Abstract
Anemia and vitamin D deficiency are conditions that both result in significant morbidity and increase with age. The potential relationship between them remains poorly understood, particularly in the elderly. We used the Third National Health and Nutrition Examination Survey to examine the association of vitamin D deficiency with anemia subtypes in persons aged ≥ 60 years. Vitamin D deficiency was defined as serum levels < 20 ng/mL, and anemia was defined according to World Health Organization criteria. Vitamin D deficiency was associated with anemia prevalence independent of age, sex, or race/ethnicity (odds ratio, 1.47; 95% confidence interval, 1.06-2.05; P = .02) and varied significantly by anemia subtype (P overall = .003). The prevalence of vitamin D deficiency was 33.3% in the nonanemic population, 56% in anemia of inflammation (AI; P = .008), and 33.0% in unexplained anemia (P = .55). Non-Hispanic blacks had a 7-fold increased risk of AI compared with whites, and this was partially attenuated after adjusting for vitamin D deficiency. These data show that vitamin D deficiency is associated with specific subtypes of anemia in the elderly, especially in those with AI. Vitamin D may suppress inflammatory pathways, and studies to determine whether vitamin D supplementation ameliorates AI are warranted.
Introduction
Anemia of any degree is increasingly recognized as an independent contributor to morbidity and mortality in the elderly.1,2 The Third National Health and Nutrition Examination Survey (NHANES III) study found the incidence of anemia in men and women older than age 65 years to be 11% and 10%, respectively.3 The prevalence of anemia rose rapidly after the age of 50 years, approaching a rate > 20% in those persons aged ≥ 85 years. Population-based studies examining anemia in various cohorts of elderly patients have shown a consistent epidemiology for anemia, with one-third of patients found to have nutritional deficiency, one-third with anemia of inflammation (AI) that was diagnosed on the basis of iron studies, and one-third with “unexplained” anemia.3,4 AI has historically been termed the “anemia of chronic disease” and is characterized biochemically by low serum iron and low iron binding capacity in the setting of an elevated serum ferritin. The antimicrobial peptide hepcidin has been implicated in the pathophysiology of AI. However, mechanistic insight into the cause of unexplained anemia is sorely lacking. Although myelodysplasia and other uncommon causes of anemia may explain a portion of those with unexplained anemia, their combined contribution is felt to be relatively low. The effect of vitamin and mineral deficiencies, beyond iron, vitamin B12, and folate deficiencies, or the potential effect of subclinical renal disease on the development of unexplained anemia remains poorly studied.
A growing body of evidence has advanced our understanding of potentially beneficial effects of vitamin D, and vitamin D supplementation among the population is becoming more commonplace in general clinical practice. It is estimated that greater than one-half of US middle-aged and older women and greater than one-third of similarly aged men have vitamin D insufficiency.5,6 Given the aging population, low vitamin D status is an increasingly important public health issue. Although data from laboratory studies, epidemiologic investigations, and small clinical trials suggest a protective effect for vitamin D against cancer7,8 and risk of cardiovascular disease,9,10 little is known about the effect of vitamin D deficiency on the development of anemia or on other aspects of hematopoietic cell function. A cross-sectional analysis of 554 patients in the Kaiser-Permanente Health System, 193 of whom had normal renal function, showed an increased prevalence of vitamin D deficiency associated with anemia as defined by a hemoglobin level < 11 g/dL.11 Lower 25-hydroxyvitamin D [25(OH)D] and higher C-reactive protein levels have been associated with lower hemoglobin concentrations in patients with chronic renal disease not requiring hemodialysis.12 Limited data have been presented suggesting that vitamin D supplementation in 25(OH)D-deficient patients receiving chronic hemodialysis may reduce the need for erythropoietin therapy in a subset of these patients.13 Another small study examining the effect of calcitriol on erythropoiesis in 33 patients with chronic uremia suggested that calcitriol promoted increased erythroid colony formation.14 However, these 2 small, preliminary studies are inconclusive and leave open the question of whether vitamin D has an effect on erythropoiesis. Therefore, we undertook to study the relationship between vitamin D status and particular subtypes of anemia commonly seen in elderly subjects.
In the present study, we used data available in the NHANES III and NHANES 2001-2006 surveys to examine the association of vitamin D deficiency with anemia subtypes in noninstitutionalized persons ≥ 60 years in the United States, with particular focus on vitamin D status in elderly subjects with either AI or unexplained anemia. Our data show that vitamin D deficiency is associated with anemia in this cohort of older persons and that among those with anemia, vitamin D deficiency is particularly associated with classic AI.
Methods
Data source
Data for the analysis of the association between vitamin D deficiency and all-cause anemia are from phases 1 and 2 of NHANES III (1988-1994) and from NHANES 2001-2006. Briefly, each NHANES survey was designed as a separate national probability sampling to assess the health and nutritional status of the noninstitutionalized population in the United States. Subjects were chosen with the use of a stratified, multistage sample design used to produce a nationally representative sample with no upper age limit. The survey included a home questionnaire, a standardized physical examination performed in mobile examination centers, and laboratory tests. NHANES was approved by the National Center for Health Statistics Institutional Review Board, and all participants gave their informed consent in accordance with the Declaration of Helsinki.
In phases 1 and 2 of NHANES III and for NHANES 2001-2006, both hemoglobin and vitamin D levels were determined for 5100 and 4575 persons aged ≥ 60 years, respectively. Data for the analysis of the association between vitamin D deficiency and anemia subtypes were limited to phase 2 of NHANES III (1991-1994) and NHANES 2001-2002 because only these years contained the full complement of laboratory tests necessary for this analysis. In phase 2 of NHANES III 2610 persons aged ≥ 60 years had complete data for anemia subtype analysis, whereas 1493 participants aged ≥ 60 years in NHANES 2001-2002 had complete data.
Laboratory variables
Detailed descriptions of laboratory methods used in NHANES III are available15 and are summarized here. Hemoglobin, mean corpuscular volume, red cell distribution width, white blood cell count, and platelet count were determined with a Coulter Counter Model S-Plus JR (Coulter Electronics). Measurements of serum ferritin were performed with the Quantimmune Ferritin IRMA kit (Bio-Rad Laboratories), whereas serum iron and iron-binding capacity were performed colorimetrically (Alpkem RFA Analyzer). Free erythrocyte protoporphyrin was measured with fluorescence extraction.16 Serum folate and vitamin B12 were measured with the Quantaphase Folate radioassay kit (Bio-Rad Laboratories). RBC folate was calculated on the basis of measured whole blood folate and serum folate.15
Serum creatinine was measured by the Jaffe reaction with the use of a Hitachi model 737 multichannel analyzer (Boehringer Mannheim Diagnostics). Glomerular filtration rate was estimated from serum creatinine with the use of the Modification of Diet in Renal Disease Study equation, and chronic kidney disease (CKD) was defined as a glomerular filtration rate < 60 mL/min.17,18 These assays were performed in NHANES 2001-2006 with the use of similar methods.
Serum 25(OH)D levels were performed in both NHANES surveys with the use of a radioimmunoassay (RIA) kit (DiaSorin). The RIA kit was reformulated between the 2 time periods. On average, 25(OH)D values from the reformulated RIA used in NHANES 2000-2004 were 12% lower than the RIA used in NHANES III. Drifts in the performance of the serum 25(OH)D assay because of changes in reagent and calibrator lots over the period 2000-2006 were also observed. The National Center for Health Statistics recommends correcting NHANES III serum 25(OH)D values to allow for comparisons with the 2000-2006 values: NHANES III 25(OH)D2000-2004 RIA assay = (0.8429 × NHANES III 25(OH)D1988-1994 RIA assay) + 2.5762 nmol/L.19
Definitions of anemia, anemia subtypes, and vitamin D deficiency
Anemia was classified according to World Health Organization criteria with hemoglobin of < 13 g/dL in men and < 12 g/dL in women. Anemia subtypes were assigned in the following hierarchical order if persons met criteria as previously described: anemia because of nutritional deficiency, anemia of CKD, AI, and, if none of these were met, unexplained anemia.3 Transferrin saturation, calculated as the serum iron divided by the total iron-binding capacity, was deemed abnormal at values < 15%. Iron deficiency was identified if subjects met ≥ 2 of the following criteria: serum ferritin < 12 ng/mL, transferrin saturation < 15%, and erythrocyte protoporphyrin concentration > 1.24μM. Folate deficiency was defined as either a serum folate < 2.6 ng/mL or a RBC folate < 102.6 ng/mL.3 Vitamin B12 deficiency was defined by serum levels < 200 pg/mL.3 Subjects were defined as having chronic renal disease-associated anemia if their estimated creatinine clearance was < 60 mL/min. AI was defined as a low serum iron (< 60 μg/dL) in the absence of evident iron deficiency as defined in “Methods.” Anemic subjects who did not meet any of the above-mentioned diagnostic criteria were deemed to have unexplained anemia. Participants were deemed to be vitamin D deficient if they had vitamin D levels < 20 ng/mL.
Demographic variables
Self-reported race/ethnicity was classified as non-Hispanic white, non-Hispanic black, Mexican American, or other.
Statistical analysis
Descriptive statistics are reported as mean and standard error or percentage and standard error, unless otherwise noted. Logistic regression was used to examine the association of vitamin D deficiency with anemia with adjustment for age, sex, and race/ethnicity, and measures of associations are reported as odds ratios (ORs). Multinomial logistic regression was used to examine the association of vitamin D deficiency with anemia subtype, with measures of associations reported as relative risk ratios (RRRs). All analyses accounted for the stratified, multistage probability design, and sample weights were used to account for oversampling and nonresponse. In statistical comparisons of anemia subtypes, group sizes were too small to allow for the use of sample weights or account for the complex sampling methods; therefore, absolute values were used. Primary analyses were performed on NHANES III data, and key findings were confirmed in NHANES 2001-2006. Analyses of anemia subtypes were also performed on the combined NHANES III and NHANES 2001-2006 datasets to increase group sizes, and absolute values were used in the analysis of this combined dataset.
Results
Effect of vitamin D deficiency on anemia prevalence
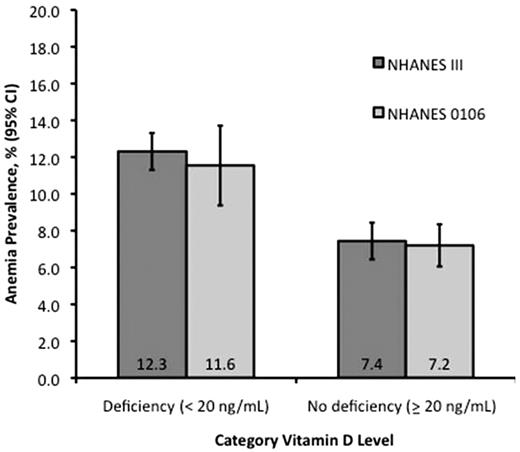
The characteristics of participants included in the analyses of vitamin D with anemia and anemia subtypes are shown in Table 1. In participants aged ≥ 60 years, the prevalence of vitamin D deficiency was 34.3% and 35.7% and of anemia was 9.1% and 8.7% in NHANES III and NHANES 2001-2006, respectively. In NHANES III, among 5100 participants aged ≥ 60 years, we found that the prevalence of anemia in subjects with vitamin D deficiency was 12.3% compared with 7.4% in persons with normal vitamin D levels (OR, 1.78; 95% confidence interval [CI], 1.34-2.39; P < .001; Figure 1). The association between anemia and vitamin D deficiency remained significant after adjustment for age, sex, and race/ethnicity (OR, 1.47; 95% CI, 1.06-2.05; P = .02). The association between anemia and vitamin D status was confirmed in the NHANES 2001-2006 survey, with the prevalence of anemia being 11.6% and 7.2% in persons with deficient or normal vitamin D levels, respectively (unadjusted OR, 1.56; 95% CI, 1.23-2.15; P < .001; adjusted OR, 1.43; 95% CI, 1.06-1.94; P = .02; Figure 1).
Demographic characteristics, hemoglobin and vitamin D levels, and prevalence of chronic conditions in persons ≥ 60 years: NHANES III and NHANES 2001-2006
| . | NHANES III* phases 1 and 2, 1988-1994 (n = 5100) . | NHANES III† phase 2 only 1991-1994 (n = 2610) . | NHANES 2001-2006 continuous* (N = 4575) . | NHANES 2001-2002 Continuous† (N = 1493) . |
|---|---|---|---|---|
| Age, y, mean (SE) | 70.6 (0.2) | 70.9 (0.4) | 71.0 (0.2) | 71.0 (0.3) |
| Female sex, % (SE) | 57.0 (0.7) | 57.4 (0.01) | 56.0 (0.8) | 56.9 (1.0) |
| Race/ethnicity, % (SE) | ||||
| Non-Hispanic white | 84.9 (1.3) | 83.3 (2.0) | 82.6 (1.6) | 82.2 (3.0) |
| Non-Hispanic black | 7.9 (0.7) | 7.8 (1.0) | 8.2 (0.9) | 7.7 (1.7) |
| Mexican American | 2.3 (0.2) | 2.4 (0.3) | 3.4 (0.7) | 3.1 (1.0) |
| Other | 4.9 (0.9) | 6.5 (1.5) | 5.9 (0.8) | 7.0 (2.4) |
| Anemia, % (SE) | 9.1 (0.5) | 8.3 (0.7) | 8.7 (0.5) | 7.9 (0.9) |
| Hemoglobin level, g/L, mean (SE) | 13.9 (0.03) | 14.0 (0.05) | 14.2 (0.04) | 14.2 (0.08) |
| Vitamin D deficiency, % (SE) | 34.3 (1.0) | 34.0 (1.6) | 35.7 (1.3) | 35.6 (2.5) |
| Vitamin D (ng/mL), mean (SE) | 24.3 (0.2) | 24.4 (0.2) | 22.8 (0.3) | 22.7 (0.5) |
| Chronic kidney disease, % (SE) | 22.4 (0.8) | 22.3 (1.3) | 29.6 (0.9) | 29.2 (1.7) |
| . | NHANES III* phases 1 and 2, 1988-1994 (n = 5100) . | NHANES III† phase 2 only 1991-1994 (n = 2610) . | NHANES 2001-2006 continuous* (N = 4575) . | NHANES 2001-2002 Continuous† (N = 1493) . |
|---|---|---|---|---|
| Age, y, mean (SE) | 70.6 (0.2) | 70.9 (0.4) | 71.0 (0.2) | 71.0 (0.3) |
| Female sex, % (SE) | 57.0 (0.7) | 57.4 (0.01) | 56.0 (0.8) | 56.9 (1.0) |
| Race/ethnicity, % (SE) | ||||
| Non-Hispanic white | 84.9 (1.3) | 83.3 (2.0) | 82.6 (1.6) | 82.2 (3.0) |
| Non-Hispanic black | 7.9 (0.7) | 7.8 (1.0) | 8.2 (0.9) | 7.7 (1.7) |
| Mexican American | 2.3 (0.2) | 2.4 (0.3) | 3.4 (0.7) | 3.1 (1.0) |
| Other | 4.9 (0.9) | 6.5 (1.5) | 5.9 (0.8) | 7.0 (2.4) |
| Anemia, % (SE) | 9.1 (0.5) | 8.3 (0.7) | 8.7 (0.5) | 7.9 (0.9) |
| Hemoglobin level, g/L, mean (SE) | 13.9 (0.03) | 14.0 (0.05) | 14.2 (0.04) | 14.2 (0.08) |
| Vitamin D deficiency, % (SE) | 34.3 (1.0) | 34.0 (1.6) | 35.7 (1.3) | 35.6 (2.5) |
| Vitamin D (ng/mL), mean (SE) | 24.3 (0.2) | 24.4 (0.2) | 22.8 (0.3) | 22.7 (0.5) |
| Chronic kidney disease, % (SE) | 22.4 (0.8) | 22.3 (1.3) | 29.6 (0.9) | 29.2 (1.7) |
Limited to participants with data available for both hemoglobin and vitamin D levels.
Limited to participants with hemoglobin, vitamin D, and all laboratory values needed for the definition of anemia subtypes (see “Methods”).
Prevalence of anemia categorized by vitamin D levels in persons ≥ 60 years in NHANES III and NHANES 2001-2006. NHANES III (n = 5100); NHANES 2001-2006 (n = 4575).
Prevalence of anemia categorized by vitamin D levels in persons ≥ 60 years in NHANES III and NHANES 2001-2006. NHANES III (n = 5100); NHANES 2001-2006 (n = 4575).
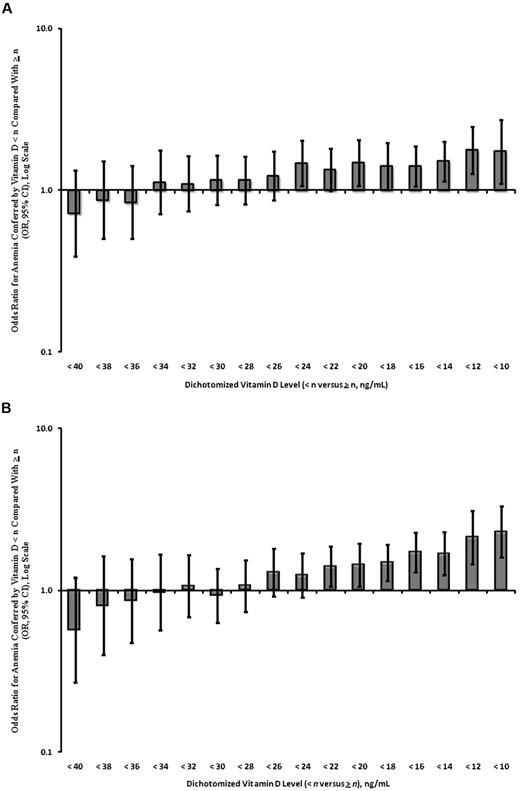
Vitamin D deficiency is classically defined as a 25(OH)D level < 20 ng/mL. Increasingly, however, 25(OH)D levels of between 20 and 30 ng/mL representing vitamin D insufficiency are thought to be relevant to the pathogenesis of various disease phenotypes. We therefore assessed the association between vitamin D insufficiency and anemia with the use of dichotomized vitamin D levels with adjustment for age, sex, and race/ethnicity. Our analysis of NHANES III showed a significant increased risk of anemia, beginning at 25(OH)D levels < 24 ng/mL (OR, 1.46; 95% CI, 1.06-2.02; Figure 2A). This analysis was confirmed in the NHANES 2001-2006 dataset, with a similar significant association noted at 25(OH)D levels < 22 ng/mL (OR, 1.40; 95% CI, 1.06-1.86; Figure 2B).
ORs for anemia by dichotomized vitamin D levels in persons ≥ 60 years adjusted for age, sex, and race/ethnicity. (A) NHANES III (n = 5100); (B) NHANES 2001-2006 (n = 4575).
ORs for anemia by dichotomized vitamin D levels in persons ≥ 60 years adjusted for age, sex, and race/ethnicity. (A) NHANES III (n = 5100); (B) NHANES 2001-2006 (n = 4575).
Association of vitamin D deficiency with anemia subtypes
Using NHANES III phase 2 data (n = 2610), we next examined the prevalence of vitamin D deficiency in anemia subtypes in men and women aged ≥ 60 years, with selected characteristics of this population shown in Table 2. The prevalence of vitamin D deficiency was 33.3% in the nonanemic population and was found to be higher in AI (56.0%; P = .008), anemia of nutrient deficiency (47.7%; P < .001), and anemia of CKD (37.1%; P = .04) but not in unexplained anemia (33.0%; P = .55; Table 2). The variation in vitamin D deficiency prevalence among anemia subtypes was statistically significant (P overall = .003). Vitamin D deficiency was associated with AI compared with unexplained anemia (RRR, 2.79; 95% CI, 1.22-6.34) even after adjustment for age, sex, and race/ethnicity (RRR, 2.47; 95% CI, 1.03-5.93; Table 3). Detailed analysis of persons with anemia of nutrient deficiency failed to show statistically significant differences in the prevalence of vitamin D deficiency among nutrient-deficient subgroups (data not shown).
Prevalence of vitamin D deficiency in anemia subtypes and in nonanemic persons ≥ 60 years: NHANES III, phase 2, 1991-1994
| . | Nonanemic . | Anemia subtypes . | P, comparison among anemia subtypes* . | |||
|---|---|---|---|---|---|---|
| Anemia of chronic inflammation . | Anemia of nutrient deficiency . | Anemia of chronic kidney disease . | Unexplained anemia . | |||
| No (%) | 2302 | 42 (11.9) | 125 (33.0) | 84 (33.4) | 57 (21.7) | NA |
| Age, y, mean | 70.6 | 70.2 | 74.3 | 73.9 | 71.9 | < .001 |
| Female sex, % | 58.3 | 48.8 | 43.6 | 53.7 | 46.1 | .99 |
| Race/ethnicity, % | < .001 | |||||
| Non-Hispanic white | 84.4 | 55.4 | 80.7 | 69.4 | 67.3 | |
| Non-Hispanic black | 6.8 | 43.9 | 16.7 | 12.9 | 21.7 | |
| Mexican American | 2.4 | 0.7 | 2.6 | 2.2 | 0.8 | |
| Other | 6.4 | 0.0 | 0.0 | 15.4 | 10.3 | |
| Hemoglobin level, g/dL, mean | 14.2 | 11.7 | 11.6 | 11.6 | 11.8 | < .001 |
| Vitamin D deficiency, % | 33.3 | 56.0 | 47.7 | 37.1 | 33.0 | .003 |
| Vitamin D level, ng/mL, mean | 24.5 | 21.5 | 21.8 | 23.6 | 24.2 | .002 |
| . | Nonanemic . | Anemia subtypes . | P, comparison among anemia subtypes* . | |||
|---|---|---|---|---|---|---|
| Anemia of chronic inflammation . | Anemia of nutrient deficiency . | Anemia of chronic kidney disease . | Unexplained anemia . | |||
| No (%) | 2302 | 42 (11.9) | 125 (33.0) | 84 (33.4) | 57 (21.7) | NA |
| Age, y, mean | 70.6 | 70.2 | 74.3 | 73.9 | 71.9 | < .001 |
| Female sex, % | 58.3 | 48.8 | 43.6 | 53.7 | 46.1 | .99 |
| Race/ethnicity, % | < .001 | |||||
| Non-Hispanic white | 84.4 | 55.4 | 80.7 | 69.4 | 67.3 | |
| Non-Hispanic black | 6.8 | 43.9 | 16.7 | 12.9 | 21.7 | |
| Mexican American | 2.4 | 0.7 | 2.6 | 2.2 | 0.8 | |
| Other | 6.4 | 0.0 | 0.0 | 15.4 | 10.3 | |
| Hemoglobin level, g/dL, mean | 14.2 | 11.7 | 11.6 | 11.6 | 11.8 | < .001 |
| Vitamin D deficiency, % | 33.3 | 56.0 | 47.7 | 37.1 | 33.0 | .003 |
| Vitamin D level, ng/mL, mean | 24.5 | 21.5 | 21.8 | 23.6 | 24.2 | .002 |
Data are presented as weighted estimates. Variance could not be estimated because of small group sizes.
Comparisons are made with multinomial logistic regression of unweighted samples because of small group sizes.
Association of vitamin D deficiency with anemia of inflammation compared with unexplained anemia in persons ≥ 60 years: NHANES III, phase 2, 1991-1994
| . | RRR (95% CI) . | P . |
|---|---|---|
| Vitamin D deficiency, unadjusted | 2.79 (1.22-6.34) | .015 |
| Adjusted for age, sex, and race/ethnicity | 2.47 (1.03-5.93) | .043 |
| . | RRR (95% CI) . | P . |
|---|---|---|
| Vitamin D deficiency, unadjusted | 2.79 (1.22-6.34) | .015 |
| Adjusted for age, sex, and race/ethnicity | 2.47 (1.03-5.93) | .043 |
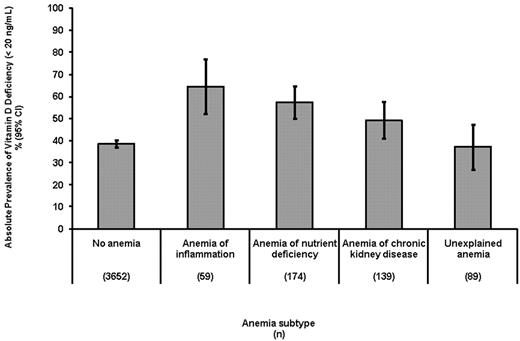
The association between vitamin D deficiency and AI was confirmed in our analysis of the NHANES 2001-2002 survey (n = 1493). The high prevalence of vitamin D deficiency in subjects with AI (64.5%) compared with subjects who were nonanemic (35.0%) was consistent in this dataset. The variation of vitamin D deficiency prevalence among anemia subtypes was not statistically significant in this smaller dataset (P = .47). However, in a combined analysis of participants in both NHANES III phase 2 and NHANES 2001-2002 (n = 4103), the prevalence of vitamin D deficiency did vary significantly among anemia subtypes (P = .001), being greatest among persons with AI (64.4%; 95% CI, 52.1%-76.8%) and least in subjects with unexplained anemia (37.1%; 95% CI, 26.9%-47.2%; Figure 3). In adjusted analysis, the relative risk for AI associated with vitamin D deficiency compared with UA was 2.59 (95% CI, 1.24-5.45).
Association of vitamin D deficiency and anemia subtypes in persons ≥ 60 years. Combined NHANES III, phase 2, 1991-1994 and NHANES 2001-2002 datasets.
Association of vitamin D deficiency and anemia subtypes in persons ≥ 60 years. Combined NHANES III, phase 2, 1991-1994 and NHANES 2001-2002 datasets.
Among persons with AI, the non-Hispanic black racial and ethnic subgroup was overrepresented, comprising 43.9% of subjects. To evaluate whether vitamin D deficiency was associated with AI in non-Hispanic blacks, we analyzed the combined NHANES III phase 2 and NHANES 2001-2002 datasets to allow for an increased sample size of this subgroup (∼ 8% of the whole cohort). After adjustment for age and sex, non-Hispanic blacks had a 7-fold greater risk of having AI than whites (RRR, 7.31; 95% CI, 4.05-13.20). After additional adjustment for vitamin D deficiency, this association was partially attenuated (RRR, 5.67; 95% CI, 3.03-10.59), suggesting that a proportion of the increased susceptibility to AI in non-Hispanic blacks may possibly result from vitamin D deficiency.
Discussion
This is the first population-based study to provide compelling evidence that vitamin D deficiency is associated with anemia in relatively healthy older persons, particularly among those with AI. Separate analyses of both NHANES III and NHANES 2001-2006 showed similar increases in the prevalence of anemia in subjects who were vitamin D deficient, and this association was found to be independent of age, sex, or race/ethnicity. The association of vitamin D deficiency with anemia became statistically significant at 25(OH)D levels < 24 ng/mL. In addition, we found that vitamin D deficiency was strongly associated with classic AI, with this subgroup exhibiting a rate of vitamin D deficiency nearly twice that of similarly aged nonanemic persons. In contrast, the prevalence of vitamin D deficiency in persons with unexplained anemia was found to be no different from nonanemic persons. The observed increased prevalence of vitamin D deficiency in persons with anemia because of nutritional deficiency is not surprising and probably reflects in large part the influence of multiple contributing factors, including poor general nutritional status and decreased outdoor activity found in many older subjects.
The strong association between vitamin D deficiency and AI raises important questions about the role of vitamin D in the suppression of specific inflammatory pathways that contribute to the development of AI. AI is most commonly seen in association with infection, rheumatologic disorders, malignancy, and other chronic illnesses. On a biochemical level, AI is classically characterized by low serum iron and low iron-binding capacity in the setting of an elevated serum ferritin. Although the cause of classic AI has been attributed to decreased red cell survival, disordered iron-limited erythropoiesis, and progressive erythropoietin (EPO) resistance of erythroid progenitors, the relative role and interplay of these 3 mechanisms and the potential common pathways that result in the development of anemia remain unknown. Although our study highlights a correlation between vitamin D deficiency and AI in older persons, further studies that examine this association are needed before any causal or mechanistic conclusions may be drawn.
Our understanding of AI has been transformed by the discovery of hepcidin.20 Hepcidin is synthesized by the liver and functions as a key regulator of iron metabolism that inhibits intestinal absorption of iron and blocks release of iron from macrophages. Overexpression of hepcidin in transgenic mice results in perinatal mortality from iron deficiency unless the mice are salvaged with intravenous iron,21 whereas mice in which the hepcidin gene is ablated develop severe iron overload.22 Patients with AI, as diagnosed by elevated ferritin and low iron and iron-binding capacity, have been shown to have elevated levels of hepcidin. Furthermore, patients with transfusional iron overload have also been shown to express elevated hepcidin levels. The NHANES III study preceded the identification of hepcidin; therefore, its potential role in the cause AI and other subtypes of elderly anemia was not assessed. Although the ability of vitamin D to modulate hepcidin expression remains unknown, our study provides intriguing epidemiologic evidence to support a potential role for vitamin D in the regulation of pathways that mediate hepcidin synthesis. Alternatively, vitamin D deficiency may merely result from similar chronic inflammatory pathways that underlie the development of anemia.
Regulation of hepcidin synthesis is complex and includes several inflammatory-mediated cellular pathways. Hepcidin is an acute phase reactant potently induced by interleukin-6 (IL-6), and hepcidin is implicated in mediating iron-limited erythropoiesis in patients with acute and chronic inflammatory states.20,23 Limited evidence suggests vitamin D may suppress IL-6–mediated signaling. Vitamin D derivatives have been shown to decrease the levels of both IL-6 and IL-8 in fibroblast cultures derived from nasal polyposis samples.24 In addition, 25(OH)D has been found to modulate expression of intracellular Toll-like receptor-9 (TLR9) in primary human monocytes, resulting in the subsequent decreased secretion of IL-6 in response to Toll-like receptor-9 challenge.25 Further evidence supporting a role for vitamin D in suppressing inflammatory pathways potentially active in AI comes from analysis of vitamin D receptor (VDR)–null mice. Vitamin D functions primarily by interaction with the VDR, which was recently shown to form a complex with the nuclear factor κB (NF-κB) p65 subunit. Studies in VDR-null mice showed that VDR expression down-regulates NF-κB transcriptional activity, serving as a negative regulator of bacterial-induced intestinal NF-κB activation and attenuating the response to infection through reduced IL-6 levels.26
A recent analysis of a subgroup of participants in the InCHIANTI (Invecchiare in Chianti, ageing in the Chianti area) study examined the association between urinary hepcidin levels, proinflammatory markers, and anemia and found that, although IL-6 and C-reactive protein were associated with anemia and low iron status, they were not associated with higher urinary hepcidin, leading the investigators of the study to postulate that increased hepcidin synthesis occurs only in situations of overt inflammation.27 However, it may also support the hypothesis that anemia may be mediated through hepcidin-independent proinflammatory pathways such as tumor necrosis factor α or IL-1β, which may induce anemia by direct suppression of erythroid colony formation rather than impairment of iron utilization. Whether adequate vitamin D levels serve to suppress IL-6–mediated increases in hepcidin or ameliorate hepcidin-independent proinflammatory pathways associated with AI remains to be determined.
Leptin, an adipokine associated with inflammation, body fat mass, and energy metabolism, has also been recently shown to induce hepcidin by Janus kinase-2/signal transducer and activator of transcription 3 signaling, potentially linking obesity to inflammation and iron homeostasis.28 Multiple polymorphic alleles within the leptin gene influence leptin expression,29 and low leptin levels have been associated with impaired erythropoietin responsiveness of erythroid progenitors and the syndrome of frailty in the elderly.30 The interrelationship between vitamin D status and adiposity appears to be complex and reciprocal. Some31-33 but not all cross-sectional34-36 studies have shown an inverse association between 25(OH)D levels and adiposity. Although the precise underlying mechanisms are not well understood, increased storage of 25(OH)D in adipose tissue in obese persons is one potential explanation, because vitamin D can be efficiently deposited in body fat stores and becomes no longer bioavailable.37 Further studies are required to investigate whether vitamin D may help to mediate common pathways that link inflammation with obesity.
Hepcidin is also down-regulated by hypoxia; however, it remains to be determined whether the body's primary hypoxia sensor, hypoxia inducible factor-1 α (HIF-1α), directly suppresses hepcidin synthesis. Although recent evidence suggests that 25(OH)D suppresses HIF-1α expression in various human cancer cells,38 the ability of vitamin D to modulate HIF-1α–mediated regulation of hepcidin remains unknown.
In our analysis we did not find a correlation between low vitamin D levels and unexplained anemia. The pathophysiology of unexplained anemia in the elderly is poorly understood and probably represents a heterogenous population with multiple causes. Impaired EPO responsiveness of the hematopoietic stem cell has been implicated in the pathophysiology of anemia in the elderly, and unexplained anemia may primarily reflect impaired EPO responsiveness of the hematopoietic stem cell.39 The Baltimore Longitudinal Study on Aging showed that EPO levels rose with age in healthy, nonanemic persons, and that the slope of the rise was greater for persons without diabetes or hypertension.40 Persons with anemia also had a lower slope of rise, suggesting that anemia reflected a failure of a normal compensatory rise in EPO levels with age. It is unclear whether vitamin D has any effect on EPO responsiveness in elderly subjects with normal renal function. However, we hypothesize that a proportion of unexplained anemia is because of hepcidin-independent proinflammatory pathways that directly suppress erythroid colony formation, albeit to an extent that is not reversed by vitamin D on the basis of the results of this study.
The high proportion of anemia, particularly AI, in elderly non-Hispanic blacks has been previously described.41 Beutler and West42 found that hemoglobin, hematocrit, and mean corpuscular volume were all lower, whereas the serum ferritin was higher in blacks than in age-matched whites, and these differences remained significant after adjusting for α-thalassemia and iron deficiency. It seems unlikely to explain all of the difference in anemia incidence among black patients, because in the Beutler and West42 study, after correcting for α-thalassemia and iron deficiency, the percentages of anemia in African Americans was still strikingly higher than in whites (11.26% in blacks vs 3.83% for whites). Ethnic groups, especially African Americans, are at increased risk of low vitamin D status because of dark skin pigmentation. Observational studies have shown that African Americans have lower circulating levels of 25(OH)D and are more likely to be vitamin D deficient than other ethnic groups.43-47 Our data suggest that a small proportion of the increased prevalence of AI in non-Hispanic blacks may correlate with vitamin D deficiency. It would be interesting to determine whether part of the increased prevalence of vitamin D deficiency seen in non-Hispanic blacks may be due to functional VDR gene polymorphisms that promote the development of AI by reduction in vitamin D–mediated signaling and decreased attenuation of NF-κB–associated inflammatory pathways.
In summary, the results of the present study suggest that moderate vitamin D deficiency is associated with anemia in general and with AI in particular in older persons. The use of single-sample results is a limitation that should be addressed in future longitudinal studies. Nevertheless, these findings have potentially broad public health implications, given the high prevalence of vitamin D deficiency in elderly populations; the ethnic, demographic, and geographic variables that contribute to reduced 25(OH)D levels; and the relative ease and cost-effectiveness of vitamin D supplementation. Further laboratory investigations, particularly those examining specific inflammatory pathways or host genetic changes that contribute to the pathogenesis of classic AI, are required before vitamin D deficiency can be implicated in the causal development of anemia. Our data support the design of randomized clinical trials to evaluate vitamin D supplementation as therapy for patients with AI.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: T.S.P. collected, analyzed, and interpreted data; performed statistical analysis; and served as first author in the writing of the manuscript. R.P. collected, analyzed, and interpreted data and performed statistical analysis. N.B. analyzed and interpreted data and assisted in writing the manuscript. G.J.V. oversaw all aspects of the project and designed research, analyzed and interpreted data, and assisted in writing the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Gary J. Vanasse, Division of Hematology/Medicine, Brigham and Women's Hospital, Harvard Medical School, 75 Francis St, Karp 5216, Boston, MA 02115; e-mail: gvanasse@partners.org

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal