Abstract
Repopulation of immunodeficient mice remains the primary method to assay human hematopoietic stem cells (HSCs). Here we report that female NOD/SCID/IL-2Rgc-null mice are far superior in detecting human HSCs (Lin−CD34+CD38−CD90+CD45RA−) compared with male recipients. When multiple HSCs were transplanted, female recipients displayed a trend (1.4-fold) toward higher levels of human chimerism (female vs male: injected femur, 44.4 ± 9.3 vs 32.2 ± 6.2; n = 12 females, n = 24 males; P = .1). Strikingly, this effect was dramatically amplified at limiting cell doses where female recipients had an approximately 11-fold higher chimerism from single HSCs (female vs male: injected femur, 8.1 ± 2.7 vs 0.7 ± 0.7; n = 28 females, n = 20 males; P < .001). Secondary transplantations from primary recipients indicate that females more efficiently support the self-renewal of human HSCs. Therefore, sex-associated factors play a pivotal role in the survival, proliferation, and self-renewal of human HSCs in the xenograft model, and recipient sex must be carefully monitored in the future design of experiments requiring human HSC assays.
Introduction
The dynamic interplay between donor hematopoietic stem cells (HSCs) and the recipient microenvironment governs the survival and proliferation of HSCs in a transplantation setting. A complex network of cells within the microenvironment regulates HSC function via direct interaction or secretion of cytokines that act in an autocrine or paracrine manner.1 However, the effects on HSCs by molecules that communicate in an endocrine fashion, such as steroid hormones, remain understudied. Interestingly, sex-associated hormones, including androgens and estrogen, have been directly implicated in regulating hematopoietic cell function.2-4 During the course of our functional analyses of HSCs using the recently developed NOD/SCID/IL-2Rgc-null (NSG) mice,5 we observed that the recipient sex plays a critical role in the engraftment and proliferation of human HSCs. Specifically, female NSG mice are far superior to their male counterparts in engrafting and detection of single human HSCs.
Methods
Collection of human CB
Human cord blood (CB) was collected according to guidelines established by Trillium Health Center and University Health Network. Mononuclear cells from pooled CBs were harvested using a Ficoll gradient and subsequently depleted of mature cells (Lin−) via negative selection using StemSep system (StemCell Technologies). Cells were viably frozen in 10% dimethyl sulfoxide solution and stored at −80°C or −150°C.
Fluorescence-activated cell sorting
Freshly thawed Lin− CB was stained with CD34 allophycocyanin, CD38 PC7, CD45RA fluorescein isothiocyanate, and CD90 phycoerythrin in phosphate-buffered saline plus 5% fetal calf serum at a concentration of 107 cells/mL for 30 minutes at 4°C. Cells were sorted on FACSAria II (BD Biosciences), and purities of more than 99% were commonly achieved in postsort analysis (data not shown). Cells were counted in Trypan blue before transplantation and resuspended in 25 μL of Iscove modified Dulbecco medium/mouse in a 28.5-gauge 0.5-mL insulin syringe.
Xenotransplantation and detection of human chimerism
All animal experiments were performed with the approval of the Animal Care Committee at the University Health Network. Ten- to 12-week-old NSG mice were sublethally irradiated (200-225 cGy) 24 hours before transplantation. Cells were transplanted intrafemorally as previously described.6 Briefly, a 27-gauge needle was used to drill a hole in the right femur, which was followed by injection of cells using a 28.5-gauge insulin syringe. Mice were killed 16 to 20 weeks after transplantation. To evaluate human engraftment, cells were collected from injected femur (IF; right femur), noninjected bones (bone marrow [BM], including left femur, right and left tibiae), spleen (SP), and thymus (TH), and stained with CD45PC7, CD45PC5 (Beckman Coulter), CD19, CD33, and CD4 (Beckman Coulter) and CD8 (all BD Biosciences, unless indicated). Threshold for human engraftment was considered to be 0.1% double-positive for CD45. All flow cytometric analysis was performed on the LSRII (BD Biosciences).
Statistical analysis
Data are mean plus or minus SEM. The significance of the differences between groups was determined using the Mann-Whitney test.
Results and discussion
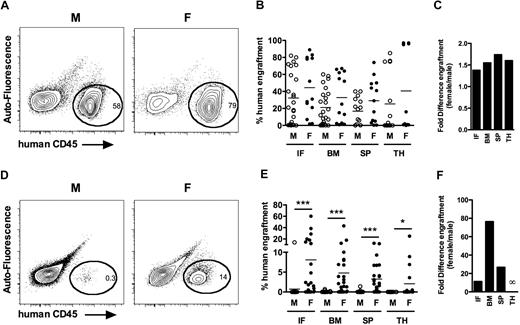
In the hematopoietic system, HSCs possess extensive self-renewal and differentiation capacities and remain the only cell type capable of long-term multilineage engraftment. Human HSCs capable of this feat reside in the Lin−CD34+CD38−CD90+CD45RA− compartment.7-10 This population was flow-sorted from Lin− CB and intrafemorally transplanted at equivalent cell doses into sublethally irradiated adult male and female NSG mice (supplemental Figure 1A, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Human chimerism was assessed by flow cytometry 16 to 24 weeks after transplantation. The cell dose varied (245-5000 cells) and was considered nonlimiting because all mice transplanted at these doses displayed human multilineage engraftment (supplemental Figure 1B-C). When multiple HSCs were transplanted, female NSG mice displayed a trend toward higher levels of human engraftment compared with males (female vs male: IF, 44.4. ± 9.3 vs 32.2 ± 6.2, P = .14; BM, 32.8 ± 7.8 vs 21.1 ± 4.3, P = .16; SP, 29.1 ± 7.1 vs 16.7 ± 4.0, P = .12; TH, 40.5 ± 15.2 vs 25.3 ± 9.5, P = .12; n = 13 females, 24 males, 3 independent experiments; Figure 1A-B). Females displayed 1.4-, 1.6-, 1.7-, and 1.6-fold increases in engraftment in the IF, BM, SP, and TH, respectively, compared with males (Figure 1C). There were no differences in lineage distribution of human cells engrafted in male or female recipients (supplemental Figure 1C). Therefore, Lin−CD34+CD38−CD90+CD45RA− CB cells repopulate at modestly higher levels in females compared with age-matched male NSG mice.
Female NSG mice more efficiently support human HSC detection and proliferation than syngeneic male mice. Male and female recipients transplanted with Lin−CD34+CD38−CD90+CD45RA− cells (supplemental Figure 1) were killed 16 to 20 weeks after transplantation, and human chimerism was assessed by flow cytometry in the IF, noninjected bones (BM, left femur, 2 tibiae), SP, and TH. (A) Representative analysis from primary male and female recipients transplanted with Lin−CD34+CD38−CD90+CD45RA− cells. (B) Mean human engraftment levels in the IF, BM, SP, and TH of male (○, n = 24) and female (●, n = 13) NSG recipients transplanted with Lin−CD34+CD38−CD90+CD45RA− cells (range, 245-5000). This analysis was consistent in 3 independent experiments. (C) Bars represent fold difference in engraftment levels between male and female recipients from panel B. (D) Representative analysis from primary male and female recipients transplanted with 25 Lin−CD34+CD38−CD90+CD45RA− cell dose. (E) Mean human engraftment levels in the IF, BM, SP, and TH of male (○, n = 20) and female (●, n = 28) NSG recipients transplanted with Lin−CD34+CD38−CD90+CD45RA− cells (range, 10-25), from 4 independent experiments. (F) Bars represent fold difference in engraftment levels between male and female recipients from panel E. *P < .05. ***P < .001.
Female NSG mice more efficiently support human HSC detection and proliferation than syngeneic male mice. Male and female recipients transplanted with Lin−CD34+CD38−CD90+CD45RA− cells (supplemental Figure 1) were killed 16 to 20 weeks after transplantation, and human chimerism was assessed by flow cytometry in the IF, noninjected bones (BM, left femur, 2 tibiae), SP, and TH. (A) Representative analysis from primary male and female recipients transplanted with Lin−CD34+CD38−CD90+CD45RA− cells. (B) Mean human engraftment levels in the IF, BM, SP, and TH of male (○, n = 24) and female (●, n = 13) NSG recipients transplanted with Lin−CD34+CD38−CD90+CD45RA− cells (range, 245-5000). This analysis was consistent in 3 independent experiments. (C) Bars represent fold difference in engraftment levels between male and female recipients from panel B. (D) Representative analysis from primary male and female recipients transplanted with 25 Lin−CD34+CD38−CD90+CD45RA− cell dose. (E) Mean human engraftment levels in the IF, BM, SP, and TH of male (○, n = 20) and female (●, n = 28) NSG recipients transplanted with Lin−CD34+CD38−CD90+CD45RA− cells (range, 10-25), from 4 independent experiments. (F) Bars represent fold difference in engraftment levels between male and female recipients from panel E. *P < .05. ***P < .001.
To assess the effects of sex on the repopulation capacity of single human HSCs, we performed limiting-dilution cell dose transplantations with Lin−CD34+CD38−CD90+CD45RA− fraction (doses, 10-25). Transplantation of doses equivalent to a single HSC unveiled striking differences in total engraftment between male and female recipients (female vs male: IF, 8.1 ± 2.7 vs 0.7 ± 0.7, P < .001; BM, 4.8 ± 1.7 vs 0.1 ± 0.04, P < .001; SP, 3.2 ± 0.8 vs 0.1 ± 0.1, P < .001; TH, 2.1 ± 1.2 vs 0, P = .04; n = 28 females, 20 males, 4 independent experiments; Figure 1D-E). Female mice had 11.3-, 76.5-, and 26.7-fold higher mean engraftment levels in IF, BM, and SP, respectively (Figure 1F). Furthermore, 23 of 28 females and 3 of 20 males were engrafted at limiting dose, indicating that females were approximately 5-fold more sensitive in detecting human HSCs. Thus, proliferation and detection of HSCs at the clonal level, but not their lineage potential (supplemental Figure 1C), are markedly enhanced in female NSG recipients. Interestingly, only clonal doses revealed these dramatic differences, indicating that transplantation of saturating levels of HSCs can mitigate sex effects on engraftment. These results are consistent with Ballen et al,11 where high numbers of CB cells transplanted in NOD/SCID mice did not reveal significant effects of recipient sex on human engraftment. Therefore, female NSG recipients more efficiently support the repopulation of single human HSCs.
In the initial phase of engraftment, transplanted HSCs home to the bone marrow and lodge in a supportive microenvironment. To determine whether this process was influenced by recipient sex, Lin− CB cells were injected intravenously and were quantified 16 hours after transplantation. Homing efficiency of both total Lin− CB and HSCs was similar between male and female mice, indicating that sex-specific factor(s) influence long-term, but not initial, phases of HSC engraftment (supplemental Figure 2A-B).
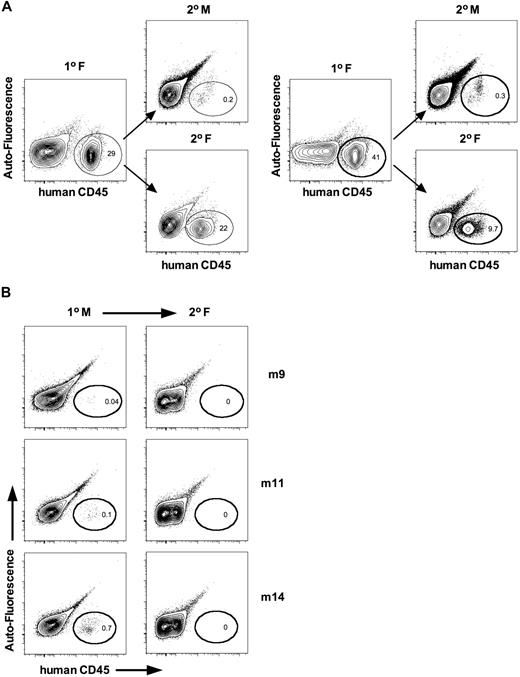
The self-renewal potential of HSCs can be evaluated using long-term repopulation assays or secondary transplantations. Long-term repopulation indirectly measures this capability because self-renewal divisions must be executed by HSCs to maintain chimerism. Historically, however, secondary transplantations remain the most commonly used technique to assay for self-renewal. We performed parallel secondary transplantations from both male and female primary recipients. Human cells from primary females generated higher levels of human engraftment in parallel secondary female, compared with male, recipients (Figure 2A). To rule out whether male mice harbored quiescent HSCs that did not proliferate efficiently, we also performed secondary transplantations into females from 12 primary males that had undetectable (< 0.1%, n = 8) or low (> 0.1%, n = 4) engraftment levels. No engraftment was observed in secondary recipients (Figure 2B), indicating that male mice do not harbor quiescent HSCs. These data support the conclusion that female NSG recipients better support the self-renewal of human HSCs.
Female NSG mice more efficiently support the self-renewal of human HSCs. (A) Whole bone marrow (IF and BM) was harvested and transplanted in parallel into secondary male and female recipients at equivalent cell dose. Mice were killed 12 weeks after transplantation, and human cell engraftment was assessed by flow cytometry. Shown is the representative analysis from 2 of 4 independent cases. (B) Whole bone marrow from primary male mice displaying low (> 0.1%, n = 4) or undetectable (< 0.1%, n = 8) levels of human engraftment were transplanted into secondary female recipients to evaluate whether males harbored quiescent HSCs. No engraftment was detected in secondary females. Shown is representative analysis from 3 cases.
Female NSG mice more efficiently support the self-renewal of human HSCs. (A) Whole bone marrow (IF and BM) was harvested and transplanted in parallel into secondary male and female recipients at equivalent cell dose. Mice were killed 12 weeks after transplantation, and human cell engraftment was assessed by flow cytometry. Shown is the representative analysis from 2 of 4 independent cases. (B) Whole bone marrow from primary male mice displaying low (> 0.1%, n = 4) or undetectable (< 0.1%, n = 8) levels of human engraftment were transplanted into secondary female recipients to evaluate whether males harbored quiescent HSCs. No engraftment was detected in secondary females. Shown is representative analysis from 3 cases.
In conclusion, we report a striking and previously unappreciated role of sex in the engraftment potential of human HSCs in the xenotransplantation model. In our perspective, 2 rationales exist to reconcile this dichotomy: (1) female NSG mice might be more immunodeficient than males, or (2) sex-associated factors, such as steroid hormones, can positively or negatively regulate human HSCs. Residual B, T, and natural killer cells could not be detected over background levels by flow cytometry, suggesting that the IL2Rgc deletion is fully penetrant in male mice (data not shown). Furthermore, cellular toxicity to the murine hematopoietic system observed after sublethal irradiation at increasing doses was comparable between age-matched male and female mice, ruling out the possibility that the increased body weight of male mice serves to mitigate the effects of sublethal irradiation (supplemental Figure 2C-E).
The role of sex hormones in immune function is well documented, and the expression of cognate receptors on HSCs implies the potential to respond to ligand binding. However, the expression of receptors, such as estrogen and progesterone receptor, varies during ontogeny.4 Consistent with murine studies, adult human BM, and not CB CD34+, expresses detectable levels of estrogen and progesterone, whereas androgen receptor is expressed in both.4 Although further experiments are required to identify sex-specific mechanisms of HSC engraftment, our study has revealed their key role and supports the concept of recipient sex as a critical variable in the context of stem cell transplantation studies.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank A. Poeppl and L. Jin for technical assistance and the rest of the Dick Laboratory for the critical review of this manuscript; K. Moore and the obstetrics unit of Trillium Hospital (Mississauga, ON) for providing cord blood samples and cooperative students for processing cord blood samples; and P. A. Penttilä, L. Jamieson, and S. Zhao at our UHN/Sickkids flow cytometry facility.
This work was supported by Canadian Institutes for Health Research studentships (F.N., S.D.), the Stem Cell Network of Canadian National Centres of Excellence, the Canadian Cancer Society and the Terry Fox Foundation, Genome Canada through the Ontario Genomics Institute, Ontario Institute for Cancer Research with funds from the province of Ontario, the Leukemia & Lymphoma Society, the Canadian Institutes for Health Research, and a Canada Research Chair. This research was supported in part by the Ontario Ministry of Health and Long Term Care.
The views expressed do not necessarily reflect those of the Ontario Ministry of Health and Long Term Care.
Authorship
Contribution: F.N., S.D., and J.E.D. designed research and wrote the manuscript; and F.N. and S.D. performed experiments.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: John E. Dick, Toronto Medical Discovery Tower, Rm 8-301, 101 College St, Toronto, ON M5G 1L7, Canada; e-mail: jdick@uhnres.utoronto.ca.
References
Author notes
F.N. and S.D. contributed equally to this study.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal