Abstract
The induction of the iron-regulatory peptide hepcidin by proinflammatory cytokines is thought to result in the withholding of iron from invading pathogens. Hfe and transferrin receptor 2 (Tfr2) are involved in the homeostatic regulation of hepcidin and their disruption causes hereditary hemochromatosis (HH). To determine whether either Hfe or Tfr2 is involved in the inflammatory pathway regulating hepcidin, we analyzed the effect of inflammation in 3 mouse models of HH. The inflammatory response and indicators of iron homeostasis were measured in wild-type, Hfe−/−, Tfr2−/−, and Hfe−/−/Tfr2−/− mice injected with lipopolysaccharide (LPS). The administration of LPS significantly reduced serum iron in wild-type and Hfe−/− mice, with smaller reductions in Tfr2−/− and Hfe−/−/Tfr2−/− mice. Low basal levels of hepcidin in the Hfe−/−/Tfr2−/− mice were increased in response to LPS, but remained significantly lower than in the other strains of mice. These results suggest that despite the absence of Hfe and Tfr2, hepcidin is responsive to inflammation; however, the low basal expression and subsequent low levels of circulating hepcidin are insufficient to reduce serum iron effectively. This suggests that in HH, the iron-withholding response to invading pathogens may be inadequate, and this is especially the case in the absence of both Hfe and Tfr2.
Introduction
Iron is an important element in biologic processes because it is required for the activity of many enzymes and proteins. Therefore, its regulation is important and a deficiency or excess of iron can be detrimental to health. Mammals have developed an intricate system for the regulation of body iron levels. At the center of this is the antimicrobial peptide and iron-regulatory hormone hepcidin. Hepcidin functions by binding to the cellular iron-exporter ferroportin, causing its internalization and degradation.1 This results in reduced release of iron from cells expressing ferroportin, such as duodenal enterocytes and cells of the reticuloendothelial system. As a consequence, intestinal iron absorption and iron recycling are reduced and serum iron levels and transferrin saturation decrease. The production of hepcidin is restricted mainly to the liver, where its expression is regulated by several important molecules involved in iron metabolism and hereditary hemochromatosis (HH).2 Hepcidin is up-regulated by increased iron stores, proinflammatory cytokines, and bone-morphogenetic proteins (BMPs). The hemojuvelin (HJV) protein is a BMP coreceptor, and its cell-associated form is required for the regulation of hepcidin through the BMP-SMAD signaling pathway.3 Lack of functional HJV results in very low hepcidin levels and early-onset juvenile hemochromatosis (type 2A).4 Adult-onset forms of HH caused by mutations in HFE and TFR2 are also characterized by low hepcidin relative to iron stores. Both of these proteins have been implicated in the regulation of hepcidin, although their exact mechanisms of action remain unresolved.5,6
We recently showed that double knockout of Hfe and Tfr2 in mice results in more severe iron loading and lower hepcidin levels than in the 2 single knockouts, with a phenotype similar to juvenile hemochromatosis.7 This suggests that the loss of Hfe and Tfr2 has a compounding effect on hepcidin regulation and that the 2 molecules can function independently to regulate hepcidin. The pathways through which Hfe and Tfr2 regulate hepcidin are not well defined, but there is some evidence that the Erk1/2 and/or Smad pathways may be involved.7,8 Recently, new evidence for the role of Hfe in the regulation of hepcidin through the Bmp/Smad pathway has been presented, showing that Hfe−/− mice have higher hepatic Bmp6 expression but lower levels of phospho-Smad1/5/8 in relation to iron stores than wild-type mice.9 In addition, low doses of Bmp6 were less effective at up-regulating hepcidin in hepatocytes isolated from Hfe−/− mice compared with wild-type mice.9 A similar pattern of increased BMP6 expression concurrent with low expression of BMP target genes and phospho-SMAD1/5/8 relative to iron stores has also been observed in liver biopsies from patients with HFE-HH.10 These studies suggest that there is an impairment in Bmp-Smad signaling in the absence of Hfe, resulting in a blunted hepcidin response. Treatment of Hfe−/− mice with high doses of Bmp6, however, can overcome this blunted hepcidin response, increasing hepcidin and reducing serum iron levels, and suggesting that Hfe is not necessary for BMP6-mediated induction of hepcidin.11
Hepcidin also plays a role in the immune response to pathogens. Originally isolated as a liver-expressed antimicrobial peptide with activity against a range of bacteria and fungi,12,13 hepcidin is induced by inflammatory stimuli and has been implicated in the anemia of inflammation.14 The administration of inflammatory agents such as lipopolysaccharide (LPS), turpentine, or Freund's complete adjuvant has been shown to increase hepatic Hamp1 (the gene encoding hepcidin) expression and reduce serum iron levels in mice.14-16 The proinflammatory cytokine interleukin-6 (IL-6) plays an important role in this inflammation-mediated regulation of Hamp1 expression.15 The proinflammatory cytokines IL-1α and IL-1β have also been shown to induce hepcidin.17 Hepcidin is regulated by IL-6 through the gp130 receptor and the Stat3 signaling pathway.18 The role of Hfe and Tfr2 in the inflammation-mediated induction of hepcidin has also been explored. It was originally reported that Hfe−/− mice were unable to mount an appropriate hepcidin response to LPS, implicating Hfe in the inflammation-mediated pathway regulating hepcidin.19 However, 2 subsequent studies indicated that Hfe was not required for inflammatory-mediated induction of hepcidin, and that Hfe−/− mice were able to increase hepcidin and reduce serum iron in response to inflammation.16,20 One of these studies also suggested that Tfr2 was not required for inflammation-mediated hepcidin expression.20
We used Hfe−/−, Tfr2−/−, and double-knockout Hfe−/−/Tfr2−/− mice on an identical genetic background to comprehensively and comparatively explore the roles of Hfe and Tfr2 in the inflammatory-mediated regulation of hepcidin and serum iron levels.
Methods
Animals
Animal studies were approved by the Queensland Institute of Medical Research Animal Ethics Committee. All animals had free access to water and food under standard conditions and received humane care. The Hfe−/−, Tfr2−/−, and Hfe−/−/Tfr2−/− mice on a C57BL/6 background have been described previously.7 Male mice were weaned at 21 days and maintained on standard laboratory chow ad libitum (iron content, 120.1 mg/kg). All mice were fasted overnight before being killed at 5 weeks and tissues collected for analysis. Control mice were injected intraperitoneally with saline 4 days before being killed at 5 weeks. LPS-treated mice were injected intraperitoneally with 1μg/g of LPS from Escherichia coli 055:B5 (Sigma-Aldrich) 6 hours before being killed at 5 weeks.
Measurement of iron indices
Serum iron and transferrin saturation were measured using an iron and iron-binding–capacity kit (Sigma-Aldrich). Nonheme hepatic and splenic iron concentrations were measured using the method of Torrance and Bothwell.21
Real-time PCR analysis of mRNA transcripts
Primer pairs for detecting hepcidin mRNA transcripts and inflammatory markers have been described previously.22,23 Primer sequences for detecting IL-6 mRNA were forward 5′-ATGGATGCTACCAAACTGGAT-3′ and reverse 5′-TGAAGGACTCTGGCTTTGTCT-3′. The quantitation of mRNA transcripts in liver and spleen was determined by real-time polymerase chain reaction (PCR) using LightCycler 480 SYBR Green Mix in a LightCycler 480 (Roche Diagnostics), as described previously.23
Western blotting
Liver and spleen samples were homogenized in phosphatase inhibitor lysis buffer (200mM Tris pH 8.0, 100mM NaCl, 1mM EDTA, 0.5% NP-40, 10% glycerol, 1mM NaF, 1mM sodium orthovanadate, 1mM sodium pyrophosphate, 1:1000 protease inhibitor cocktail, 2mM phenylmethanesulfonyl fluoride, and 10μg/mL of DNase). Samples (25 μg) were electrophoresed on 10% Tris-glycine sodium dodecyl sulfate–polyacrylamide gels, transferred onto Hybond-C+ membranes, and blocked in Western blocking buffer (10% skim milk powder, 0.5% Tween 20 in Tris-buffered saline [TBS-T]) overnight at 4°C. Blots were incubated with the primary antibodies anti-ferritin (1:2000; Sigma-Aldrich), anti–phospho-Stat3 (1:1000; Cell Signaling Technology), anti-Stat3 (1:1000; Cell Signaling Technology), anti-ferroportin (1:2000; Alpha Diagnostics International), and anti–glyceraldehyde-3-phosphate dehydrogenase (anti-GAPDH; 1:100 000; Millipore) in Western blocking buffer overnight. Blots were washed in TBS-T and incubated with anti–rabbit or anti–mouse horseradish peroxidase (1:10 000; Invitrogen) for 1 hour at room temperature. After washing in TBS-T, blots were incubated with Immobilon Western Chemiluminescent Horseradish Peroxidase Substrate (Millipore) and exposed to film. Bands were quantitated by densitometry using the GeneGenius Imaging System (Syngene). Blots probed with phospho-Stat3 were stripped by incubating with stripping buffer (1% sodium dodecyl sulfate; 62.5mM Tris-HCl, pH 6.8; 0.7% 2-mercaptoethanol) at 50°C for 30 minutes. Blots were then rinsed with 3 10-minute washes and reblocked with Western blocking buffer at room temperature for 1 hour before incubation with antibodies to total Stat3.
IL-6 measurement
The concentration of IL-6 was measured in serum samples using an enzyme-linked immunosorbent assay (ELISA; Life Research) according to the manufacturer's instructions.
Statistical analysis
Variables were compared between groups using 1-way ANOVA or 2-tailed Student t test in Prism 5 software (GraphPad). A P value < .05 was considered statistically significant. Graphs were prepared using Prism 5.
Results
Reduction in serum iron and transferrin saturation is blunted in Hfe−/−/Tfr2−/− mice exposed to LPS
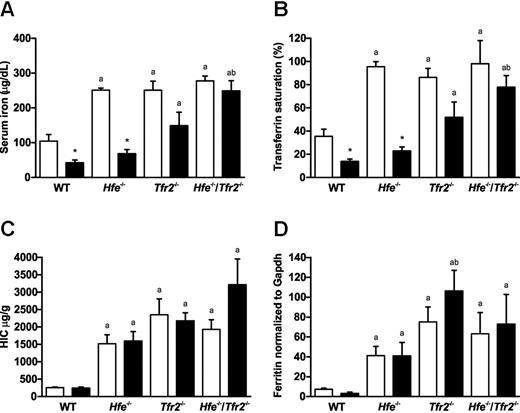
The effect of inflammation on iron homeostasis was assessed in 5-week-old wild-type, Hfe−/−, Tfr2−/−, and Hfe−/−/Tfr2−/− mice by injection with LPS. The effect of LPS on serum iron and transferrin saturation was measured. In all mice, there was a close correlation between serum iron and transferrin saturation (Figure 1A-B). In wild-type mice, serum iron and transferrin saturation were significantly reduced after administration of LPS. Similarly, in the 3 knockout strains, the serum iron and transferrin saturation were reduced in mice exposed to LPS (Figure 1A-B). However, the magnitude of reduction varied between the strains. Hfe−/− mice had a large reduction in serum iron and transferrin saturation that reached statistical significance, Tfr2−/− mice had an intermediate reduction that did not quite reach statistical significance, and Hfe−/−/Tfr2−/− mice had a small reduction that was not statistically significant (Figure 1A-B).
Iron indices in wild-type (WT), Hfe−/−, Tfr2−/−, and Hfe−/−/Tfr2−/− mice treated with LPS. (A) Serum iron, (B) transferrin saturation, (C) HIC, and (D) hepatic ferritin levels were measured in 5-week-old male WT, Hfe−/−, Tfr2−/−, and Hfe−/−/Tfr2−/− mice injected with saline (white bars) or LPS (black bars) for 6 hours (n = 3-4 per group). Data are shown as the mean; error bars indicate SEM. Statistical comparisons were performed using the Student t test. Significant differences (P < .05) are denoted between control and LPS treatments (*) and between strains compared with WT (a), with Hfe−/− (b), and with Tfr2−/− (c).
Iron indices in wild-type (WT), Hfe−/−, Tfr2−/−, and Hfe−/−/Tfr2−/− mice treated with LPS. (A) Serum iron, (B) transferrin saturation, (C) HIC, and (D) hepatic ferritin levels were measured in 5-week-old male WT, Hfe−/−, Tfr2−/−, and Hfe−/−/Tfr2−/− mice injected with saline (white bars) or LPS (black bars) for 6 hours (n = 3-4 per group). Data are shown as the mean; error bars indicate SEM. Statistical comparisons were performed using the Student t test. Significant differences (P < .05) are denoted between control and LPS treatments (*) and between strains compared with WT (a), with Hfe−/− (b), and with Tfr2−/− (c).
Hepatic iron concentration (HIC) and hepatic ferritin protein expression were also measured in all mice. As expected, all 3 knockout strains had significantly elevated HIC and hepatic ferritin expression compared with wild-type mice (Figure 1B-C). There were no significant changes in HIC or hepatic ferritin after exposure to LPS in any of the mouse strains (Figure 1B-C).
The hepcidin response to LPS is inadequate in Hfe−/−/Tfr2−/− mice
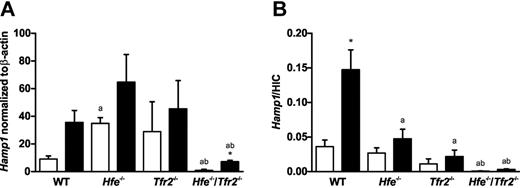
To investigate the response to LPS in the mouse models of HH, we analyzed the expression of hepatic hepcidin mRNA. In saline-injected mice, we found a significant increase in Hamp1 mRNA expression in Hfe−/− mice and a significant decrease in Hamp1 mRNA expression in Hfe−/−/Tfr2−/− mice compared with wild-type (Figure 2A). We previously observed a similar increase in Hamp1 mRNA in 5-week-old Hfe−/− mice on the C57BL6 background; however, when iron stores were taken into account, these mice actually had a relative reduction in Hamp1 mRNA that was probably insufficient to keep iron stores within normal limits.7 When iron stores were taken into account by calculating the ratio of Hamp1 to HIC, we saw a decreasing gradient from wild-type to Hfe−/− to Tfr2−/− to Hfe−/−/Tfr2−/− mice (Figure 2B). LPS administration resulted in an increase in Hamp1 expression in wild-type mice that almost reached statistical significance (P = .052). Increases in Hamp1 were also observed in Hfe−/−, Tfr2−/−, and Hfe−/−/Tfr2−/− mice after LPS administration, but the increase was statistically significant only in the Hfe−/−/Tfr2−/− mice (Figure 2A). The basal Hamp1 expression levels in the saline-injected Hfe−/−/Tfr2−/− mice were more than 10-fold lower than in the other strains; therefore, although there was a 7-fold increase in Hamp1 expression after LPS administration in the Hfe−/−/Tfr2−/− mice, the actual expression levels were still below that seen in the wild-type saline-injected mice. When iron stores were taken into account, the Hamp1/HIC ratio was increased after LPS administration in all strains of mice, but this only reached statistical significance in the wild-type mice (Figure 2B). Again, there was a decreasing gradient in Hamp1/HIC values from wild-type to Hfe−/− to Tfr2−/− to Hfe−/−/Tfr2−/−. This suggests that although the ability to up-regulate Hamp1 in response to LPS was retained in the Hfe−/−/Tfr2−/− mice, the increased levels were insufficient to reduce serum iron levels effectively. This was likely because of the low basal levels of Hamp1 in relation to iron stores. This was also the case, but to a lesser extent, in Tfr2−/− mice, and although the Hfe−/− mice had a robust response to LPS in terms of serum iron reduction, the hepcidin response was smaller compared with wild-type mice.
Hepatic hepcidin (Hamp1) mRNA expression in wild-type (WT), Hfe−/−, Tfr2−/−, and Hfe−/−/Tfr2−/− mice treated with LPS. Real-time PCR was used to quantitate hepatic Hamp1 mRNA transcript levels in 5-week-old male WT, Hfe−/−, Tfr2−/−, and Hfe−/−/Tfr2−/− mice injected with saline (white bars) or LPS (black bars) for 6 hours (n = 3-4 per group). (A) Hamp1 mRNA transcript levels are shown normalized to β-actin. (B) Hamp1 mRNA transcript levels normalized to β-actin are shown relative to iron stores as a ratio to HIC. Data are shown as the mean; error bars indicate SEM. Statistical comparisons were performed using the Student t test. Significant differences (P < .05) are denoted between control and LPS treatments (*) and between strains compared with WT (a), with Hfe−/− (b), and with Tfr2−/− (c).
Hepatic hepcidin (Hamp1) mRNA expression in wild-type (WT), Hfe−/−, Tfr2−/−, and Hfe−/−/Tfr2−/− mice treated with LPS. Real-time PCR was used to quantitate hepatic Hamp1 mRNA transcript levels in 5-week-old male WT, Hfe−/−, Tfr2−/−, and Hfe−/−/Tfr2−/− mice injected with saline (white bars) or LPS (black bars) for 6 hours (n = 3-4 per group). (A) Hamp1 mRNA transcript levels are shown normalized to β-actin. (B) Hamp1 mRNA transcript levels normalized to β-actin are shown relative to iron stores as a ratio to HIC. Data are shown as the mean; error bars indicate SEM. Statistical comparisons were performed using the Student t test. Significant differences (P < .05) are denoted between control and LPS treatments (*) and between strains compared with WT (a), with Hfe−/− (b), and with Tfr2−/− (c).
Splenic ferroportin protein is unaffected by LPS in Hfe−/−/Tfr2−/− mice
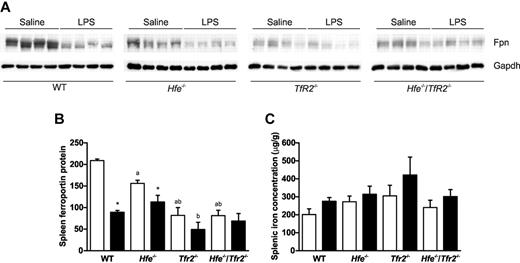
Hepcidin indirectly controls serum iron levels by affecting the cell-surface expression of the iron-exporter ferroportin by inducing its internalization and degradation. We investigated the mechanism underlying the LPS-mediated reduction in serum iron by analyzing the levels of ferroportin protein in the spleen. The spleen was chosen because it has a high proportion of macrophage cells and high expression of ferroportin. The expression of ferroportin protein was analyzed by Western blotting and the levels quantitated (Figure 3A-B). There was a statistically significant reduction in ferroportin protein after LPS administration in the spleens of wild-type and Hfe−/− mice, but no significant reduction in Tfr2−/− and Hfe−/−/Tfr2−/− mice (Figure 3A-B). The decrease in spleen ferroportin protein in wild-type and Hfe−/− mice exposed to LPS is consistent with the observed reduction in serum iron and the increase in hepatic Hamp1 expression. The splenic iron concentration was also determined, and although an increase was observed in all strains, there were no significant changes after LPS administration (Figure 3C). This may be because of the relatively short time course of the experiment (6 hours).
Splenic ferroportin expression and splenic iron concentration in WT, Hfe−/−, Tfr2−/−, and Hfe−/−/Tfr2−/− mice treated with LPS. (A) Western blotting was used to determine the expression of ferroportin in the spleens of 5-week-old male WT, Hfe−/−, Tfr2−/−, and Hfe−/−/Tfr2−/− mice injected with saline or LPS for 6 hours. The blots in panel A were quantitated and results are shown relative to Gapdh for saline-injected (white bars) and LPS-injected (black bars) mice (B). Splenic iron concentration was determined in all mice (C). Data are shown as the means; error bars indicate SEM. Statistical comparisons were performed using the Student t test. Significant differences (P < .05) are denoted between control and LPS treatments (*) and between strains compared with WT (a), with Hfe−/− (b), and with Tfr2−/− (c).
Splenic ferroportin expression and splenic iron concentration in WT, Hfe−/−, Tfr2−/−, and Hfe−/−/Tfr2−/− mice treated with LPS. (A) Western blotting was used to determine the expression of ferroportin in the spleens of 5-week-old male WT, Hfe−/−, Tfr2−/−, and Hfe−/−/Tfr2−/− mice injected with saline or LPS for 6 hours. The blots in panel A were quantitated and results are shown relative to Gapdh for saline-injected (white bars) and LPS-injected (black bars) mice (B). Splenic iron concentration was determined in all mice (C). Data are shown as the means; error bars indicate SEM. Statistical comparisons were performed using the Student t test. Significant differences (P < .05) are denoted between control and LPS treatments (*) and between strains compared with WT (a), with Hfe−/− (b), and with Tfr2−/− (c).
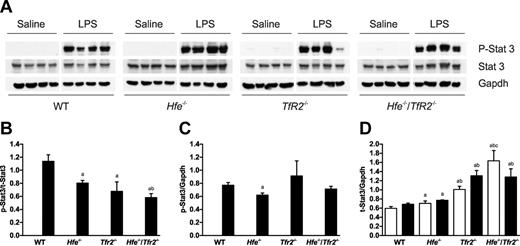
The inflammatory response to LPS was similar in all mice
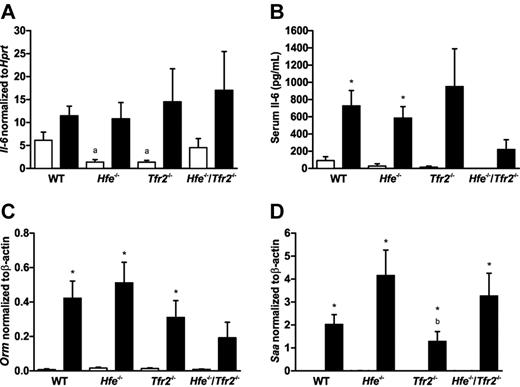
To determine whether the differences in hepcidin response to LPS in the knockout mice was because of an impaired inflammatory response, the levels of the inflammatory cytokine IL-6 and other markers of the inflammatory response were measured. The expression of IL-6 mRNA in the spleen was measured by real-time PCR and serum concentrations of IL-6 were measured by ELISA. The levels of IL-6 were elevated in the groups treated with LPS, and there were no significant differences between genotype groups (Figure 4A-B). To assess the inflammatory response in the liver, the mRNA expression of 2 inflammatory markers was measured by real time PCR. Orosomucoid (Orm), also known as α-1-acid glycoprotein (Agp) is an acute phase plasma glycoprotein expressed predominantly in the liver. Serum amyloid A (Saa) is also an acute-phase plasma apolipoprotein that is produced in the liver during inflammation. The expression of these 2 inflammatory markers was increased markedly in mice treated with LPS (Figure 4C-D). There did not appear to be any consistent differences in the induction of these 2 inflammatory markers between the wild-type and knockout strains of mice. This suggests that the inflammatory response in the liver is similar in all strains of mice. The induction of hepcidin by the inflammatory cytokine IL-6 involves the activation of the Stat3 transcription factor through gp130.18 Stat3 also plays an important role in the cytokine-mediated induction of Saa.24 Western blots were used to determine the levels of phosphorylated and total Stat3 in mouse livers (Figure 5A). Phospho-Stat3 was undetectable in control saline-injected mice. LPS administration led to a massive increase in the levels of phospho-Stat3 in wild-type and all 3 knockout strains (Figure 5). Compared with the levels of total Stat3, there was a significant decline in the ratio of phospho-Stat3 in the 3 knockout strains compared with wild-type mice (Figure 5B). However, the amount of phospho-Stat3 (relative to Gapdh) was actually similar across all mouse strains, with the decreasing ratio to total-Stat3 resulting from increased levels of total-Stat3 (relative to Gapdh) in the knockout strains (Figure 5C-D). The reason for the increase in total-Stat3 in the knockout mice is unknown, and the effect of this, if any, on signaling is unclear. Overall, our results suggest that the Hfe−/−, Tfr2−/−, and Hfe−/−/Tfr2−/− mice have a normal cytokine-mediated response to inflammation through the Stat3 pathway.
Expression of IL-6 and hepatic inflammatory markers in wild-type (WT), Hfe−/−, Tfr2−/−, and Hfe−/−/Tfr2−/− mice treated with LPS. Real-time PCR was used to quantitate mRNA transcript levels in 5-week-old male WT, Hfe−/−, Tfr2−/−, and Hfe−/−/Tfr2−/− mice injected with saline (white bars) or LPS (black bars) for 6 hours (n = 3-4 per group). In the spleen, IL-6 mRNA transcripts levels were measured relative to Hprt (A). An ELISA was used to determine IL-6 concentrations in the serum of all mice (B). In the liver, Orm (C) and Saa (D) mRNA transcript levels were measured relative to β-actin. Data are shown as the means; error bars indicate SEM. Statistical comparisons were performed using the Student t test. Significant differences (P < .05) are denoted between control and LPS treatments (*) and between strains compared with WT (a), with Hfe−/− (b), and with Tfr2−/− (c).
Expression of IL-6 and hepatic inflammatory markers in wild-type (WT), Hfe−/−, Tfr2−/−, and Hfe−/−/Tfr2−/− mice treated with LPS. Real-time PCR was used to quantitate mRNA transcript levels in 5-week-old male WT, Hfe−/−, Tfr2−/−, and Hfe−/−/Tfr2−/− mice injected with saline (white bars) or LPS (black bars) for 6 hours (n = 3-4 per group). In the spleen, IL-6 mRNA transcripts levels were measured relative to Hprt (A). An ELISA was used to determine IL-6 concentrations in the serum of all mice (B). In the liver, Orm (C) and Saa (D) mRNA transcript levels were measured relative to β-actin. Data are shown as the means; error bars indicate SEM. Statistical comparisons were performed using the Student t test. Significant differences (P < .05) are denoted between control and LPS treatments (*) and between strains compared with WT (a), with Hfe−/− (b), and with Tfr2−/− (c).
Expression of activated Stat3 in the livers of wild-type (WT), Hfe−/−, Tfr2−/− and Hfe−/−/Tfr2−/− mice treated with LPS. (A) Western blotting was used to determine the expression of phosphorylated Stat3 in the livers of 5-week-old male WT, Hfe−/−, Tfr2−/−, and Hfe−/−/Tfr2−/− mice injected with saline or LPS for 6 hours. The blots in panel A were quantitated using SynGene GeneTools, and the results are shown as ratios of phospho-Stat3/total-Stat3 (B), phospho-Stat3/Gapdh (C), and total-Stat3/Gapdh (D) for saline-injected (white bars) and LPS-injected (black bars) mice. Data are shown as the means; error bars indicate SEM. Statistical comparisons were performed using the Student t test. Significant differences (P < .05) are denoted between control and LPS treatments (*) and between strains compared with WT (a), with Hfe−/− (b), and with Tfr2−/− (c).
Expression of activated Stat3 in the livers of wild-type (WT), Hfe−/−, Tfr2−/− and Hfe−/−/Tfr2−/− mice treated with LPS. (A) Western blotting was used to determine the expression of phosphorylated Stat3 in the livers of 5-week-old male WT, Hfe−/−, Tfr2−/−, and Hfe−/−/Tfr2−/− mice injected with saline or LPS for 6 hours. The blots in panel A were quantitated using SynGene GeneTools, and the results are shown as ratios of phospho-Stat3/total-Stat3 (B), phospho-Stat3/Gapdh (C), and total-Stat3/Gapdh (D) for saline-injected (white bars) and LPS-injected (black bars) mice. Data are shown as the means; error bars indicate SEM. Statistical comparisons were performed using the Student t test. Significant differences (P < .05) are denoted between control and LPS treatments (*) and between strains compared with WT (a), with Hfe−/− (b), and with Tfr2−/− (c).
Discussion
The regulation of iron homeostasis is essential for maintaining body iron levels within safe limits and is also important in the immune response to pathogens. Hepcidin, the master regulator of iron homeostasis, is implicated in both of these processes. The important role of the HH-associated proteins Hfe and Tfr2 in regulating hepcidin, and ultimately iron absorption and body iron stores, has been shown by us and others.5,7,22,25-27 Hepcidin is also up-regulated by proinflammatory cytokines during inflammation, and this aspect of hepcidin regulation has implicated it in the anemia of chronic disease. Hepcidin causes the sequestration of iron within cells, reducing serum iron levels and essentially withholding iron from potential invading pathogens. The roles of Hfe and Tfr2 in the immune response to pathogens are less clear. Studies in Hfe−/− mice16 and hepatocytes deficient in Hfe or Tfr220 suggest that these genes are not involved in the inflammatory pathway regulating hepcidin, because hepcidin was up-regulated after exposure to an inflammatory stimulus or to IL-6 in a pattern similar to that of wild-type controls. In Hfe−/− mice, this was accompanied by a significant reduction in serum transferrin saturation.16
In this study, we have shown that the iron-withholding response after an inflammatory stimulus is blunted in mice deficient in both Hfe and Tfr2, and to a lesser extent in mice deficient in Tfr2 alone. The inflammatory response in the knockout mice appears to be intact, as evidenced by the up-regulation of IL-6 mRNA in the spleen and increased levels of IL-6 in the serum of mice exposed to LPS. Inflammatory markers (Orm and Saa) in the livers of the knockout mice also suggest that the response to inflammation in the liver is not abrogated. There was also robust activation of Stat3 in the livers of all mice treated with LPS. Although absolute levels of phospho-Stat3 were similar between the strains, the levels of total-Stat3 were increased in the knockout stains of mice. The reasons for the increase in total-Stat3 are unclear, and whether it had an impact on Stat3 activation and the inflammatory response in the knockout mice is uncertain. The response of Saa, a gene known to be regulated by Stat3, to LPS suggests that Stat3 activity is not impaired in the knockout mice. Our observation of a normal inflammatory response to LPS in our mouse models of HH contrasts with another study that observed an attenuated inflammatory response in Hfe−/− mice. In that study, Hfe−/− mice infected orally with Salmonella typhimurium showed reduced macrophage expression of tumor necrosis factor-alpha and IL-6.28 In agreement with our results, however, another study that infected mice intraperitoneally with S typhimurium observed no differences between the expression of inflammatory cytokines in macrophages from wild-type and Hfe−/− mice.29
The blunting of the iron-withholding response in the Hfe−/−/Tfr2−/− mice appears to be due to inadequate levels of the iron-regulatory hormone hepcidin. Whereas mice deficient in both Hfe and Tfr2 are capable of up-regulating the expression of Hamp1 in response to an inflammatory stimulus, the absolute levels, although elevated from baseline, remain low. This low level of circulating hepcidin is probably insufficient to adequately down-regulate the cell-surface expression of ferroportin and to reduce iron release from cells. Indeed, we have shown that the levels of ferroportin protein in the spleen are significantly reduced after exposure to LPS in wild-type and Hfe−/− mice, but not in Tfr2−/− or Hfe−/−/Tfr2−/− mice. The higher basal Hamp1 expression in the Tfr2−/− mice compared with Hfe−/−/Tfr2−/− mice would allow greater hepcidin expression during inflammation and a larger decrease in serum iron, but would still be inadequate to reduce serum iron down to wild-type levels. The even higher basal Hamp1 levels in Hfe−/− mice would allow a more marked decrease in serum iron levels, close to that observed in wild-type mice and similar to that observed in other studies.16 However, the Hfe−/− mice exposed to LPS did have lower Hamp1 in relation to iron stores compared with wild-type, and this may suggest a slight deficiency in the iron-withholding response to pathogens in Hfe−/− mice.
The low basal hepcidin expression in HH and Hfe−/− mice is likely because of deficiencies in the BMP-SMAD signaling pathway, as has been suggested by several recent studies.9-11 Our demonstration of an attenuated hepcidin response to inflammation in Hfe−/−/Tfr2−/− mice suggests that a functional BMP-SMAD pathway is also required for an effective iron-withholding response during inflammation. This has implications for patients with HH because it may lead to a poorer protective response to invading pathogens. It has been documented that HH patients are more susceptible to infection with the pathogenic gram-negative bacteria Vibrio vulnificus, which has been attributed to the higher availability of iron in the blood of patients with HH.30,31 Patients with iron overload are also more susceptible to other aggressive bacteria that require iron for growth and enhanced virulence, such as Listeria monocytogenes, Klebsiella sp, and Yersinia sp32 Our results suggest that a slightly blunted hepcidin response to inflammation in HH patients infected with V vulnificus or other iron-requiring bacteria may also contribute to the greater susceptibility to infection. In contrast, Hfe−/− mice may be protected against infection with bacteria whose pathogenicity depends on their ability to invade the host's macrophages (such as S typhimurium).29 The relative iron deficiency of Hfe−/− macrophages and the high expression of ferroportin has been proposed to partially underlie the protection against S typhimurium and other intramacrophage pathogens in HH.33-36 Other mechanisms for the protection in Hfe−/− mice have been proposed, such as the up-regulation of lipocalin 2, a molecule capable of capturing iron-laden bacterial siderophores.29 In another study, an attenuated immune response observed in Hfe−/− macrophages suggested that the absence of Hfe does not protect against these bacteria.28
In conclusion, we have shown that in the absence of Hfe and Tfr2, the low basal expression of hepcidin contributes to an inadequate iron-withholding response during inflammation. This is the case to a lesser extent in the absence of Tfr2 and possibly Hfe alone. This has implications for patients with HH and may partly explain their greater susceptibility to infection with some iron-requiring pathogens.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Emily Crampton for assistance with the animal experiments.
This work was supported in part by a program grant from the National Health and Medical Research Council (NHMRC) of Australia (no. 339400 to V.N.S.) and by an NHMRC R. D. Wright Career Development Award (no. 443026 to D.F.W.).
Authorship
Contribution: D.F.W. designed and performed the research, analyzed the data, and wrote the paper; C.J.M. and L.O. performed the research and analyzed the data; and V.N.S. designed the research, analyzed the data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Assoc Prof V. Nathan Subramaniam, Membrane Transport Laboratory, The Queensland Institute of Medical Research, 300 Herston Rd, Herston, Brisbane, QLD 4006, Australia; e-mail: Nathan.Subramaniam@qimr.edu.au.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal