increased risk of developing DVT, with greatest risk during the first year after diagnosis. MGUS cases had stable 3-fold increased risk of DVT over time; however, we found no statistical association between DVT and risk of multiple myeloma transformation. Future studies should be directed toward resolving the underlying pathogenesis behind our findings as well as potential implications for DVT prophylaxis in the clinical setting.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank the Medical Administration Service of the Veterans Health Services and Research Administration, which provided the data on which this study is based, and Ms Heather Morris of Information Management Services (Silver Spring, MD) for computer programming support.
This work was supported by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, Division of Cancer Epidemiology and Genetics (DCEG) and by grants from the Swedish Cancer Society, Stockholm County Council, and Karolinska Institutet Foundations.
National Institutes of Health
Authorship
Contribution: G.G. obtained data; T.R.F. analyzed data; S.K. and O.L. initiated this work and wrote the report; and all authors were involved in the interpretation of the results, read, gave comments, and approved the final version of the manuscript. T.R.F. had full access to the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Ola Landgren, National Cancer Institute, National Institutes of Health, DCEG, GEB, Department of Health & Human Services, 6120 Executive Blvd, Bldg EPS/Rm 7110, Bethesda, MD 20892-7236; e-mail: landgreo@mail.nih.gov.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal