Abstract
Aurora-A is a mitotic kinase that regulates mitotic spindle formation and segregation. In multiple myeloma (MM), high Aurora-A gene expression has been correlated with centrosome amplification and proliferation; thus, inhibition of Aurora-A in MM may prove to be therapeutically beneficial. Here we assess the in vitro and in vivo anti-MM activity of MLN8237, a small-molecule Aurora-A kinase inhibitor. Treatment of cultured MM cells with MLN8237 results in mitotic spindle abnormalities, mitotic accumulation, as well as inhibition of cell proliferation through apoptosis and senescence. In addition, MLN8237 up-regulates p53 and tumor suppressor genes p21 and p27. Combining MLN8237 with dexamethasone, doxorubicin, or bortezomib induces synergistic/additive anti-MM activity in vitro. In vivo anti-MM activity of MLN8237 was confirmed using a xenograft-murine model of human-MM. Tumor burden was significantly reduced (P = .007) and overall survival was significantly increased (P < .005) in animals treated with 30 mg/kg MLN8237 for 21 days. Induction of apoptosis and cell death by MLN8237 were confirmed in tumor cells excised from treated animals by TdT-mediated dUTP nick end labeling assay. MLN8237 is currently in phase 1 and phase 2 clinical trials in patients with advanced malignancies, and our preclinical results suggest that MLN8237 may be a promising novel targeted therapy in MM.
Introduction
Multiple myeloma (MM) is a B-cell disease characterized by accumulation of malignant plasma cells in the bone marrow (BM), bone lesions, and immunodeficiency. Genetic analysis shows that approximately 55% to 60% of MM patients have a hyperdiploid karyotype, which confers a better prognosis than nonhyperdiploid disease.1 The most frequent chromosomal abnormalities observed in nonhyperdiploid MM are translocations between immunoglobulin heavy chain gene located on chromosome 14q32 and an oncogene chromosome 11q13 (CYCLIN D1), 4p16.3 (FGFR3 and MMSET), 6p21 (CYCLIN D3), 16q23 (MAF), or 20q11 (MAFB); or less frequently, the immunoglobulin light chain locus (2p12,κ or 22q11λ).2 During cell proliferation, activation of each cell-cycle phase is dependent on the progress and completion of the previous one. Regulation of the cell cycle involves detecting and repairing genetic damage, as well as controlling various checkpoints to prevent uncontrolled cell division. MM cells are further influenced by the BM microenvironment because adhesion of MM cells to extracellular-matrix proteins supports cell adhesion-mediated drug resistance. In addition, binding of MM cells to BM accessory cells induces secretion of cytokines, which further promote growth, survival, and migration of tumor cells, as well as resistance to conventional chemotherapy.2,3
Aberrant or overexpression of D-type cyclins is ubiquitous in MM,4,5 and Aurora kinases regulate cell-cycle checkpoints6 and cell cycle–regulatory molecules, including cyclins and cyclin-dependent kinases.7-9 Aurora serine/threonine kinases localize in the centrosome and play a crucial role in cell division by regulating chromatid segregation in mitotic cells10 ; moreover, defective chromatid segregation causes genetic instability, leading to tumorigenesis.11 They were first identified in Xenopus Eg2, yeast Ipl1, and Drosophila aurora. The human genome expresses 3 members of the mitotic Aurora kinase family: Aurora-A serine/threonine kinases, Aurora-B serine/threonine kinases, and Aurora-C serine/threonine kinases. Aurora-A localizes to centrosomes in early S phase, and a fraction associates with spindle microtubules proximal to the spindle poles during mitosis.10,12,13 Aurora-B localizes to the spindle midzone at anaphase7 and Aurora-C localizes to centrosomes. The expression and activity of all Aurora kinases increase in mitosis and decrease rapidly during mitotic exit in proliferating cells. Because Aurora-A is required for cytokinesis, defective Aurora-A may cause aneuploidy characteristic of tumors. During normal cell proliferation, expression of Aurora-A is regulated by tumor suppressor gene p53. Aurora-A is activated by phosphorylation during G2 to M phase transition in the cell cycle.14 Although little is known about Aurora-C kinases, Aurora-A and Aurora-B kinases have been extensively studied and found to be overexpressed in tumor cells.15-18 Because high Aurora-A gene expression has been correlated with centrosome amplification and a worse prognosis in MM, inhibition of Aurora-A may prove to be therapeutically beneficial.2,6,9,19-24
Recently, we and others have shown that inhibition of Aurora-A kinase gene expression in MM cells by RNAi induces apoptosis and cell death, as well as abrogates G2/M cell-cycle progression in MM cell lines.9,19 Small molecule inhibitors of pan-Aurora (-A and -B) and Aurora-B kinases are undergoing evaluation in clinical trials in patients with MM and other cancers9,20,25-27 ; however, these inhibitors of pan-Aurora kinases induce a phenotype consistent with Aurora-B, rather than Aurora-A, inhibition.9,20,25,28,29 MLN8237 is the first orally available small molecule selective inhibitor of Aurora-A kinase, which is currently in early-phase clinical testing in patients with advanced solid tumors and acute myelogenous leukemia. In this study, we assess the in vitro and in vivo anti-MM activity of MLN8237 in preclinical models of human MM to provide the framework for its use, alone and in combination with conventional and novel anti-MM agents, to improve patient outcome in MM.
Methods
Cell culture
Human MM cell lines RPMI8226 and U266 were obtained from ATCC. Dexamethasone-sensitive (MM.1S) and -resistant (MM.1R) cells were kindly provided by Dr Steven Rosen (Northwestern University). Melphalan-resistant (LR5) and doxorubucin-resistant (RPMI-DOX40) cells were kindly provided by Dr William Dalton (Lee Moffitt Cancer Center); and OPM1, OPM2, and interleukin-6 (IL-6)-dependent INA-6 plasma cell leukemia cell lines were kindly provided by Dr Edward Thompson (University of Texas Medical Branch). MM cell lines were maintained in a complete media-RPMI 1640 medium (Sigma-Aldrich) and BM stroma cells in Dulbecco modified Eagle medium (Sigma-Aldrich) supplemented with 10% fetal bovine serum, 2mM l-glutamine (Invitrogen), 100 units/mL penicillin, and 100 mg/mL streptomycin (Invitrogen). Peripheral blood and BM samples from patients with MM and healthy donors were obtained with informed consent per the Declaration of Helsinki and approval by the Institutional Review Board of the Dana-Farber Cancer Institute. Peripheral blood and BM mononuclear cells were separated by Ficoll-density gradient centrifugation, and CD138+ plasma cells were purified from BM aspirates using anti-CD138 magnetic-labeled selection antibody (Miltenyi Biotec).
Reagents and compounds
Small molecule inhibitor of Aurora-A kinase MLN8237 (10mM; Millennium Pharmaceuticals) and nocodazole (10 mg/mL; Sigma-Aldrich) were dissolved in dimethyl sulfoxide (DMSO) and stored at −20°C until use. Recombinant human IL-6 and insulin growth factor-1 (IGF-1) were obtained from R&D Systems. INA-6 cells were cultured with 2 ng/mL recombinant human (rh) IL-6. rhIL-6 (10 ng/mL) and rhIGF-1 (25 ng/mL) were used to induce MM cell growth and proliferation.
Measurement of cell viability and proliferation
MM cell lines, CD138+ tumor cells purified from BM aspirates of patients with MM, and peripheral blood mononuclear cells (PBMCs) obtained from healthy donors were seeded in triplicate 96-well plates in 100 μL complete media at a density of 20 × 104cells/well. MLN8237 was added to each well to give a range of concentrations (0.0001-4μM) in a final volume of 200 μL. Cell viability was measured using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT; Sigma-Aldrich), and cell proliferation was measured using 3[H]-thymidine incorporation at 24, 48, and 72 hours of incubation. The absorbance was measured at 570/630 nm by a spectrophotometer (Molecular Devices).
MM cells were incubated in 96-well plates, alone or in the presence of BM stroma cells, rhIL-6 (10 ng/mL), or rhIGF-1 (25 ng/mL), and then exposed to MLN8237 (0.0001-4μM) for 24, 48, and 72 hours. Cells were pulsed with 3[H]-thymidine (0.5 μCi) for the last 8 hours of incubation, harvested onto glass filters (Cambridge Technology), and counted using LKB Betaplate scintillation counter (Wallac).
MM cell lines were incubated with DMSO or MLN8237 (0.125-0.5μM) in combination with conventional anti-MM agents melphalan (2.5-5μM), doxorubicin (50-100nM), or dexamethasone (50-100nM); and with novel anti-MM agents bortezomib (2.5-5nM) or lenalidomide (0.5-1μM) for 72 hours. Cell viability was measured by MTT assay. The combination index (CI) was determined by isobologram analysis using CalcuSyn software, Version 2.0 (Biosoft; CI < 1 indicates synergistic effect; CI = 1, additive effect; and CI > 1, no significant combination effect).
Detection of apoptosis and senescence
Induction of cell death in MM cells triggered by MLN8237 was measured by fluorescein-conjugated annexin V and propidium iodide (PI) costaining (BD Biosciences). Cells were incubated with 0.5 to 1μM of MLN8237 or DMSO for 24 to 72 hours and stained with fluorescein isothiocyanate-annexin V and PI, according to the manufacturer's protocol. Apoptotic cells were determined by flow cytometric analysis using BDFACS-Canto II (BD Biosciences) and FlowJo Version 7.0 software (TreeStar).
Induction of cell senescence was detected in MM1.S cells and OPM1 cells treated with 0.5μM of MLN8237 for 48 hours using the Senescence β-Galactosidase Staining Kit (Cell Signaling Technology), according to the manufacturer's protocol. β-Galactosidase positive cells were visualized using a light microscope (original magnification ×20; Leica DMIL) at room temperature.
Cell-cycle analysis
MM cells were exposed to DMSO or 0.5 to 1μM of MLN8237 for 24 to 72 hours, permeabilized by 70% ethanol at −20°C, and incubated with 50 μg/mL PI and 20 units/mL RNase-A (Roche Diagnostics). DNA content was analyzed by flow cytometry using BDFACS-Canto II (BD Biosciences) and FlowJo software.
Immunofluorescence staining and flow cytometry
MM cells were cultured on L-lysine-coated glass plates in the absence or presence of MLN8237 (0.5μM) for 24 hours and then stained with anti–phosphorylated Aurora-A kinase at Thr288 (pAurora-A kinase) and α-tubulin, according to the manufacturer's protocol (Cell Signaling Technology). Briefly, cells were fixed in 4% formaldehyde–phosphate-buffered saline for 15 minutes at room temperature and then permeabilized with ice-cold methanol-acetone (1:1 vol/vol) for 10 minutes. Cells were incubated with Alexa Fluor–conjugated (red) anti–pAurora-A kinase (Thr288) and Alexa Fluor–conjugated (green) α-Tubulin Abs (Cell Signaling Technology) at 4°C for 16 hours. Nuclei were stained with 4,6-diamidino-2-phenylindole (blue) for 10 minutes, and fluorescent cells were analyzed and visualized using fluorescein microscopy (original magnification ×40), digital CCD camera (AxioCam ICc1), Axiovision, Version 4.7 software at room temperature (Zeiss Axioplan). Inhibition of Aurora-A was determined by counting the number of pAurora-A kinase (Thr288) stained cells in multiple areas. The percentage of pAurora-A kinase (Thr288) stained cells was determined relative to total mitotic cell number.
Intracellular expression of proteins was detected according to the manufacturer's protocol (Cell Signaling Technology). Cells treated with MLN8237 (0.5μM) for 1 to 72 hours were harvested, fixed in 4% formaldehyde–phosphate-buffered saline for 10 minutes at room temperature, and permeabilized with ice-cold methanol for 10 minutes at room temperature. Cells were then incubated with anti–phospho-HisH3 (Ser10), HisH3, phospho-Chk1 (Ser345), anti–phospho-Chk2 (Thr68), anti–phospho p38 MAPK (Thr180/Tyr182), anti–phospho SAPK/JNK (Thr183/Tyr185), and anti–phospho Cdc2 (Tyr15) antibodies (Cell Signaling Technology) at 4°C for 1 hour. After incubation with Alexa Fluor–IgG for 30 minutes, cells were analyzed by flow cytometry using FACSCanto II (BD Biosciences) and FlowJo software.
Immunoblotting
To synchronize mitotic cells, MM cells were incubated in the absence or presence of nocodazole (400 ng/mL) for 16 hours; and both unsynchronized and synchronized cells were then exposed to MLN8237 (0.5-1μM) for 1 to 72 hours. Cells were lysed in radioimmunoprecipitation assay–lysis buffer: 50mM Tris-HCl (pH 7.4), 150mM NaCl, 1% NP-40, 5mM ethylenediaminetetraacetic acid, 10mM sodium pyrophosphate, 1mM ethyleneglycoltetraacetic acid, 2mM sodium orthovanadate, 5mM sodium fluoride, 5 μg/mL aprotinin, 5 μg/mL leupeptin, and 1mM phenylmethylsulfonyl fluoride. Proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, transferred onto polyvinylidene difluoride membranes (Bio-Rad), and immunoblotted with anti–pAurora-A kinase (Thr288; Millennium Pharmaceuticals), Aurora-A kinase, pHisH3 (Ser10), HisH3, p53, p21, p27, caspase-3, caspase-8, caspase-9, poly (ADP ribose) polymerase (PARP; Cell Signaling Technology), glyceraldehyde-3-phosphate dehydrogenase (GAPDH), and actin antibodies (Santa Cruz Biotechnology). Immunoreactive bands were detected by Western Blot chemiluminescence reagents (NEN Life Sciences) and exposed on Kodak-XAR film.
Xenograft murine model
Fox Chase severe combined immune-deficient (SCID) mice (18-20 weeks old) were purchased from Charles River Laboratories. All animal studies were conducted according to protocols approved by the Animal Ethics Committee of the Dana-Farber Cancer Institute. Mice were irradiated (200 cGy), and then 5 × 106 MM1.S cells were inoculated subcutaneously in the right flank. When tumor growth was measurable (∼ 2 weeks after the injection), mice were assigned into 4 groups (10 mice each) receiving vehicle orally (100 μL of 10% 2-hydroxypropyl-β-cyclodextrin [Sigma-Aldrich]/1% sodium bicarbonate) or MLN8237 (7.5 mg/kg, 15 mg/kg, and 30 mg/kg in a final formulation in 10% 2-hydroxypropyl-β-cyclodextrin/1% sodium bicarbonate) for 21 consecutive days. The maximal tolerated dose of MLN8237 in most mouse strains (continuous dosing for 21 days) is approximately 20 mg/kg twice a day (40 mg/kg per day). Tumor volumes were measured by a Vernier caliper every alternate day and calculated using the following formula: length × width2 × 0.5. Tumor growth inhibition (TGI) was calculated using the formula (Δcontrol average volume − Δtreated average volume) × 100/(Δcontrol average volume). Mice were killed at the end of the treatment, 2 hours after the last treatment, or when tumor reached 2 cm3 ; tumors were immediately collected from mice and evaluated for induction of apoptosis and cell death by TdT-mediated dUTP nick end labeling (TUNEL) assay.
Statistics
All in vitro experiments were performed in triplicate and repeated 3 times; a representative experiment is demonstrated in the figures. Statistical significance of the data was determined by Student t tests, with minimal significance level P less than .05. The median effect dose Dm (analogous to 50% inhibitory concentration [IC50]) values and CI values were determined by isobologram analysis using CalcuSyn software, Version 2.0 (Biosoft; CI < 1 indicates synergistic effect; CI = 1, additive effect; and CI > 1, no significant combination effect).30 In the in vivo experiments, overall survival was determined using the Kaplan-Meier method, and the results are presented as the median overall survival, with 95% confidence intervals.
Results
MLN8237 inhibits activation of Aurora-A kinase in MM cells in vitro
MLN8237 is a novel kinase small molecule inhibitor similar in structure to MLN8054,26,31,32 which is designed to avoid benzodiazepine-like effects (somnolence) observed with MLN8054 (supplemental Figure 1A, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). It exhibits potent activity against Aurora-A kinase (IC50 = 1nM) in both enzyme and cell-based assays.32 MLN8237 shows 200-fold more selective activity against Aurora-A than related kinase Aurora-B. In addition, MLN8237 does not have any significant activity against a Novascreen panel of receptors and ion channels.32
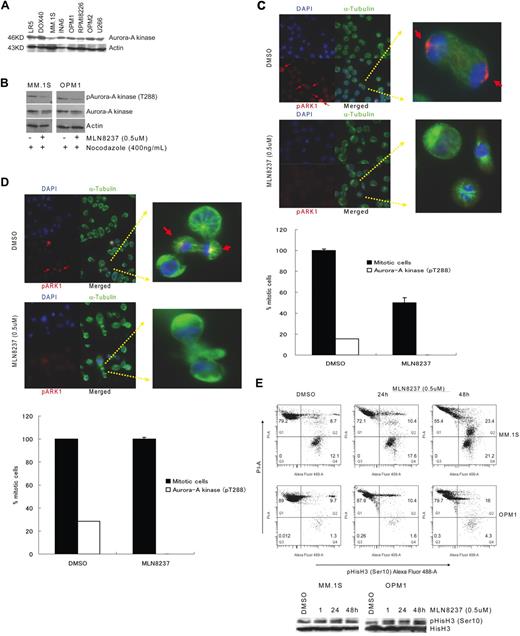
We have shown that Aurora-A kinase protein is expressed in MM cell lines, as well as BM plasma cells from healthy donors and patients with monoclonal gammopathy of undetermined significance and MM patients, by immunohistochemical staining and immunoblotting.19 In this study, we confirmed that all MM cell lines tested expressed Aurora-A kinase protein (Figure 1A). We next examined whether MLN8237 inhibits the activation and function of Aurora-A kinase in MM cells in vitro. To determine the inhibitory effect of MLN8237 on the mitotic cell population, cell division in MM cell lines was first synchronized by treatment with nocodazole, and then phosphorylation of Aurora-A kinase at threonine 288 (pThr288) was measured by Western blotting. Decreased phosphorylation of Aurora-A kinase was shown in MLN8237-treated MM1.S and OPM1 MM cells (Figure 1B), compared with the DMSO and nocodazole-treated cells. To further analyze inhibition of Aurora-A kinase phosphorylation by MLN8237 in unsynchronized MM cell lines, immunofluorescein staining was performed in MM1.S and OPM1 cells. Costaining with α-tubulin, pAurora-A kinase (Thr288), and 4,6-diamidino-2-phenylindole (nucleus) showed that DMSO-treated MM1.S (Figure 1C) and OPM1 (Figure 1D) cells were proliferating with phosphorylated Aurora-A kinase (Thr288), whereas all MLN8237-treated cells accumulated at the nondividing prometaphase and metaphase stages, with no detectable phosphorylation of Aurora-A kinase. In addition, analysis of DMSO-treated cells by fluorescein microscopy demonstrated ongoing mitosis, within the 15% MM1.S cells and 28.5% OPM-1 cells mitotic cell population expressing pAurora-A kinase (Thr288). Importantly, after 24 hours of MLN8237 treatment, there was an accumulation of prometaphase and metaphase cells, with no detectable expression of pAurora-A kinase (Thr288), in both MM cell lines. In addition, Figure 1E shows that MLN8237 does not inhibit histone H3 phosphorylation at ser10 in these cells, indicating mitosis and active Aurora-B kinase in the MLN8237-treated cells.
Inhibition of Aurora-A kinase (Thr288) phosphorylation in MM cells by MLN8237. (A) Expression of Aurora-A kinase protein in a panel of MM cell lines by immunoblotting of equal amounts of total protein (30 μg) with anti–Aurora-A kinase antibody. (B) Immunoblotting with anti–phospho (Thr288)-Aurora-A kinase antibody showed inhibition of Aurora-A kinase autophosphorylation (Thr288) in MM1.S and OPM1, with or without synchronizing with nocodazole. Expression of phospho (Thr288)-Aurora-A kinase protein was shown relative to total expression of Aurora-A kinase protein. Actin was used as a loading control. MM1.S (C) and OPM1 (D) MM cell lines were treated with DMSO or MLN8237 (0.5μM) for 24 hours and then stained with anti–phospho (Thr288)-Aurora-A kinase antibody (red), αtubulin (green), and DNA (blue). Overlapping localization is shown in the merged images. Arrow indicates Aurora-A autophosphorylation on Thr288 in the centrosome (original magnification ×40). Magnified single mitotic cell image is shown in the right panel. The number of mitotic cells with phosphorylated Aurora-A kinase (pT288) relative to total number of mitotic cells are shown. (E) To assess the effect of MLN8237 on Aurora-B kinase and mitosis, MM1.S and OPM1 cells were treated for 1, 24, and 48 hours with DMSO or MLN8237 (0.5μM). Flow cytometric (top panel) and Western immunoblot (bottom panel) analyses in representative MM cell lines show mitotic cells and active Aurora-B with increased DNA, costained with phospho-(Ser10)-histone H3 (Alexa Fluor 488) and PI.
Inhibition of Aurora-A kinase (Thr288) phosphorylation in MM cells by MLN8237. (A) Expression of Aurora-A kinase protein in a panel of MM cell lines by immunoblotting of equal amounts of total protein (30 μg) with anti–Aurora-A kinase antibody. (B) Immunoblotting with anti–phospho (Thr288)-Aurora-A kinase antibody showed inhibition of Aurora-A kinase autophosphorylation (Thr288) in MM1.S and OPM1, with or without synchronizing with nocodazole. Expression of phospho (Thr288)-Aurora-A kinase protein was shown relative to total expression of Aurora-A kinase protein. Actin was used as a loading control. MM1.S (C) and OPM1 (D) MM cell lines were treated with DMSO or MLN8237 (0.5μM) for 24 hours and then stained with anti–phospho (Thr288)-Aurora-A kinase antibody (red), αtubulin (green), and DNA (blue). Overlapping localization is shown in the merged images. Arrow indicates Aurora-A autophosphorylation on Thr288 in the centrosome (original magnification ×40). Magnified single mitotic cell image is shown in the right panel. The number of mitotic cells with phosphorylated Aurora-A kinase (pT288) relative to total number of mitotic cells are shown. (E) To assess the effect of MLN8237 on Aurora-B kinase and mitosis, MM1.S and OPM1 cells were treated for 1, 24, and 48 hours with DMSO or MLN8237 (0.5μM). Flow cytometric (top panel) and Western immunoblot (bottom panel) analyses in representative MM cell lines show mitotic cells and active Aurora-B with increased DNA, costained with phospho-(Ser10)-histone H3 (Alexa Fluor 488) and PI.
MLN8237 inhibits proliferation of MM cells and overcomes the protective effect of the BM environment on MM cells in vitro
We next assessed the antitumor effect of MLN8237 in dexamethasone-resistant (MM.1R), melphalan-resistant (LR5), and doxorubucin-resistant (RPMI-DOX40) MM cells, as well as OPM1, OPM2, and interleukin-6-dependent INA-6 plasma cell leukemia cell lines. Although cytotoxic activity of MLN8237 was detected in several cell lines as early as 24 to 48 hours (Figure 2A) of exposure to less than 0.1μM/L, more potent cytotoxicity in all cell lines occurred at 72 hours (Figure 2A). MLN8237 also induced antiproliferative effects in all cell lines at 72 hours (Figure 2B). For example, MLN8237 induced inhibition of cell viability (40%-60%) and proliferation (70%-90%) in MM1.R, LR5, and Dox40 cells. Half-maximal effect values (Dm), of MLN8237 (analogous to IC50) was determined in each MM cell line for inhibition of both cell viability and cell proliferation using CalcuSyn software, Version 2.0 (Table 1). Higher concentrations (> 0.5μM) of MLN8237 were required to induce anti-MM effects in purified CD138+ tumor cells from BM aspirates of MM patients than MM cell lines (Figure 2C).
MLN8237 inhibits cell viability and proliferation in MM cells in the presence or absence of BM stroma cells. A panel of MM cell lines and tumor cells obtained from patients with MM were treated with DMSO or increasing doses of MLN8237 (0.0001-4μM) for 48 to 72 hours. (A) Inhibition of cell line viability was determined by MTT assay. Inhibition of cell proliferation was determined by 3[H]thymidine incorporation assay in MM cell lines (B) and in tumor cells obtained from MM patients (C). MM1.S and RPMI8226 cell lines and tumor cells from patients with MM were cocultured with either BM stroma cells from patients with MM or cytokines (IL-6, 10 ng/mL; IGF-1, 25 ng/mL), in the absence or presence of MLN8237 (0.001-4μM) for 24 to 48 hours. (D) MM cell proliferation was measured by 3[H]thymidine incorporation assay in the cocultures with BM stroma cells. (E) MM cell viability was also assessed by MTT assay, in the absence or presence of IL-6 or IGF-1. Data represent mean ± SD of triplicate cultures. Statistical significance indicated (t test, 1-tailed distribution, P < .05).
MLN8237 inhibits cell viability and proliferation in MM cells in the presence or absence of BM stroma cells. A panel of MM cell lines and tumor cells obtained from patients with MM were treated with DMSO or increasing doses of MLN8237 (0.0001-4μM) for 48 to 72 hours. (A) Inhibition of cell line viability was determined by MTT assay. Inhibition of cell proliferation was determined by 3[H]thymidine incorporation assay in MM cell lines (B) and in tumor cells obtained from MM patients (C). MM1.S and RPMI8226 cell lines and tumor cells from patients with MM were cocultured with either BM stroma cells from patients with MM or cytokines (IL-6, 10 ng/mL; IGF-1, 25 ng/mL), in the absence or presence of MLN8237 (0.001-4μM) for 24 to 48 hours. (D) MM cell proliferation was measured by 3[H]thymidine incorporation assay in the cocultures with BM stroma cells. (E) MM cell viability was also assessed by MTT assay, in the absence or presence of IL-6 or IGF-1. Data represent mean ± SD of triplicate cultures. Statistical significance indicated (t test, 1-tailed distribution, P < .05).
Median effect values of MLN8237 in MM cell lines
| Cell line . | Dm, μM (median effective dose) . | |
|---|---|---|
| Cell viability . | Cell proliferation . | |
| MM1.S | 0.13 | 0.006 |
| OPM1 | 0.03 | 0.003 |
| RPMI 8226 | 10.32 | 0.004 |
| INA6 | 0.002 | 0.0002 |
| OPM2 | 4.37 | 1.71 |
| MM1.R | 1.68 | 0.04 |
| DOX40 | 5.48 | 0.10 |
| LR5 | 2.53 | 0.009 |
| U266 | 1.43 | 0.08 |
| Cell line . | Dm, μM (median effective dose) . | |
|---|---|---|
| Cell viability . | Cell proliferation . | |
| MM1.S | 0.13 | 0.006 |
| OPM1 | 0.03 | 0.003 |
| RPMI 8226 | 10.32 | 0.004 |
| INA6 | 0.002 | 0.0002 |
| OPM2 | 4.37 | 1.71 |
| MM1.R | 1.68 | 0.04 |
| DOX40 | 5.48 | 0.10 |
| LR5 | 2.53 | 0.009 |
| U266 | 1.43 | 0.08 |
Half-maximal effect values (Dm) of MLN8237 in each MM cell line were determined using CalcuSyn software, Version 2.0 (Biosoft). MM cells were incubated in the absence or presence of MLN8237 at the indicated concentrations (0.0001-4μM) for 72 hours. Dm values were determined using cell viability and cell proliferation data.
Because we and others have demonstrated that BM stroma cells and cytokines in the BM microenvironment trigger and maintain MM cell homing, proliferation, and drug resistance, we next examined whether MLN8237 has an impact on MM cell viability and proliferation in the presence of BM stroma cells and cytokines IL-6 and IGF-1 in vitro. MLN8237 strongly inhibits viability and proliferation of both primary MM cells and MM cell lines in the presence of stroma cells (Figure 2D), as well as IL-6 and IGF-1 (Figure 2E), as early as 24 hours. Importantly, these data indicate that MLN8237 is more potent against MM cell growth and proliferation in the presence of BM cells and cytokines than against MM cells alone. Although higher concentrations of MLN8237 induced antiproliferative effects in proliferating PBMCs from healthy donors (supplemental Figure 1B), there was no significant effect on their viability (supplemental Figure 1C).
MLN8237 induces G2/M arrest, apoptosis, and senescence in MM cells in vitro
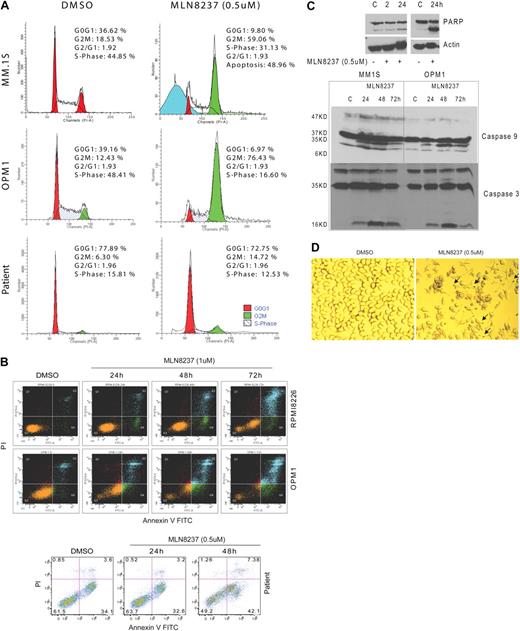
Because Aurora-A kinase is a cell cycle–regulatory protein, we next examined the effects of MLN8237 on the cell cycle in MM cells in vitro. DNA content analysis by flow cytometry after treatment with MLN8237 (0.5μM) for 24 hours demonstrated that MLN8237 induces marked accumulation of cells with G2/M DNA content in both MM cell lines and plasma cells purified from BM of patients with MM (Figure 3A). Specifically, there was a 2- to 6-fold increase in G2/M phase in MLN8237-treated primary MM cells and cell lines compared with DMSO-treated control cells. Costaining with annexin V and PI showed significant induction of apoptosis by MLN8237 treatment (0.5-1μM) in both MM cell lines (Figure 3B) and primary tumor cells as early as 24 hours. We also showed that cleavage of PARP, caspase 9, and caspase 3 (Figure 3C) was induced in MM cells treated with MLN8237. Interestingly, increased nuclear and cell body size, as well as staining for β-galactosidase, a molecular marker of cell senescence, revealed that MLN8237 induces more senescence in MM cell lines at 72 hours compared with DMSO (22% vs 3%, respectively; Figure 3D).
MLN8237 induces accumulation of G2/M phase and apoptosis in MM cells. (A) DNA profiles of MM cell lines, MM1.S and OPM1, as well as tumor cells from MM patient BM treated with DMSO or MLN8237 (0.5μM) for 24 hours were evaluated by flow cytometry. The percentage of cells in G0G1, G2M, and S phases are shown. (B) MM cell lines, RPMI8226 and OPM1, as well as tumor cells from MM patient BM were cultured with DMSO or 0.5 to 1μM of MLN8237 for 24, 48, and 72 hours. Induction of apoptosis and cell death were determined by flow cytometric analysis in MM cells costained with annexin V and PI. (C) Induction of PARP, caspase-9, and caspase-3 cleavage by MLN8237 was measured by immunoblotting in MM cell lines. (D) Representative photomicrograph of β-galactosidase activation in MLN8237 (0.5μM, 48 hours) treated MM1.S cells. Arrows indicate activated β-galactosidase (blue) in cytoplasm. The senescent MM1.S cells (β-galactosidase active, blue stain) were visualized using a light microscope (original magnification ×20).
MLN8237 induces accumulation of G2/M phase and apoptosis in MM cells. (A) DNA profiles of MM cell lines, MM1.S and OPM1, as well as tumor cells from MM patient BM treated with DMSO or MLN8237 (0.5μM) for 24 hours were evaluated by flow cytometry. The percentage of cells in G0G1, G2M, and S phases are shown. (B) MM cell lines, RPMI8226 and OPM1, as well as tumor cells from MM patient BM were cultured with DMSO or 0.5 to 1μM of MLN8237 for 24, 48, and 72 hours. Induction of apoptosis and cell death were determined by flow cytometric analysis in MM cells costained with annexin V and PI. (C) Induction of PARP, caspase-9, and caspase-3 cleavage by MLN8237 was measured by immunoblotting in MM cell lines. (D) Representative photomicrograph of β-galactosidase activation in MLN8237 (0.5μM, 48 hours) treated MM1.S cells. Arrows indicate activated β-galactosidase (blue) in cytoplasm. The senescent MM1.S cells (β-galactosidase active, blue stain) were visualized using a light microscope (original magnification ×20).
MLN8237 effects on MM cells in combination with conventional and novel anti-MM agents in vitro
Isobologram analysis using CalcuSyn software demonstrated that MLN8237, in combination with conventional (melphalan, dexamethasone, doxorubucin) or novel (bortezomib, lenalidomide) therapeutic agents, showed diverse outcomes. For example, there was no synergistic or additive effect of MLN8237 with either conventional or novel agents against dexamethasone-sensitive MM1.S cells (data not shown), However, MLN8237 was synergistic with dexamethasone (CI < 1) and additive/synergistic with doxorubucin (CI ± 1) against OPM1 cells (supplemental Figure 2A-B). Moreover, MLN8237 showed additive effect with bortezomib (CI ± 1) against OPM1 cells (supplemental Figure 2A,C).
Molecular changes induced by MLN8237 in cell cycle–regulatory and antitumor pathways in vitro
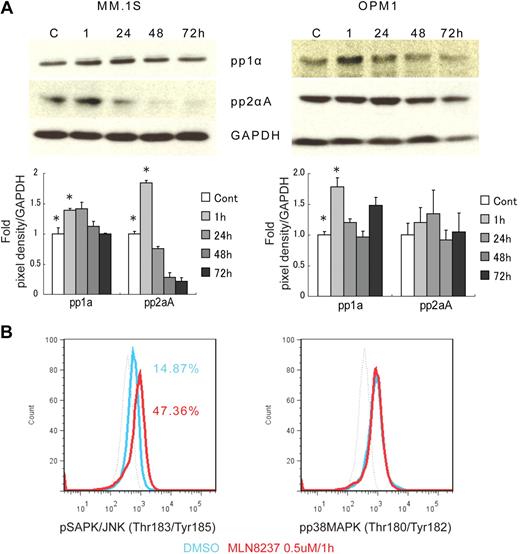
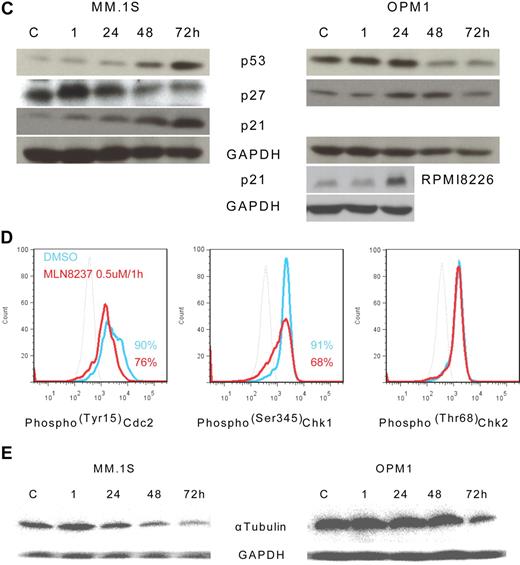
To further define the mechanism of anti-MM activity of MLN8237, we next analyzed its effect on cell cycle–regulatory and checkpoint molecules in MM cells. Cell cycle–negative regulatory molecules, as well as members of the PP2C family of Ser/Thr protein phosphatases protein phosphatase 1α (pp1α) and protein phosphatase 2αA (pp2αA) subunit, were both up-regulated after 1-hour exposure to MLN8237 in all MM cell lines and decreased with longer exposure (Figure 4A). We next examined target molecules of PP2C family members, as well as apoptosis and DNA-damage markers. Stress-activated protein kinase (pSAPK/JNK; Thr183/Tyr185) phosphorylation was activated by MLN8237, whereas there was no change in mitogen-activated protein kinase phosphorylation (pp38 MAPK; Thr180/Tyr182; Figure 4B). In addition, other downstream signaling molecules of PP2C family members, as well as p53, p27, and p21, were activated by MLN8237 in MM cells (Figure 4C). In terms of cell-cycle checkpoint-regulatory molecules, phosphorylation of cell division cycle 2 (pCdc2; Tyr15) and checkpoint 1 (pChk1; Ser345) was down-regulated by MLN8237 in MM cells (Figure 4D). In contrast, there was no significant change in activation of checkpoint 2 molecule triggered by MLN8237. Finally, there was also decreased expression of α-tubulin in MM cells after MLN8237 exposure (Figure 4E).
Molecular changes induced by MLN8237 in cell cycle–regulatory and antitumor pathways. Molecular changes induced by MLN8237 in cell cycle–regulatory and antitumor pathways were analyzed in MM cell lines. MM cell lines were cultured in the absence or presence of DMSO or MLN8237 (0.5-1μM) for various times. Protein level changes were determined by either Western blotting or flow cytometry. (A) Relative expression of negative cell cycle–regulatory molecules PP1α and PP2αA in MM cell lines treated with MLN8237 (0.5μM) for the indicated times. Pixel density of each protein band was measured using ImageJ software (1.37v; National Institutes of Health, http://rsb.info.nih.gov/ij/) and normalized with GAPDH expression. Fold expression per control for each band is shown. *Statistical significance (t test, one-tailed distribution, P < .05; n = 3 independent experiments). (B) Intracytoplasmic expression of phospho(Thr183/Tyr185) SAPK/JNK and phospho(Thr180/Tyr182) MAPK in MM1.S cells after 1-hour exposure to DMSO or MLN8237 (0.5μM). Dotted line indicates isotypic control; blue line, DMSO; and red line, MLN8237 (0.5μM for 1 hour). (C) Molecular changes induced by MLN8237 in p53 pathway were determined by Western blotting in MM cell lines. MLN8237 induced expression of p53, p27, and p21 in MM cell lines. MM cell line OPM1 does not express p21. GAPDH was used as a control to determine total protein expression. (D) Intracytoplasmic expression of cell-cycle checkpoint molecules phospho(Tyr15) Cdc2, phospho(Ser345) Chk1, and phospho(Thr68) Chk2 were analyzed in MM1.S cells after 1-hour exposure to DMSO or MLN8237 (0.5μM). Dotted line indicates isotypic control; blue line, DMSO; and red line, MLN8237 (0.5μM for 1 hour). (E) Molecular changes induced by MLN8237 in α-tubulin activation and formation were analyzed by Western blotting in MM cell lines. MLN8237 induced expression of α-tubulin in MM cell lines at early exposure time, whereas there was decreased expression of α-tubulin after 24 hours of MLN8237 treatment. GAPDH was used as a control to determine total protein expression.
Molecular changes induced by MLN8237 in cell cycle–regulatory and antitumor pathways. Molecular changes induced by MLN8237 in cell cycle–regulatory and antitumor pathways were analyzed in MM cell lines. MM cell lines were cultured in the absence or presence of DMSO or MLN8237 (0.5-1μM) for various times. Protein level changes were determined by either Western blotting or flow cytometry. (A) Relative expression of negative cell cycle–regulatory molecules PP1α and PP2αA in MM cell lines treated with MLN8237 (0.5μM) for the indicated times. Pixel density of each protein band was measured using ImageJ software (1.37v; National Institutes of Health, http://rsb.info.nih.gov/ij/) and normalized with GAPDH expression. Fold expression per control for each band is shown. *Statistical significance (t test, one-tailed distribution, P < .05; n = 3 independent experiments). (B) Intracytoplasmic expression of phospho(Thr183/Tyr185) SAPK/JNK and phospho(Thr180/Tyr182) MAPK in MM1.S cells after 1-hour exposure to DMSO or MLN8237 (0.5μM). Dotted line indicates isotypic control; blue line, DMSO; and red line, MLN8237 (0.5μM for 1 hour). (C) Molecular changes induced by MLN8237 in p53 pathway were determined by Western blotting in MM cell lines. MLN8237 induced expression of p53, p27, and p21 in MM cell lines. MM cell line OPM1 does not express p21. GAPDH was used as a control to determine total protein expression. (D) Intracytoplasmic expression of cell-cycle checkpoint molecules phospho(Tyr15) Cdc2, phospho(Ser345) Chk1, and phospho(Thr68) Chk2 were analyzed in MM1.S cells after 1-hour exposure to DMSO or MLN8237 (0.5μM). Dotted line indicates isotypic control; blue line, DMSO; and red line, MLN8237 (0.5μM for 1 hour). (E) Molecular changes induced by MLN8237 in α-tubulin activation and formation were analyzed by Western blotting in MM cell lines. MLN8237 induced expression of α-tubulin in MM cell lines at early exposure time, whereas there was decreased expression of α-tubulin after 24 hours of MLN8237 treatment. GAPDH was used as a control to determine total protein expression.
In vivo anti-MM activity of MLN8237
We next investigated the in vivo efficacy of MLN8237 in a MM xenograft murine model. MM.1S cells were inoculated subcutaneously into γ-irradiated CB-17 SCID mice (n = 40, 8-10 mice each group). When tumors were measurable (> 100 mm3) 2 weeks after MM cell injection, mice were treated with daily oral doses of vehicle alone or 7.5 mg/kg, 15 mg/kg, and 30 mg/kg MLN8237 for 21 days. Mice were monitored regularly for changes in tumor size, body weight, and signs of infections. All animal studies were performed according to the regulations approved by Dana-Farber Cancer Institute Institutional Animal Care and Use Committee. Overall survival (defined as time between injection of tumor cells and sacrifice or death) was compared in vehicle versus MLN8237-treated mice by the Kaplan-Meier method. Tumor burden was significantly reduced (P = .007 unpaired, one-tailed t test) in animals treated with 30 mg/kg MLN8237 compared with controls (Figure 5A). TGI was significant in the 15 mg/kg (TGI = 42%, P < .025) and 30 mg/kg (TGI = 80%, P < .001) treated animals compared with vehicle controls. TGI was maintained in the 30 mg/kg MLN8237-treated group 2 weeks after treatment was completed, but tumor regrowth was observed in 2 animals thereafter. Importantly, overall survival from the time of injection of tumor cells was significantly prolonged (P > .004, log-rank test) in animals treated with MLN8237 compared with vehicle controls (Figure 5B). Median overall survival of treated animals (7.5 mg/kg = 50 days, 15 mg/kg = 52 days, and 30 mg/kg = 58 days) was significantly longer than control animals (41.5 days; P > .004, log-rank test). Interestingly, there was no measurable tumor at 58 days in one animal that received (30 mg/kg) treatment. Importantly, there were no significant changes in weight or other appearance of toxicity and infection in animals that received treatment (Figure 5C), suggesting a favorable toxicity profile. In vivo cytotoxic effects of MLN8237 were then validated by performing TUNEL apoptosis assay in tumor tissues excised from treated versus control animals. There was a significant dose-related increase in apoptotic cells in tumors harvested from MLN8237-treated, but not vehicle-treated, animals (Figure 5D).
MLN8237 induces inhibition of tumor growth in MM xenograft murine model. MM1.S cells were injected subcutaneously into SCID mice. After 2 weeks of tumor engraftment, mice were treated orally with vehicle or MLN8237 (7.5 mg/kg, 15 mg/kg, and 30 mg/kg) for 21 consecutive days. Tumor size, survival, and changes in weight were measured. (A) Tumor growth was significantly inhibited (t test, one-tailed distribution, P < .007). Mean tumor volumes (cubic millimeter [volume]) ± SD (n = 8-10 animals/group) are shown from the beginning of treatment. (B) The overall survival after the injection of tumor cells was significantly increased (log-rank, Mantel-Cox test, P < .03; log-rank test for trend, P < .005) in treated compared with control animals. (C) Treatment with MLN8237 was not associated with significant changes in weight (t test, one-tailed distribution, P = not significant). Mean weight (grams) ± SD (n = 8-10 animals/group) is shown from the beginning of treatment. (D) Induction of apoptosis and cell death in tumors excised from vehicle or MLN8237 (7.5 mg/kg, 15 mg/kg, and 30 mg/kg) treated animals are shown by TUNEL assay. Photomicrographs show apoptotic cells indicated by arrows (brown) with nucleus (blue; original magnification ×20) using light microscopy. The average number of TUNEL-positive cells was determined in 93 mm2 area.
MLN8237 induces inhibition of tumor growth in MM xenograft murine model. MM1.S cells were injected subcutaneously into SCID mice. After 2 weeks of tumor engraftment, mice were treated orally with vehicle or MLN8237 (7.5 mg/kg, 15 mg/kg, and 30 mg/kg) for 21 consecutive days. Tumor size, survival, and changes in weight were measured. (A) Tumor growth was significantly inhibited (t test, one-tailed distribution, P < .007). Mean tumor volumes (cubic millimeter [volume]) ± SD (n = 8-10 animals/group) are shown from the beginning of treatment. (B) The overall survival after the injection of tumor cells was significantly increased (log-rank, Mantel-Cox test, P < .03; log-rank test for trend, P < .005) in treated compared with control animals. (C) Treatment with MLN8237 was not associated with significant changes in weight (t test, one-tailed distribution, P = not significant). Mean weight (grams) ± SD (n = 8-10 animals/group) is shown from the beginning of treatment. (D) Induction of apoptosis and cell death in tumors excised from vehicle or MLN8237 (7.5 mg/kg, 15 mg/kg, and 30 mg/kg) treated animals are shown by TUNEL assay. Photomicrographs show apoptotic cells indicated by arrows (brown) with nucleus (blue; original magnification ×20) using light microscopy. The average number of TUNEL-positive cells was determined in 93 mm2 area.
Discussion
We and others have shown, similar to other cancers, that Aurora kinases are expressed at various levels and frequencies at the transcriptional and translational levels in both newly diagnosed and relapsed patients with MM and MM cell lines.6,19,24,27,33 A recent analysis showed no association between genetic instability and expression of Aurora kinases in MM.9,20 In this preclinical study, we investigated the anti-MM activity of MLN8237, an orally available novel small molecule selective inhibitor of Aurora-A kinase. Aurora-A kinase activity is necessary for proper mitotic progression11,12,26 : expression of Aurora-A kinase protein peaks during mitosis and is activated by phosphorylation at Thr288.34 Our study shows that MLN8237 mediates its antitumor activity by inhibition of Aurora-A kinase activation via phosphorylation at T288 amino acid residue,26 whereas Aurora-B remains intact and functional, as suggested by the increased phosphorylation of histone H3 at Ser10 in treated MM cell lines. MLN8237 has more than 200-fold higher selectivity for Aurora-A than the structurally related kinase Aurora-B.32 Thus, inhibition of Aurora-A kinase with MLN 8237 may represent a novel therapeutic strategy in MM.
In our in vitro study, we have shown that MLN8237 inhibits cell growth and proliferation in a large panel of MM cell lines and primary tumor cells at nanomolar levels, associated with mitotic spindle abnormalities and accumulation of G2/M cells consistent with Aurora-A kinase inhibition. Potent cytotoxic activity was also observed by MTT assay in primary MM cells. Importantly, we detected no significant growth inhibition triggered by MLN8237 in phytohemagglutinin-stimulated, proliferating healthy PBMCs compared with MM cells. Moreover, drug-related inhibition of proliferation in healthy PBMCs was observed only at concentration of MLN8237 (0.1-4μM) higher than those active against MM cell lines (0.01-0.1μM).
The BM microenvironment plays a crucial role in promoting MM growth and cell adhesion-mediated drug resistance, and we next therefore determined whether MLN8237 is able to overcome the protective effect of the BM microenvironment on MM cells. Importantly, we observed that MLN8237 has more potent anti-MM activity in the presence of BM stroma cells and cytokines IL-6 and IGF-1 than against MM cells alone. In addition, MLN8237 induces apoptosis and cell death in MM cells in a shorter time (24 hours) and at lower concentrations (< 100nM) than other Aurora kinase inhibitors.6,9,20 Consistent with the phenotype induced by Aurora-A kinase inhibition, we confirmed that MLN8237 induces accumulation of cells at G2/M, resulting in cell death via apoptosis in cultured MM cells. However, the induction of G2/M arrest and apoptosis were less pronounced in purified tumor cells from BM of patients with MM compared with cell lines, perhaps because of the low proliferation of purified patient MM cells in vitro. PARP, caspase 3, and caspase 9 cleavage was induced by MLN8237 in MM cell lines, confirming apoptosis. Interestingly, β-galactosidase staining indicated that MLN8237 induces not only apoptosis and MM cell death, but also cell senescence. Moreover, MLN8237 shows strong synergistic anti-MM effect with dexamethasone, as well as additive effect with doxorubucin and bortezomib. These preclinical studies suggest that MLN8237 may have clinical utility, both alone and combined with other clinically available anti-MM agents.
Aurora-A kinase is positioned in the centrosome early in G2 and activates Cdc2 and several other proteins by phosphorylation to induce centrosome maturation, proper chromosome segregation, and mitotic bipolar spindle formation.35,36 Regulation of its kinase activity depends on autophosphorylation of Thr288; conversely, Thr288 phosphorylation is inhibited by type-1 protein phosphatase 1 (PP1).36 PP1 together with protein phosphatase 2A (PP2A) are major eukaryotic serine/threonine protein phosphatases regulating cell functions. PP1 regulates Aurora-A kinase activity, is associated with Aurora-A kinase in vivo, and can inactivate Aurora-A in vitro by preventing the phosphorylation of Thr288.36-39 In this preclinical study, we found that PP1α and PP2αA expression was up-regulated in MM cells early after treatment with MLN8237 (≤ 1 hour) and decreased thereafter. These data suggest that, in addition to its direct inhibition of autophosphorylation effect, MLN8237 may also directly or indirectly regulate PP1 activation to prevent autophosphorylation of Aurora-A kinase, thereby resulting in G2/M arrest and cell death in MM cells. Finally, G2/M arrest is associated with decreased phosphorylated Cdc2 (Tyr15) and Cdc25B in cancer.40 We here show that MLN8237 decreases pCdc2 (Tyr15), consistent with previous studies showing that inhibition of β-catenin using RNAi also down-regulates expression of both Aurora-A kinase protein and Cdc25B in MM cells.19
It has been reported that Aurora-A kinase localizes in the centrosome late in the G1 and early S phase of mitosis, where it regulates mitotic spindle formation by recruiting: TACC,41 a centrosomal microtubule stabilizing microtubule-associated protein; Kinesin 5, a motor protein that is essential for the formation of the bipolar mitotic spindle; and tubulins, structural molecules to polymerize centrosomal microtubules.12 Aurora-A is necessary for centrosome separation after mitotic spindle formation. Conversely, as we have shown in this study, inhibition of Aurora-A leads to accumulation of tubulin in centrosomes, causing immature centrosomes. Mitotic spindles will therefore not be separated, resulting in unseparated centrosomes; and cytokinesis, separation of parent cytoplasm into 2 daughter cells, will not be completed. In our study, inhibition of PP1 activity after longer exposures to MLN8237 resulted in decreased expression of tubulin in MM cells, suggesting an indirect effect of MLN8237 on PP1 activity and tubulin formation.
It has been reported that Chk1 is functionally associated with G2/M transition, mitotic spindle formation, and conversely, that inhibition of Chk1 results in abrogation of checkpoints induced by DNA-damaging chemotherapy and radiation, thereby leading to enhanced tumor cell killing.42-44 Although Chk2 function can be mimicked by Chk1, Chk1 cannot be replaced by Chk2.42 We here show that there was no change in phoshorylation of Chk2 (pChk2; Thr68) in MLN8237-treated MM cell lines, but there was a significant decrease in phosphorylated Chk1 (pChk1; Ser345). These data suggest, in addition to its direct anti-MM activity, that MLN8237 may render MM cells susceptible to chemotherapy- and radiotherapy-induced DNA damage. Finally, similar to previous reports that induction of apoptosis and cell death as well as cell senescence are associated with changes in the expression and activation of antitumor molecule p53 pathway, we here detected increased expression of p53, p27, and p21 in MLN8237-treated MM cell lines. There was also decreased activation of stress-activated protein kinases (pSAPK/JNK; Thr183/Tyr185), without modulation of the p38MAPK pathway (pp38MAPK; Thr180/Tyr182).
We have previously developed xenograft models of MM to assess in vivo efficacy of novel agents. In these models, we here observed significant tumor growth inhibition and prolonged survival after initiation of treatment with MLN8237, suggesting robust anti-MM activity of MLN8237 in vivo. Importantly, analysis of excised tumors from treated animals confirmed apoptosis, and therapy was well tolerated.
In conclusion, we here show that MLN8237, an orally active small molecule inhibitor of Aurora-A kinase, induces accumulation of MM cells at G2/M, mitotic spindle abnormalities, aneuploidy, apoptosis, and senescence. It inhibits proliferation by regulating both mitotic checkpoints and antitumor pathways in MM. MLN8237 is currently in phase 1 and phase 2 clinical trials in patients with advanced malignancies, and our studies provide the preclinical rationale for its clinical evaluation in MM.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the National Institutes of Health/National Cancer Institute Specialized Program of Research Excel-lence in Myeloma (P50 CA100707; K.C.A.), National Institutes of Health/National Cancer Institute Host-Tumor Cell Interactions in Myeloma (Therapeutic Applications P01 CA78378; K.C.A.), and National Institutes of Health/National Cancer Institute Molecular Sequelae of Myeloma-Bone Marrow Interactions (Therapeutic Applications R01 CA50947; K.C.A.).
National Institutes of Health
Authorship
Contribution: G.G., E.C., T.H., M.M., G.P., H.I., G.B., Y.H., D.C., L.S., Y.-T.T., M.Z., S.N., R.D.C., and M.B. performed experiments; N.R., N.M., P.R., and K.C.A. provided the clinical samples; G.G. analyzed results and made the figures; and G.G., J.E., and K.C.A. designed the research and wrote the paper.
Conflict-of-interest disclosure: K.C.A., N.M., P.R., and N.R. are members of the Board of Directors or advisory committees for Millennium, Celgene, and Novartis. N.R. is a consultant for AstraZeneca (research grants). J.E. is employed by Millennium Pharmaceuticals. The remaining authors declare no competing financial interests.
Correspondence: Güllü Görgün, Dana-Farber Cancer Institute, Department of Medical Oncology, 44 Binney St, M557, Boston, MA 02115; e-mail: gullu_gorgun@dfci.harvard.edu.

![Figure 2. MLN8237 inhibits cell viability and proliferation in MM cells in the presence or absence of BM stroma cells. A panel of MM cell lines and tumor cells obtained from patients with MM were treated with DMSO or increasing doses of MLN8237 (0.0001-4μM) for 48 to 72 hours. (A) Inhibition of cell line viability was determined by MTT assay. Inhibition of cell proliferation was determined by 3[H]thymidine incorporation assay in MM cell lines (B) and in tumor cells obtained from MM patients (C). MM1.S and RPMI8226 cell lines and tumor cells from patients with MM were cocultured with either BM stroma cells from patients with MM or cytokines (IL-6, 10 ng/mL; IGF-1, 25 ng/mL), in the absence or presence of MLN8237 (0.001-4μM) for 24 to 48 hours. (D) MM cell proliferation was measured by 3[H]thymidine incorporation assay in the cocultures with BM stroma cells. (E) MM cell viability was also assessed by MTT assay, in the absence or presence of IL-6 or IGF-1. Data represent mean ± SD of triplicate cultures. Statistical significance indicated (t test, 1-tailed distribution, P < .05).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/115/25/10.1182_blood-2009-12-259523/4/m_zh8999105417002a.jpeg?Expires=1769201439&Signature=ETWK2tKxI7HhO2DnlJplzSaSHfM3tl8sDjPgBxxShIsBPLKqvsF2tQBo~7vWlpiRU5gjuSjmTxLfKc0xClKqtD1HbwldAlWn~TEhP5bE0Om8mVSPWyqPrHMTATLHMFWFtgjVQWjvi-qA5Gpwqk19-JvXwzSmN24Fog-ud~zvHdomT-wLWUyilpb2xV00ScjuW27Dlf8nk3X5F2BckIs6uRwLOB7CptITHmrGIQM9IVuNKMZZ~92oF~Cz0IjxWEGtC2PZNb2YhNyZH50S46tp66CnvhIJi7dwCUupyNoACXVIS-KCqPGXQ5kLWQc0JBUc4BYX9iVEpmXLXHtCIgb75A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 2. MLN8237 inhibits cell viability and proliferation in MM cells in the presence or absence of BM stroma cells. A panel of MM cell lines and tumor cells obtained from patients with MM were treated with DMSO or increasing doses of MLN8237 (0.0001-4μM) for 48 to 72 hours. (A) Inhibition of cell line viability was determined by MTT assay. Inhibition of cell proliferation was determined by 3[H]thymidine incorporation assay in MM cell lines (B) and in tumor cells obtained from MM patients (C). MM1.S and RPMI8226 cell lines and tumor cells from patients with MM were cocultured with either BM stroma cells from patients with MM or cytokines (IL-6, 10 ng/mL; IGF-1, 25 ng/mL), in the absence or presence of MLN8237 (0.001-4μM) for 24 to 48 hours. (D) MM cell proliferation was measured by 3[H]thymidine incorporation assay in the cocultures with BM stroma cells. (E) MM cell viability was also assessed by MTT assay, in the absence or presence of IL-6 or IGF-1. Data represent mean ± SD of triplicate cultures. Statistical significance indicated (t test, 1-tailed distribution, P < .05).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/115/25/10.1182_blood-2009-12-259523/4/m_zh8999105417002b.jpeg?Expires=1769201439&Signature=WNO2-rjQ5ZL0qcYamAs-5gTc6YbgQjYr0SrWr2OHkXmTWMnckN4ONqWOL977hZgLaed3NHVNYlcywMdcRx0KZ7lCYw4a6O-O1NDPJ0z2Q1S4D8B0wAiWDwk0iOAfV7zuPcbhJKQ-SiPZhYwa2YeEcvJxdNWrrCVXJ1Tm6A9vQ8ZVUtRPsgJF388fQmLAAMXwv~BdclyZMq7-7ZZfJEvoqBFx-cbbwcgb-sVQp-g70a5iHoyWbpxf34L1uU7fJ654oRGsT3sAsv73kryY9t86oDi-wnQEe0erv3FKga9I1CJPKTJnwcVKfGcHxW03YfCcS9H-kecuYfiIaoAiHMsd9A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 5. MLN8237 induces inhibition of tumor growth in MM xenograft murine model. MM1.S cells were injected subcutaneously into SCID mice. After 2 weeks of tumor engraftment, mice were treated orally with vehicle or MLN8237 (7.5 mg/kg, 15 mg/kg, and 30 mg/kg) for 21 consecutive days. Tumor size, survival, and changes in weight were measured. (A) Tumor growth was significantly inhibited (t test, one-tailed distribution, P < .007). Mean tumor volumes (cubic millimeter [volume]) ± SD (n = 8-10 animals/group) are shown from the beginning of treatment. (B) The overall survival after the injection of tumor cells was significantly increased (log-rank, Mantel-Cox test, P < .03; log-rank test for trend, P < .005) in treated compared with control animals. (C) Treatment with MLN8237 was not associated with significant changes in weight (t test, one-tailed distribution, P = not significant). Mean weight (grams) ± SD (n = 8-10 animals/group) is shown from the beginning of treatment. (D) Induction of apoptosis and cell death in tumors excised from vehicle or MLN8237 (7.5 mg/kg, 15 mg/kg, and 30 mg/kg) treated animals are shown by TUNEL assay. Photomicrographs show apoptotic cells indicated by arrows (brown) with nucleus (blue; original magnification ×20) using light microscopy. The average number of TUNEL-positive cells was determined in 93 mm2 area.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/115/25/10.1182_blood-2009-12-259523/4/m_zh89991054170005.jpeg?Expires=1769201439&Signature=f7fLpdl4TkmO3XOdSyH~NIFUbavNhfD5Kh8Op2ZlgnEzD93uu7xzFI0DaVgkHIBt4d9DS88ezMjwhNnQbQ0O6Yn7cLruwbF1QxIlMuO4SyNCG3tj0rs3y5Ybta7LDVEr5QE6J~9XGN65rklqBl-eSvfgQAph1xlfDdEiFHg9w1DaEanAlaumGwA4Zc7VQN4qk48AO95NKv1jDXTXKIgDBlktFC47v68pG0hcbPlFggzfrMnK8hPVGTML08jrIy9~mwK6pG4lxDGJbqfrqcNOuoy5cUC12LxWtiAHsCY273MTe0BMnntg0XmM9dZzXyoWl8tc1EA-91IyNs2FNdqomQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal