To the editor:
Hepcidin inhibits iron uptake from the gut and release of iron from macrophages in the reticuloendothelial system. Suppression of plasma hepcidin occurs in lowlanders after ascent to high altitude,1,2 reflecting the increased iron demand for erythropoiesis, but the mechanism and primary stimulus for hepcidin suppression in this setting remains unclear.
It has been suggested that hypoxia per se, possibly via the hypoxia-inducible factor family of transcription factors, provides a stimulus for transcriptional suppression of hepcidin.3 This is supported by the hepcidin suppression seen in patients with congenital upregulation of hypoxia-inducible factor proteins, even after correction for hemoglobin and iron status.4 However, others have argued that hepcidin suppression does not result from hypoxia directly,5,6 but rather from the hypoxia-induced increase in erythropoietic drive. Hepcidin levels are suppressed in patients with excessive ineffective erythropoiesis at sea level,7 although they remain normal in healthy high-altitude residents exposed to long-term hypoxia and in patients with polycythemia vera at sea level, in whom erythropoietic drive is likely to be relatively stable.8,9 In recent years, numerous mediators have been proposed as the link between erythropoiesis and hepcidin suppression (the so-called “erythroid regulator”). Candidates include growth-differentiation factor 15 (GDF-15), soluble transferrin receptor (sTfR), erythropoietin (EPO), and the novel hormone erythroferrone, recently described in mice and reported to be the product of the gene Fam132b.10 The protein product of the homologous human gene (FAM132B) is known as FAM132B, CTRP15, or myonectin.
We studied patients with chronic mountain sickness (CMS), a condition characterized by excessive erythrocytosis and elevated blood viscosity in high-altitude residents.11 Venesection provides symptomatic relief in such patients, and therefore presents an opportunity to examine the effects of acutely enhanced erythropoietic drive on a background of chronic, steady-state hypoxia.
Ten male patients (age 44 ± 10 years, mean ± standard deviation) were recruited in Cerro de Pasco, Peru (4340 m above sea level) as part of a wider study into iron supplementation at high altitude.12 All were high-altitude natives with CMS, as defined by excessive erythrocytosis (hemoglobin ≥21 g/dL) and hypoxemia with no other medical explanation.11 At baseline (day 0), arterial oxygen saturation was measured and venous blood was sampled for assessment of hematocrit, ferritin, transferrin saturation, hepcidin, EPO, sTfR, GDF-15, and FAM132B. Patients then underwent isovolemic venesection of 500 mL on each of days 1 to 4 (total volume, 2000 mL), with repeat pulse oximetry and blood sampling on days 5, 12, and 19.
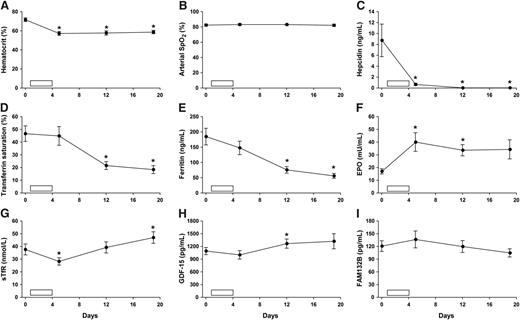
The results are shown in Figure 1. Despite significant hypoxemia (arterial SpO2 83 ± 1%, mean ± standard error of the mean) and exaggerated erythropoiesis, baseline hepcidin levels in CMS patients (8.8 ± 3.0 ng/mL) were similar to those reported both for healthy Peruvian lowlanders at sea level2 and healthy high-altitude populations in Ethiopia.8 This seems likely to reflect the relatively stable, albeit elevated, erythropoietic activity in these groups, and is in keeping with the classical concept of an erythroid regulator that signals imbalance between erythropoietic drive and iron supply, rather than absolute marrow activity.13
Effect of venesection in high-altitude residents with CMS. (A-I) Open bar represents 500-mL isovolemic venesection on each of 4 consecutive days (days 1-4; total volume, 2000 mL). Hematocrit was estimated by microcentrifugation (mean of 2 measurements). Arterial oxygen saturation was measured by pulse oximetry (Nonin Onyx; Nonin Medical, Plymouth, MN). Serum for analysis of ferritin, iron, and total iron-binding capacity was stored at 4°C and analyzed within 72 hours at sea level (Medlab, Lima, Peru). Transferrin saturation was calculated as 100(serum iron/total iron-binding capacity). Serum was stored at −20°C for EPO enzyme-linked immunosorbent assay (ELISA) (Medlab). Plasma was stored at −20°C for hepcidin (Bachem, St Helens, United Kingdom), GDF-15 and sTfR (R&D Systems, Abingdon, United Kingdom), and FAM132B ELISAs. In the case of FAM132B, samples were assayed using 2 independent ELISA kits (Cusabio Biotech, Wuhan, China, and Aviscera Bioscience, Santa Clara, CA). Results are provided for the Cusabio Biotech assay because the detection range most closely matched the range of FAM132B values in our samples, but neither assay revealed any evidence of an effect of venesection on FAM132B. Individual time points (mean ± standard error of the mean) were compared with baseline (day 0) using paired Student t tests. *Statistically significant difference (P < .05).
Effect of venesection in high-altitude residents with CMS. (A-I) Open bar represents 500-mL isovolemic venesection on each of 4 consecutive days (days 1-4; total volume, 2000 mL). Hematocrit was estimated by microcentrifugation (mean of 2 measurements). Arterial oxygen saturation was measured by pulse oximetry (Nonin Onyx; Nonin Medical, Plymouth, MN). Serum for analysis of ferritin, iron, and total iron-binding capacity was stored at 4°C and analyzed within 72 hours at sea level (Medlab, Lima, Peru). Transferrin saturation was calculated as 100(serum iron/total iron-binding capacity). Serum was stored at −20°C for EPO enzyme-linked immunosorbent assay (ELISA) (Medlab). Plasma was stored at −20°C for hepcidin (Bachem, St Helens, United Kingdom), GDF-15 and sTfR (R&D Systems, Abingdon, United Kingdom), and FAM132B ELISAs. In the case of FAM132B, samples were assayed using 2 independent ELISA kits (Cusabio Biotech, Wuhan, China, and Aviscera Bioscience, Santa Clara, CA). Results are provided for the Cusabio Biotech assay because the detection range most closely matched the range of FAM132B values in our samples, but neither assay revealed any evidence of an effect of venesection on FAM132B. Individual time points (mean ± standard error of the mean) were compared with baseline (day 0) using paired Student t tests. *Statistically significant difference (P < .05).
Venesection produced a 20% fall in hematocrit by day 5 (P < .01, paired Student t test), accompanied by a rise in plasma EPO (P < .05) and a marked fall in plasma hepcidin (P < .05). This fall occurred without any significant rise in GDF-15, sTfR, or FAM132B, and before the subsequent reduction in serum ferritin and transferrin saturation. Hepcidin suppression persisted for the duration of the study period, with undetectable plasma levels in most patients on days 12 and 19.
These findings support erythroid activity, rather than hypoxia per se, as the major stimulus for hepcidin suppression at high altitude. Our results show a temporal association between changes in EPO and hepcidin following venesection, but do not identify a clear role for systemic GDF-15, sTfR, FAM132B, or iron availability in linking erythropoietic drive to hepcidin suppression in this setting.
Authorship
Acknowledgments: The authors are grateful to the participants in this study, which was approved by the Oxford Tropical Research Ethics Committee (Oxford, United Kingdom) and the Universidad Peruana Cayetano Heredia Research Ethics Committee (Lima, Peru). All volunteers gave written informed consent.
This work was supported by The Wellcome Trust.
Contribution: N.P.T. and T.G.S. planned and performed research, analyzed and interpreted data, and wrote the manuscript; S.L.-L., C.G., and M.R.-C. planned and performed research; and K.L.D., D.R.M., and P.A.R. planned the research and interpreted the data.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Nick P. Talbot, Department of Physiology, Anatomy & Genetics, University of Oxford, Sherrington Building, Oxford OX1 3PT, United Kingdom; e-mail: nick.talbot@dpag.ox.ac.uk.
References
Author notes
N.P.T. and T.G.S. contributed equally.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal