To the editor:
We read with interest the report by Lausten-Thomsen et al in this issue of Blood.1 The study challenges the previous report by Mori et al describing ∼ 1% frequency of TEL/AML1 (ETV6/RUNX1)–positive cord blood in healthy newborns and questions the hypothesis of TEL/AML1 being a frequent prenatal first hit in childhood acute lymphoblastic leukemia (ALL).2
Between 1998 and 2002, we analyzed the cord blood of 253 healthy newborns for the presence of TEL/AML1 (n = 253), MLL/AF4 (n = 103), and BCR/ABL (n = 103) fusion transcripts using standard end-point reverse transcription–polymerase chain reaction (RT-PCR; 197 samples) or quantitative RT-PCR (TEL/AML1 only; 56 samples). While all tested samples proved to be BCR/ABL and MLL/AF4 negative,3 we found that of the cord blood tested, 5 of 253 (2%) bore the TEL/AML1 fusion. Moreover, we tested tissues taken from aborted fetuses (1 × spleen, 11 × liver) for the presence of TEL/AML1 and we found the spleen tissue positive (abortion for sepsis in 29th week of gestation).
We have several reasons to consider the positively tested samples genuinely positive rather than contaminated.
First, although strict precautions are routinely taken in the laboratory to prevent carryover contamination, some extra measures were introduced for this particular study. Procedures were performed in the new laboratories where leukemic samples had never before been processed. Therefore, all cord blood material was processed separately from the material that might contain any of the tested transcripts. Negative controls were included at all steps and positive controls were diluted. The positive control cDNA was added in a separate room when all other PCR vessels were tightly closed.
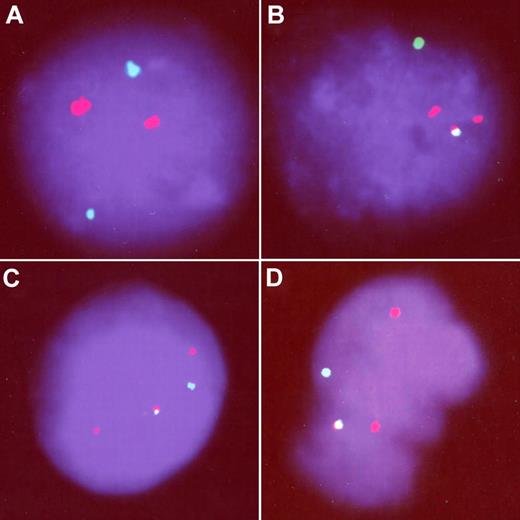
Second, we confirmed the RT-PCR positivity at the chromosomal level using fluorescence in situ hybridization (FISH) in 1 sample with adequate amount of material. Although none of the 5000 nuclei screened by FISH in the negative control showed the TEL/AML1 fusion, we detected 3 of 3000 cells with the fusion signal in cord blood positively tested by RT-PCR (Figure 1). This sample was the only one showing an atypical TEL/AML1 transcript (the TEL exon 5 was truncated and the 3′ end of the AML1 exon 1 was included in the fusion). While we agree that this fusion might not result in the typical TEL/AML1-positive ALL, the presence of this unique atypical transcript cannot be a result of contamination and proves that the fusion between the TEL and AML1 genes do occur prenatally.
Third, the positive spleen tissue presented with the less common variant of the TEL/AML1 transcript (TEL exon 5/AML1 exon 3) which was never used as a positive control.
Fourth, we also tested the hypothesis that the TEL/AML1 transcript arises as an artefact in cells under an apoptotic stress. Thus, we tested peripheral blood of 30 children with a nonmalignant febrile condition (fever > 38°C) and normal mononuclear cells under apoptotic stress (ultraviolet, serum starvation, delayed sample processing; altogether 30 samples). All samples remained TEL/AML1 negative.
FISH analysis of TEL/AML1-positive cord blood. One negative (A) and 3 TEL/AML1-positive (B-D) cells. All the positive cells show the TEL/AML1 fusion signal (yellow), presence of the nontranslocated TEL allele (green), and 2 red signals demonstrating 1 normal AML1 and 1 signal from the disrupted AML1 allele.
FISH analysis of TEL/AML1-positive cord blood. One negative (A) and 3 TEL/AML1-positive (B-D) cells. All the positive cells show the TEL/AML1 fusion signal (yellow), presence of the nontranslocated TEL allele (green), and 2 red signals demonstrating 1 normal AML1 and 1 signal from the disrupted AML1 allele.
In conclusion, our results support the original report by Mori et al and the hypothesis that TEL/AML1 is a relatively frequent prenatal first hit in leukemogenesis.
Authorship
Acknowledgment: This work was supported by grant MSM0021620813 from the Czech Ministry of Education, Youth and Sports.
Contribution: J.Z., O.H., and J.T. designed research; J.Z. and J.T. analyzed data and wrote the paper; J.M, O.K., Z.Z., M.K., K.M., M.Z., and J.S. performed experiments; and J.H. was involved in data collection.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jan Zuna, MD, PhD, CLIP (Childhood Leukaemia Investigation Prague), Department of Paediatric Haematology and Oncology, 2nd Faculty of Medicine, Charles University Prague, V Uvalu 84, 150 06–Prague 5, Czech Republic; e-mail: jan.zuna@lfmotol.cuni.cz.