Abstract
Iron is tightly connected to oxygen homeostasis and erythropoiesis. Our aim was to better understand how hypoxia regulates iron acquisition for erythropoiesis in humans, a topic relevant to common hypoxia-related disorders. Forty-seven healthy volunteers participated in the HIGHCARE project. Blood samples were collected at sea level and after acute and chronic exposure to high altitude (3400-5400 m above sea level). We investigated the modifications in hematocrit, serum iron indices, erythropoietin, markers of erythropoietic activity, interleukin-6, and serum hepcidin. Hepcidin decreased within 40 hours after acute hypoxia exposure (P < .05) at 3400 m, reaching the lowest level at 5400 m (80% reduction). Erythropoietin significantly increased (P < .001) within 16 hours after hypoxia exposure followed by a marked erythropoietic response supported by the increased iron supply. Growth differentiation factor-15 progressively increased during the study period. Serum ferritin showed a very rapid decrease, suggesting the existence of hypoxia-dependent mechanism(s) regulating storage iron mobilization. The strong correlation between serum ferritin and hepcidin at each point during the study indicates that iron itself or the kinetics of iron use in response to hypoxia may signal hepcidin down-regulation. The combined and significant changes in other variables probably contribute to the suppression of hepcidin in this setting.
Introduction
Iron is an essential element required as a cofactor for proteins managing oxygen transport, such as hemoglobin, myoglobin, and cytochromes. Hypoxia, which occurs in a broad range of pathologic or environmental conditions, is primarily sensed in vertebrates by the hypoxia inducible family of transcription factors (hypoxia inducible factors), which activate or repress genes involved in adaptations to low oxygen pressure.1 In the erythropoietic compartment, which contains approximately 70% of the body iron, hypoxia stimulates erythropoiesis and promotes hemoglobin synthesis, which largely depends on iron availability.2-6 Accordingly, both hypoxia and anemia induce the synthesis of erythropoietin (EPO) and are the 2 main signals that increase iron absorption independently of iron stores.6-9 Thus, iron metabolism is tightly connected to oxygen homeostasis and erythropoiesis.
The liver peptide hepcidin, codified by HAMP, is the key regulator of iron homeostasis. It acts by inhibiting intestinal iron absorption and release by macrophages, and its production is regulated by different stimuli, including inflammatory cytokines,10 iron,11 erythropoietic activity,12-14 anemia, and hypoxia,15-17 mainly at the transcriptional level as described in recent reviews.18,19 Anemia could mediate hepcidin suppression through multiple mechanisms, including increased EPO or erythropoietic activity, increased iron demand, or liver hypoxia.3 The nature of the erythropoietic regulator of hepcidin is still uncharacterized but may include one or more proteins released during active erythropoiesis. One of these regulators, GDF15, a divergent member of the transforming growth factor-β superfamily, has been identified also as an oxygen-regulated transcript responding to hypoxia,20 suggesting that some homeostatic systems for iron and oxygen are responsive to both stimuli.
Previous studies reported a decrease of hepcidin expression in response to hypoxia17 ; however, the physiologic relevance and the mechanisms of hepcidin regulation by hypoxia are still uncertain and conflicting.12,15,16,21,22 There is limited information on the hypoxia-induced modifications of mediators of iron homeostasis in vivo in humans. High altitude induces several physiologic changes resulting from hypobaric hypoxia, and healthy subjects exposed to high altitude represent a convenient model to study these changes after acute or chronic exposure to hypoxia.4 The present study exploits a unique human resource: the availability of biologic material from normal volunteers participating in the HIGHCARE (HIGH altitude Cardiovascular Research) 2008 project on Mount Everest south slopes in Himalaya. We planned to investigate the modifications induced by acute and chronic exposure to hypobaric hypoxia on serum iron indices, EPO, markers of increased erythropoietic activity and inflammation, and hepcidin itself. Our aim was to better understand how hypoxia regulates iron acquisition for the erythropoietic response, a topic relevant to several common hypoxia-related human disorders, which include both acute conditions and chronic disorders of aging, as heart failure, sleep-related breathing disorders, chronic bronchitis, and anemia. To the best of our knowledge, this is the first study that examined in humans the temporal association between high altitude-induced changes in circulating hepcidin and serum iron indices, markers of increased erythropoietic demand, and known soluble regulators of iron homeostasis to obtain information on how hypoxia modulates in vivo the biologic response aimed to supply iron essential for erythropoiesis.
Methods
Participants
We enrolled 47 healthy volunteers (32 males and 15 females) in good general health, living permanently at less than 500 m above sea level. Exclusion criteria were: known cardiovascular disease, any chronic cardiovascular therapy, repeated prolonged exposures to altitudes more than 3000 m above sea level in the 8 months preceding the expedition, history of severe mountain sickness, history of angioedema, and pregnancy. Professional athletes were not included in the study. Subjects underwent a general health checkup, including exercise test and echocardiography before the expedition. All subjects gave their written informed consent to the study procedures. The study protocol was approved by the Ethics Committee of Istituto Auxologico Italiano, and the study was conducted in agreement with the Declaration of Helsinki.
Six volunteers (5 women and one man) were excluded from the study because they were iron-deficient resulting from menstrual blood loss (4 women) or blood donations (one man and one woman). They showed undetectable hepcidin levels at baseline and throughout the entire study.
Study design and procedures
Figure 1 summarizes the different steps of the study. The participants traveled by air from sea level (Milan, 140 m) to Kathmandu (Nepal, 1355 m) where they stayed for 3 days. Then they were brought by air transportation in a few hours time to Namche Bazaar (3400 m), where they stayed for another 3 days. From Namche Bazaar, they trekked over 5 days to Mt Everest south Base Camp (BC, 5400 m) where they remained for 11 days. They returned to sea level (Milan) over the next 6 days. During the study, all volunteers were on the same standard diet. Samples were collected at 5 time points: sea level baseline (SL1), during acute (days 1 and 2) exposure to high altitude (3400 m) at Namche Bazaar (Namche), during acute (days 10-12) and prolonged (days 19 and 20) exposure to very high (5400 m) altitude at Mt Everest Base Camp (BC1 and BC2, respectively), and again 3 to 4 months after returning to sea level (SL-return). To evaluate the different timing of EPO, hepcidin, and hepcidin regulator response to acute hypoxia stimulation, volunteers were divided into 2 subgroups, matched for age and sex, which were tested at 16 (first morning after arrival at Namche) and 40 hours (next morning) after acute exposure at 3400 m above the sea level. These 2 groups are classified as Namche group A (n = 20) and group B (n = 21), respectively.
Time course of the HIGHCARE project: altitude, locations, means of transportation between locations, days of ascent, staying and descent, and blood sampling.
Time course of the HIGHCARE project: altitude, locations, means of transportation between locations, days of ascent, staying and descent, and blood sampling.
Experiments in Namche Bazaar were performed in comfortable hotel rooms, whereas at BC they were performed in heated tents. Both in Namche Bazaar and in BC, laboratory temperature and barometric pressure were recorded by a microclimatic station. In the morning of the first and second day after arrival at Namche Bazaar as well as on days 10 to 12 and days 19 and 20 at BC, we measured several parameters, including heart rate, systemic blood pressure, respiratory rate, body temperature, and oxygen saturation. Blood samples were always obtained in the morning (between 7:00 am and 9.30 am) at fast, after the subject has been sitting for at least 15 minutes. Samples were immediately centrifuged and sera stored at −80°C at sea level, whereas during the expedition they were stored in 2-mL cryogenic tubes and frozen in nitrogen vapor containers (Cryoshippers MVE Biologic Systems) for the whole period until the return in Italy, when they were definitively stored at −80°C.
We investigated the modifications induced by acute and chronic exposure to hypobaric hypoxia on serum iron indices, EPO, soluble transferrin receptor, and GDF15 as markers of erythropoietic activation, interleukin-6 (IL-6) as a marker of inflammation, and hepcidin. Serum iron, transferrin, and ferritin were measured by standard methods. EPO was measured by Immulite EPO (Siemens Solutions Diagnostic Ltd), soluble transferrin receptor and GDF15 by Quantikine Human Immunoassay (R&D Systems). All these tests were performed at the laboratory of the Consortium for Human Molecular Genetics located in the University of Milano-Bicocca, Faculty of Medicine building, Monza, Italy. Serum hepcidin was measured by the immunoassay developed by Intrinsic LifeSciences LLC.23 Hematocrit was measured with a portable instrument (Radiometer ABL77).
Statistical analysis
Because of the skewed nature of the parameters under evaluation, data were log-transformed. Results of the descriptive analysis are reported in terms of geometric means with 95% confidence intervals and quartiles. We remark that at Namche, 2 volunteers, both belonging to Namche group A, showed a marked increase of serum hepcidin (from 5.3 to 171 ng/mL and 72 to 184 ng/mL, respectively), which paralleled marked IL-6 increments (from 49.1 to 267.54 pg/mL and from 16.4 to 107.5 pg/mL, respectively). These alterations were probably the result of an upper airway infection they developed in the first days after the arrival in Nepal. Accordingly, both hepcidin and IL-6 levels rapidly decreased at subsequent tests. This justified the exclusion of these values from all the longitudinal analyses, as explicitly indicated in “Results” and tables.
The evaluation of the changes in the log-measurements over altitude was done by fitting mean response profiles models,24 testing the effect of altitude, sex, and the interaction of altitude by sex. Results were reported for the final model, including only significant effects (cut-point for significance = .01). An unstructured form of the variance-covariance matrix was used as it minimized Akaike information criterion, compared with a set of competing forms (ie, compound symmetry, heterogeneous autoregressive, exponential). When a significant overall effect of altitude was detected, contrasts between baseline (SL1) and the subsequent measurements (Namche, BC1 and BC2), and between the mean values at Namche and at BC1 were evaluated. Moreover, in case of a significant interaction term, the comparisons were performed separately for males and females. The same modeling approach was used for the analysis of changes in parameters after acute exposure to altitude at Namche as measured after 16 or 40 hours of exposure to hypoxia (groups A and B).
To evaluate the influence of regulators of hepcidin, a multivariate linear regression model was considered. Regressors were screened in a univariate analysis for each parameter on log-hepcidin at each altitude. The multivariate model included those parameters that were significant in at least 1 univariate analysis (P < .01) and were adjusted for age and sex.
Results
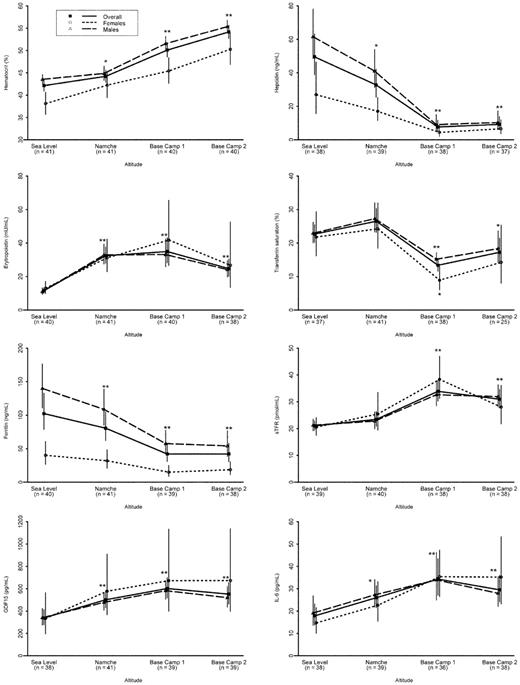
Table 1 shows volunteers' characteristics at baseline (SL1) divided according to sex. As expected, hematocrit, serum ferritin, and hepcidin were lower in women than in men. Means and medians of the parameters studied at SL1, Namche, BC1 and BC2, and SL-return are reported in Table 2. All the changes that occurred at high altitude had disappeared by the time of measurement at sea level 3 months after return. Figure 2 shows the time course of each parameter at baseline and during the period of hypoxic exposure with geometric means and 95% confidence intervals. We observed overall a significant increase of hematocrit from baseline (42.1%; 95% confidence interval, 40.9%-43.4%) to BC2 (54.1%; 95% confidence interval, 52.7%-55.6%). This increase was evident both in men (from 43.5% to 55.5%) and women (from 38.2% to 50.4%), but women always maintained significantly lower values than men. We observed a significant increase of hematocrit at Namche, but this was probably because of the rapid decrease of plasma volume associated with acute exposure to hypoxia25 not adequately compensated by increased water assumption. This is supported by the fact that the increase was found in Namche group A (41.7% at baseline and 45.6% after 16 hours of high altitude exposure), but not in volunteers belonging to Namche group B (42.5% at baseline and 42.9% after 40 hours of altitude exposure; Table 3).
Baseline characteristics at sea level (SL1) by sex
| Variable . | Females . | Males . | ||||||
|---|---|---|---|---|---|---|---|---|
| n . | Mean . | Median . | 1st-3rd quartile . | n . | Mean . | Median . | 1st-3rd quartile . | |
| Age, y | 10 | 35.5 | 33.0 | 26.0-40.0 | 31 | 41.1 | 40.0 | 33.0-48.0 |
| Weight, kg | 10 | 56.6 | 55.5 | 52.0-57.0 | 31 | 74.3 | 74.0 | 68.0-80.0 |
| BMI, kg/m2 | 10 | 20.9 | 20.3 | 19.7-21.0 | 31 | 23.6 | 23.5 | 21.8-25.3 |
| Hematocrit, % | 10 | 38.1 | 38.4 | 34.0-40.9 | 31 | 43.5 | 43.8 | 41.5-45.3 |
| Hepcidin, ng/mL | 10 | 27.0 | 22.8 | 15.3-35.1 | 30 | 57.0 | 67.0 | 40.3-93.5 |
| Erythropoietin, mU/mL | 10 | 13.0 | 11.9 | 9.4-17.8 | 30 | 11.1 | 10.7 | 8.8-13.7 |
| Serum iron, μg/dL | 9 | 75.5 | 83.0 | 67.0-87.0 | 30 | 75.9 | 73.5 | 63.0-95.0 |
| Transferrin, mg/dL | 8 | 262.5 | 245.0 | 221.5-311.5 | 29 | 237.8 | 242.0 | 218.0-259.0 |
| Transferrin saturation, % | 8 | 21.8 | 23.0 | 15.9-28.4 | 29 | 22.9 | 22.4 | 18.7-29.0 |
| Ferritin, ng/mL | 10 | 40.1 | 34.5 | 31.0-69.0 | 30 | 139.9 | 139.0 | 104.0-209.0 |
| Soluble transferrin receptor, pmol/mL | 10 | 20.5 | 19.2 | 17.4-23.4 | 29 | 21.2 | 21.7 | 17.2-24.0 |
| GDF15, pg/mL | 10 | 332.9 | 368.4 | 187.3-444.7 | 28 | 340.8 | 386.8 | 223.4-494.2 |
| IL-6, pg/mL | 10 | 14.7 | 16.2 | 9.2-18.9 | 30 | 19.7 | 16.8 | 12.8-29.0 |
| Variable . | Females . | Males . | ||||||
|---|---|---|---|---|---|---|---|---|
| n . | Mean . | Median . | 1st-3rd quartile . | n . | Mean . | Median . | 1st-3rd quartile . | |
| Age, y | 10 | 35.5 | 33.0 | 26.0-40.0 | 31 | 41.1 | 40.0 | 33.0-48.0 |
| Weight, kg | 10 | 56.6 | 55.5 | 52.0-57.0 | 31 | 74.3 | 74.0 | 68.0-80.0 |
| BMI, kg/m2 | 10 | 20.9 | 20.3 | 19.7-21.0 | 31 | 23.6 | 23.5 | 21.8-25.3 |
| Hematocrit, % | 10 | 38.1 | 38.4 | 34.0-40.9 | 31 | 43.5 | 43.8 | 41.5-45.3 |
| Hepcidin, ng/mL | 10 | 27.0 | 22.8 | 15.3-35.1 | 30 | 57.0 | 67.0 | 40.3-93.5 |
| Erythropoietin, mU/mL | 10 | 13.0 | 11.9 | 9.4-17.8 | 30 | 11.1 | 10.7 | 8.8-13.7 |
| Serum iron, μg/dL | 9 | 75.5 | 83.0 | 67.0-87.0 | 30 | 75.9 | 73.5 | 63.0-95.0 |
| Transferrin, mg/dL | 8 | 262.5 | 245.0 | 221.5-311.5 | 29 | 237.8 | 242.0 | 218.0-259.0 |
| Transferrin saturation, % | 8 | 21.8 | 23.0 | 15.9-28.4 | 29 | 22.9 | 22.4 | 18.7-29.0 |
| Ferritin, ng/mL | 10 | 40.1 | 34.5 | 31.0-69.0 | 30 | 139.9 | 139.0 | 104.0-209.0 |
| Soluble transferrin receptor, pmol/mL | 10 | 20.5 | 19.2 | 17.4-23.4 | 29 | 21.2 | 21.7 | 17.2-24.0 |
| GDF15, pg/mL | 10 | 332.9 | 368.4 | 187.3-444.7 | 28 | 340.8 | 386.8 | 223.4-494.2 |
| IL-6, pg/mL | 10 | 14.7 | 16.2 | 9.2-18.9 | 30 | 19.7 | 16.8 | 12.8-29.0 |
Geometric mean values are given for mean for all parameters except age, weight, and BMI.
Overall description of the relevant parameters as measured at different altitudes
| Variable . | SL1 (140 m), month −1 . | Namche (3400 m), days 1 and 2 . | BC1 (5400 m), days 10-12 . | BC2 (5400 m), days 19 and 20 . | SL-return (140 m), months 3 and 4 . | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n . | Mean . | Median . | n . | Mean . | Median . | n . | Mean . | Median . | n . | Mean . | Median . | n . | Mean . | Median . | |
| Hematocrit, % | 41 | 42.1 | 43.0 | 41 | 44.2 | 43.0 | 40 | 50.1 | 50.5 | 40 | 54.1 | 55.0 | 35 | 42.1 | 41.6 |
| Hepcidin,* ng/mL | 38 | 49.4 | 60.7 | 39 | 32.7 | 35.9 | 38 | 7.6 | 5.1 | 37 | 9.2 | 8.4 | 29 | 42.9 | 53.4 |
| Erythropoietin, mU/mL | 40 | 11.5 | 11.0 | 41 | 32.4 | 34.2 | 40 | 34.9 | 38.0 | 38 | 24.7 | 25.7 | 35 | 13.2 | 12.2 |
| Serum iron, μg/dL | 39 | 75.8 | 74.0 | 41 | 102.8 | 106.0 | 38 | 55.9 | 58.0 | 26 | 78.2 | 83.5 | 32 | 92.8 | 925.0 |
| Transferrin, mg/dL | 37 | 242.9 | 242.0 | 41 | 273.0 | 274.0 | 38 | 294.5 | 292.0 | 25 | 315.3 | 318.0 | 32 | 267.7 | 261.5 |
| Transferrin saturation, % | 37 | 22.7 | 22.4 | 41 | 26.5 | 27.7 | 38 | 13.4 | 13.9 | 25 | 17.2 | 17.9 | 35 | 24.3 | 23.6 |
| Ferritin, ng/mL | 40 | 102.4 | 115.0 | 41 | 80.6 | 88.0 | 39 | 42.2 | 42.0 | 38 | 42.1 | 41.5 | 31 | 91.8 | 97.0 |
| Soluble transferrin receptor, pmol/mL | 39 | 21.1 | 21.3 | 40 | 23.4 | 22.7 | 38 | 33.9 | 32.7 | 38 | 31.0 | 29.6 | 32 | 18.4 | 18.0 |
| GDF15, pg/mL | 38 | 338.7 | 386.8 | 41 | 500.7 | 488.6 | 39 | 601.6 | 588.3 | 39 | 551.6 | 577.4 | 28 | 383.8 | 401.8 |
| IL-6,* pg/mL | 38 | 17.9 | 16.2 | 39 | 25.8 | 26.7 | 36 | 34.3 | 31.7 | 38 | 29.5 | 28.4 | 28 | 19.0 | 17.2 |
| Variable . | SL1 (140 m), month −1 . | Namche (3400 m), days 1 and 2 . | BC1 (5400 m), days 10-12 . | BC2 (5400 m), days 19 and 20 . | SL-return (140 m), months 3 and 4 . | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n . | Mean . | Median . | n . | Mean . | Median . | n . | Mean . | Median . | n . | Mean . | Median . | n . | Mean . | Median . | |
| Hematocrit, % | 41 | 42.1 | 43.0 | 41 | 44.2 | 43.0 | 40 | 50.1 | 50.5 | 40 | 54.1 | 55.0 | 35 | 42.1 | 41.6 |
| Hepcidin,* ng/mL | 38 | 49.4 | 60.7 | 39 | 32.7 | 35.9 | 38 | 7.6 | 5.1 | 37 | 9.2 | 8.4 | 29 | 42.9 | 53.4 |
| Erythropoietin, mU/mL | 40 | 11.5 | 11.0 | 41 | 32.4 | 34.2 | 40 | 34.9 | 38.0 | 38 | 24.7 | 25.7 | 35 | 13.2 | 12.2 |
| Serum iron, μg/dL | 39 | 75.8 | 74.0 | 41 | 102.8 | 106.0 | 38 | 55.9 | 58.0 | 26 | 78.2 | 83.5 | 32 | 92.8 | 925.0 |
| Transferrin, mg/dL | 37 | 242.9 | 242.0 | 41 | 273.0 | 274.0 | 38 | 294.5 | 292.0 | 25 | 315.3 | 318.0 | 32 | 267.7 | 261.5 |
| Transferrin saturation, % | 37 | 22.7 | 22.4 | 41 | 26.5 | 27.7 | 38 | 13.4 | 13.9 | 25 | 17.2 | 17.9 | 35 | 24.3 | 23.6 |
| Ferritin, ng/mL | 40 | 102.4 | 115.0 | 41 | 80.6 | 88.0 | 39 | 42.2 | 42.0 | 38 | 42.1 | 41.5 | 31 | 91.8 | 97.0 |
| Soluble transferrin receptor, pmol/mL | 39 | 21.1 | 21.3 | 40 | 23.4 | 22.7 | 38 | 33.9 | 32.7 | 38 | 31.0 | 29.6 | 32 | 18.4 | 18.0 |
| GDF15, pg/mL | 38 | 338.7 | 386.8 | 41 | 500.7 | 488.6 | 39 | 601.6 | 588.3 | 39 | 551.6 | 577.4 | 28 | 383.8 | 401.8 |
| IL-6,* pg/mL | 38 | 17.9 | 16.2 | 39 | 25.8 | 26.7 | 36 | 34.3 | 31.7 | 38 | 29.5 | 28.4 | 28 | 19.0 | 17.2 |
Geometric mean values are given for mean.
Two subjects were excluded from the analysis of hepcidin and IL-6 because of upper airway infection.
Longitudinal profiles of the relevant parameters at different altitudes. Data are expressed in terms of geometric means and 95% confidence intervals (vertical lines), by sex, and overall. Significant comparison with baseline: **P < .0001; *P < .006. Two subjects were excluded from the analysis of hepcidin and IL-6 because of upper airway infection.
Longitudinal profiles of the relevant parameters at different altitudes. Data are expressed in terms of geometric means and 95% confidence intervals (vertical lines), by sex, and overall. Significant comparison with baseline: **P < .0001; *P < .006. Two subjects were excluded from the analysis of hepcidin and IL-6 because of upper airway infection.
Effect of acute hypoxia (Namche) on the relevant parameters by group A and group B: 16 and 40 hours of exposure to altitude, respectively
| Variable . | Group A . | Group B . | ||||||
|---|---|---|---|---|---|---|---|---|
| n . | Sea level, mean . | Namche, mean . | P . | n . | Sea level, mean . | Namche, mean . | P . | |
| Hematocrit, % | 20 | 41.7 | 45.6 | < .0001 | 21 | 42.5 | 43.0 | .5826 |
| Hepcidin,* ng/mL | 18 | 50.4 | 38.5 | .0869 | 21 | 48.9 | 28.5 | .0003 |
| Erythropoietin, mU/mL | 20 | 11.8 | 35.5 | < .0001 | 20 | 11.3 | 30.0 | < .0001 |
| Serum iron, μg/dL | 19 | 80.6 | 89.1 | .3014 | 20 | 70.8 | 116.7 | < .0001 |
| Transferrin, mg/dL | 18 | 244.7 | 275.9 | .0007 | 19 | 239.8 | 270.4 | .0004 |
| Transferrin saturation, % | 18 | 24.1 | 22.9 | .6515 | 19 | 21.3 | 30.6 | .0002 |
| Ferritin, ng/mL | 19 | 116.8 | 84.8 | < .0001 | 21 | 90.9 | 76.7 | .0035 |
| Soluble transferrin receptor, pmol/mL | 19 | 20.3 | 23.3 | .1777 | 20 | 21.8 | 23.6 | .4084 |
| GDF15, pg/mL | 19 | 361.4 | 528.5 | < .0001 | 19 | 317.4 | 473.4 | < .0001 |
| IL-6,* pg/mL | 18 | 20.2 | 25.2 | .2107 | 21 | 16.0 | 25.6 | .0035 |
| Variable . | Group A . | Group B . | ||||||
|---|---|---|---|---|---|---|---|---|
| n . | Sea level, mean . | Namche, mean . | P . | n . | Sea level, mean . | Namche, mean . | P . | |
| Hematocrit, % | 20 | 41.7 | 45.6 | < .0001 | 21 | 42.5 | 43.0 | .5826 |
| Hepcidin,* ng/mL | 18 | 50.4 | 38.5 | .0869 | 21 | 48.9 | 28.5 | .0003 |
| Erythropoietin, mU/mL | 20 | 11.8 | 35.5 | < .0001 | 20 | 11.3 | 30.0 | < .0001 |
| Serum iron, μg/dL | 19 | 80.6 | 89.1 | .3014 | 20 | 70.8 | 116.7 | < .0001 |
| Transferrin, mg/dL | 18 | 244.7 | 275.9 | .0007 | 19 | 239.8 | 270.4 | .0004 |
| Transferrin saturation, % | 18 | 24.1 | 22.9 | .6515 | 19 | 21.3 | 30.6 | .0002 |
| Ferritin, ng/mL | 19 | 116.8 | 84.8 | < .0001 | 21 | 90.9 | 76.7 | .0035 |
| Soluble transferrin receptor, pmol/mL | 19 | 20.3 | 23.3 | .1777 | 20 | 21.8 | 23.6 | .4084 |
| GDF15, pg/mL | 19 | 361.4 | 528.5 | < .0001 | 19 | 317.4 | 473.4 | < .0001 |
| IL-6,* pg/mL | 18 | 20.2 | 25.2 | .2107 | 21 | 16.0 | 25.6 | .0035 |
Geometric mean values are given for mean.
Two subjects in group A were excluded from the analysis of hepcidin and IL-6 because of upper airway infection.
As shown in Figure 2 and Table 2, hepcidin levels significantly decreased after acute exposure to hypoxia (Namche), reaching the nadir at BC1 and BC2, when its levels were reduced by more than 80%. Although males and females started from different baseline values, their decreasing profile did not differ significantly according to the longitudinal analysis (supplemental Table 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). EPO rapidly increased at Namche, maintaining a plateau at BC1 and beginning to decrease at BC2. Transferrin saturation augmented at Namche exclusively resulting from transient serum iron increase because serum transferrin showed a rapid and gradual increase probably related to iron depletion. It significantly decreased at BC1 because of the increased iron use by the bone marrow and remained fairly similar at BC2. The decrease of transferrin saturation was more evident for females than males (with a significant interaction term in the longitudinal model; supplemental Table 1). Serum ferritin showed a rapid and progressive decrease that was already evident at Namche, indicating massive mobilization of iron from stores. This progressive decrease was similar in males and females so that a significant difference by sex was maintained in the longitudinal profile. The 3 remaining parameters did not behave differently by sex but were affected by altitude. Specifically, soluble transferrin receptor showed a marked increase from Namche to BC1 according to erythropoietic expansion, whereas GDF15 showed a progressive, but slight, increase from SL1 to Namche and BC1, both maintaining a plateau at BC2. IL-6 levels increased at Namche and remained elevated at BC1 and BC2. As shown by the results of the model on longitudinal profiles (supplemental Table 1), changes from acute (Namche) versus chronic (BC1) hypoxia were significant for all parameters, except for EPO, which showed the major increase at Namche.
To better evaluate the time course of each parameter in response to acute hypoxia, we examined the results at 16 (Namche group A) and 40 (Namche group B) hours after acute hypoxia exposure (Table 3). Groups A and B had comparable baseline values for all parameters (P > .05). EPO, GDF15, transferrin, and ferritin significantly changed in both Namche group A and group B. After 40 hours, serum iron significantly augmented, causing transferrin saturation increase. Hepcidin did decrease after 16 hours but not significantly so (P = .0869), whereas it significantly decreased after 40 hours of exposure. Accordingly, only 9 of the 18 volunteers (50%) of group A showed a reduction of hepcidin levels compared with 17 of 21 (81%) in group B. Changes in IL-6 were more marked after a longer exposure, whereas serum transferrin receptor did not show significant modifications in both groups
Trying to evaluate the relations between hepcidin and its possible regulators (erythropoietic activity, iron stores, cytokine activation), we performed univariate and multivariate analyses at different times of exposure to hypoxia. Results of univariate analyses were used to screen for relevant factors to be included in the multivariate analysis. In the univariate analysis (data not shown), serum ferritin correlated with serum hepcidin at each point, whereas EPO inversely correlated with hepcidin only at BC1. Results of the multivariate model at different altitudes are reported in supplemental Table 2. Serum ferritin seems to be the only independent predictor for hepcidin: a 10% decrease in serum ferritin is estimated to be related to a decrease in hepcidin of 6.5%, 7.3%, 7.6%, and 8.2% at SL1, Namche, BC1, and BC2, respectively.
Discussion
During the 20-day period of high altitude exposure, we observed a marked increase of hematocrit, which required approximately 700 mg iron in men and 400 mg in women (based on approximate calculation of hemoglobin increase, hemoglobin iron content, and subjects' weight and blood volume26 ). To compensate for this massive demand of iron by erythropoietic precursors, iron release from stores and intestinal absorption had to increase dramatically. Our results strongly suggest that hepcidin down-regulation was central to these modifications.
Hepcidin suppression began some hours after acute hypoxia exposure, reaching its nadir in the following week of exposure to high altitude. The timing of hepcidin suppression we observed is similar to that recently reported in healthy subjects who received EPO injections, starting 24 hours after EPO administration.27,28 The delay and progression in hepcidin down-regulation contrast with the hypothesis that hypoxia might directly be involved in hepcidin suppression and suggest that other factors are involved. Hypoxia generates a detectable increase in serum EPO within 90 minutes,9 which peaks within 2 days and thereafter declines over a period of 1 to 2 weeks in volunteers exposed to high altitude.6 Accordingly, we observed a rapid and marked increase of EPO, which was maintained at BC1 and began to decline at BC2. It has been suggested that hypoxia could act on hepcidin down-regulation via EPO signaling,27,29 but animal studies indicated that the decrease of hepcidin expression by experimentally induced anemia was dependent on erythropoiesis and not directly mediated by EPO, anemia, or tissue hypoxia.12,14 The present study could not clarify this issue, but considering that hepcidin is a fast-responding hormone and that the timing of hepcidin suppression was gradual, we suggest that it could be mediated by circulating factor(s) released by cells in response to hypoxia-induced erythropoietic activation. Recent observations have indicated that GDF15 and twisted gastrulation 1 could be erythropoietic regulators of hepcidin,13,30 possibly opposing the effect of BMPs.31 GDF15 is expressed during erythroblast maturation mainly in more mature, hemoglobinized erythroblasts,32 whereas twisted gastrulation 1 is produced during the earlier stages of erythropoiesis.30 GDF15 is highly expressed in thalassemia,13 congenital diserythropoietic, and sideroblastic anemias,32,33 and less in other forms of ineffective erythropoiesis.13,32-34 In addition, GDF15 was up-regulated in response to iron depletion in in vitro and human studies,20 but to a smaller extent (≤ 2-fold). A similar magnitude of increase was observed in our volunteers who showed a progressive GDF15 increase, which peaks at BC2. This was probably the result of the combined action of erythropoiesis activation, iron depletion, but also of tissue hypoxia, inflammation, or enhanced oxidative stress induced by high altitude exposure.20,35 However, GDF15 levels were below the threshold that down-regulated HAMP mRNA in primary hepatocytes in vitro13 and did not show any correlation with hepcidin, suggesting that GDF15 could cooperate to hepcidin suppression but cannot be a main regulator in this setting.
Among all the parameters analyzed, serum ferritin was the one showing the best correlation with hepcidin at each point during the study, suggesting that iron itself, or the kinetics of iron use in response to hypoxia, may signal the suppression of hepcidin. At variance with that observed in patients with ineffective erythropoiesis in whom the hepcidin store regulator is overwhelmed by the erythroid signal,3 our results indicate that in healthy persons exposed to acute and chronic hypoxia, hepcidin production is modulated by the iron store regulator, even in the presence of increased erythropoietic demand.
Unexpectedly, we observed a significant decrease of serum ferritin concentration in the very early stages of hypoxia exposure (16 hours) when there was no evident increase of erythropoiesis and hepcidin was not suppressed yet. This finding contrasts with those induced by prolonged recombinant human EPO treatment in healthy subjects showing a later decrease of serum ferritin secondary to erythropoiesis expansion and increased iron demand.27,28 In animal models, stabilization of hypoxia inducible factor in hepatocytes increases ferroportin expression possibly because of a hypoxia inducible factor-dependent ferroportin mRNA up-regulation and/or to a posttranslational stabilization of ferroportin.16,36 Thus, we can hypothesize that acute hypoxia might induce a rapid mobilization of iron from storage cells to plasma to ensure enough iron to the mounting red blood cell hemoglobinization. The increase of serum iron and transferrin saturation observed at 40 hours might indicate that iron mobilization was even higher than bone marrow request in this phase. Further studies are needed to confirm and extend all these findings.
At BC1 and BC2, the increased rate of erythropoiesis was paralleled by the marked decrease of tissue iron stores and increased iron use by the erythropoietic bone marrow, as shown by the marked reduction of serum ferritin and transferrin saturation, which probably cooperated to further suppress hepcidin synthesis.18 A recent study suggested that, besides liver and spleen, skeletal muscle tissue can be another source of iron during high altitude-induced erythropoiesis.37 This finding differs from that observed in healthy volunteers treated with recombinant human EPO at sea level in whom skeletal muscle accumulated iron, despite systemic iron deficiency secondary to recombinant human EPO-induced erythropoiesis.27 It was hypothesized that a role of hypoxia in triggering muscle iron mobilization by increasing ferroportin mRNA and decreasing transferrin receptor mRNA expression.27 These findings further emphasize the difference between accelerated erythropoiesis induced by recombinant human EPO administration and hypoxia.
In agreement with previous studies,35 we found an early and stable increase of IL-6 concentrations, which remained below the range expected in inflammatory diseases. The mild systemic inflammation induced by exposure to high altitude, however, did not substantially counteract hypoxia-induced hepcidin suppression. Indeed, only the 2 volunteers who developed an upper respiratory infection at the very beginning of hypoxia exposure showed a marked increase of both IL-6 and hepcidin. The progressive decrease of hepcidin, despite the persistent slight increase of IL-6, and the absence of correlation between hepcidin and IL6 suggest that iron and erythroid signals prevailed on inflammation for hepcidin regulation in this setting as also shown in mouse models combining inflammation and erythropoiesis stimulation.38
In conclusion, our results provide new insights into the interaction between hypoxia, erythropoiesis, and iron metabolism in humans. In Figure 3, we summarized the physiologic modifications induced by acute and chronic hypoxia exposure on iron homeostasis aimed at supplying enough iron to the bone marrow, mainly based on the present findings. Hypoxia induced a marked suppression of hepcidin, which appeared to result from the combined action of hypoxia-induced increased erythropoiesis and iron depletion. Serum ferritin was a major determinant of serum hepcidin concentration, whereas the role of EPO and GDF15 seemed less important. This entails new efforts aimed to discover other erythroid regulator(s) of hepcidin expression. Last, the very early decrease of serum ferritin suggests the existence of hypoxia-dependent and hepcidin-independent mechanisms regulating storage iron mobilization. These results could have implications for hypoxia-related acute and chronic disorders, contributing to explain the role of hepcidin in the pathophysiology of hypoxia adaptation. They represent a first innovative step and should pave the way to future studies aimed at providing additional insights in this complex field and, hopefully, new markers of clinical utility.
Hypoxia-induced modifications of iron homeostasis. Erythropoietin (EPO) activation is the first event occurring after acute hypoxia exposure, inducing erythropoietic expansion, which requires more iron for red blood cell hemoglobinization. Hepcidin down-regulation is central for this function: it begins within 16 to 40 hours and peaks within a few days of exposure, and thereafter persists for a long time. Iron and erythroid signals cooperate in hepcidin suppression, increasing intestinal iron absorption and cellular iron release. Iron mobilization from stores occurs early, possibly involving a hypoxia-mediated event. Erythroid signal probably requires soluble mediators from bone marrow to the liver. Transferrin saturation (TS) decreased only in the phase of expansion of the erythropoietic mass contributing to hepcidin suppression.
Hypoxia-induced modifications of iron homeostasis. Erythropoietin (EPO) activation is the first event occurring after acute hypoxia exposure, inducing erythropoietic expansion, which requires more iron for red blood cell hemoglobinization. Hepcidin down-regulation is central for this function: it begins within 16 to 40 hours and peaks within a few days of exposure, and thereafter persists for a long time. Iron and erythroid signals cooperate in hepcidin suppression, increasing intestinal iron absorption and cellular iron release. Iron mobilization from stores occurs early, possibly involving a hypoxia-mediated event. Erythroid signal probably requires soluble mediators from bone marrow to the liver. Transferrin saturation (TS) decreased only in the phase of expansion of the erythropoietic mass contributing to hepcidin suppression.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Viviana Mauri from Centro Analisi Monza for technical support in measuring EPO, serum transferrin receptor, and serum iron indices, and Margherita Tamplenizza for technical support in collecting samples at high altitude.
The HIGHCARE project was made possible by an unrestricted research grant from Boehringer Ingelheim, Germany, and Banca Intesa San Paolo, Italy. This work was also supported by A. DeMari, CutAway srl, Sport Specialist, EuroTech, Moccagatta Pogliani & Associati, DiaTecne, FMS, GE Healthcare, InterCure, Microlife, Omron, Oridion, Pollution, Rotem, Sapio life, Seda SpA, SensorMedics, Spacelabs Healthcare, srLabs, Tecnoel srl, TensioMed, and Webbit srl companies, as well as the Cariplo Foundation (project 2009-2483) and Ministry of Instruction, University and Research (PRIN 2008N73CJ5-004; A.P.).
Authorship
Contribution: A.P. designed and performed research, analyzed data, and wrote the paper; S.G. performed the statistical analysis and wrote the paper; R.M. collected data and wrote the paper; S.P. and G.R. collected data and performed the analysis of serum parameters; C.L., G.B., M.R., A.G., A.F., and V.M. performed research and collected samples and data; M.W. and T.G. contributed to the design of the study, measured serum hepcidin, and reviewed data; M.G.V. reviewed statistical analysis and wrote the paper; G.M. reviewed the paper; and G.P. designed the HIGHCARE project, performed research, reviewed data, and reviewed the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
A complete list of the IGHCARE participants appears in the supplemental Appendix.
Correspondence: Alberto Piperno, University of Milano-Bicocca, Clinical Medicine, San Gerardo Hospital, Via Pergolesi 33, 20052, Monza, Italy; e-mail: alberto.piperno@unimib.it.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal