Abstract
Human mast cells are tissue resident cells with a principal role in allergic disorders. Cross-linking of the high-affinity receptor for immunoglobulin E (FcϵRI) results in release of inflammatory mediators initiating the clinical symptoms of allergy and anaphylaxis. Much of our knowledge regarding the mechanisms of mast cell activation comes from studies of mouse bone marrow–derived mast cells. However, clear differences have been identified between human and mouse mast cells. Studies of human mast cells are hampered by the limited sources available for their isolation, the resistance of these cells to genetic manipulation, and differences between cultures established from different persons. To address this limitation, we developed a simple coculture-free method for obtaining mast cells from human embryonic stem cells (hES). These hES-derived mast cells respond to antigen by releasing mast cell mediators. Moreover, the cells can be generated in numbers sufficient for studies of the pathways involved in their effector functions. Genetically modified mast cells, such as GFP-expressing cells, can be obtained by introduction and selection for modification in hES cells before differentiation. This direct coculture-free differentiation of hES cells represents a new and unique model to analyze the function and development of human mast cells.
Introduction
Mast cell activation plays a critical role in the protective response to certain parasites and in the pathogenesis of allergic diseases. Mast cells are derived from hematopoietic precursors that migrate from the bone marrow and complete their differentiation in the microenvironment of peripheral tissues under the influence of stem cell factor and other cytokines derived from resident cells.1 Mast cell effector functions depend on their capacity to bind antigen-specific immunoglobulin E (IgE) via high-affinity IgE receptors (FcϵRI) and subsequent cross-linking of these receptors with multivalent antigen. Cross-linking of FcϵRI initiates a series of signaling events, including phosphorylation of intracellular proteins and intracellular calcium mobilization, leading to mast cell degranulation and release of preformed proteases, biogenic amines, and the biosynthesis of cytokines, chemokines, and lipid mediators. The importance of this effector cell in allergic diseases makes the understanding of mast cell function essential for the development of new therapeutics for these disorders.2
Much of our understanding of mast cell biology comes from mouse models because of the ease with which these cells can be cultured from mouse bone marrow (bone marrow–derived mast cells [BMMCs]), and the ability to use these BMMCs, especially populations obtained from genetically manipulated mice, to reconstitute mast cell-deficient mouse lines. However, many differences have been noted between mouse and human mast cells, including differential cytokine requirements for development and proliferation,3 regulation of FcϵRI expression by Th2 cytokines,4 the ability of mediators such as prostaglandins to regulate mast cell function,5,6 and response to antiallergic drugs.7 Human mast cells can be isolated in their mature form from a few human tissues, including lung and skin.8,9 Alternatively, human mast cells can be derived from isolated CD34+ hematopoietic precursors from bone marrow, cord blood, or peripheral blood. CD34+ cells are cultured in medium supplemented with recombinant human stem cell factor and recombinant human interleukin 6.10-12 Although human mast cells isolated using this approach are valuable sources for many studies, there are a number of limitations. First, mast cells cannot be cultured indefinitely; thus, a continuous source of primary tissue/blood is required. Second, genetic differences are present between each population, as they are isolated from different persons. Finally, primary mast cells cannot be easily genetically manipulated; therefore, studies with these cultured mast cells are generally limited to the use of pharmacologic approaches. Together, these limitations have proven an obstacle in the study of human mast cell function, development, and biology.
Human embryonic stem (hES) cells are capable of both self-renewal and differentiation into cells of germ layers, that is, ectoderm, endoderm, and mesoderm. hES cells therefore offer an attractive alternative for establishing human mast cell cultures. If a reliable method for obtaining functional mast cell populations can be established, the genetic makeup of the cells will remain consistent between experiments and genetic manipulations could be carried out in the hES cells, a cell type far more amenable to these maneuvers. Previous work has shown that many cell lineages, including hematopoietic progenitors, can be generated from hES cells in vitro and, furthermore, that hES cell–derived hematopoietic progenitors can be differentiated into T cells, neutrophils, macrophages, and dendritic cells.13
In general, differentiation of hES cells into hematopoietic progenitors requires the coculture of hES cells with cell lines derived from aorta-gonad-mesonephros or cell lines, such as S17 or OP9.14-17 Alternatively, hematopoietic precursors have also been isolated from hES cell–derived embryoid bodies (EBs), structures composed of all 3 germ layers, growing in a complex mixture of cytokines.18-20 These approaches have been successfully used to induce the differentiation of mouse, human, and primate embryonic stem cells into hematopoietic CD34+ cells. However, hematopoietic precursors are rare, and the establishment of primary cultures of mature immune cells using these strategies has required isolation of the CD34+ hematopoietic precursors before differentiation into specific lineages. It is also not clear whether this methodology will yield precursors capable of differentiating into mature human mast cells at a frequency sufficient for experimental studies.
A number of reports, however, suggest that it should be possible to identify conditions optimal for supporting the generation of human mast cells from embryonic stem (ES) cells. First, previous studies have reported isolation of mast cells from mouse ES cells.21,22 Evidence suggesting that similar differentiation may be possible for human cells comes from a study in which cells expressing tryptase and chymase, enzymes found in mast cells, were identified after coculture of hES cells with murine fetal liver–derived stromal cells.23 More recently, coculture of cynomolgus monkey embryonic stem cells with the murine aorta-gonad-mesonephros–derived stromal cell line AGM-S1 yielded clusters of hematopoietic progenitors which, when mechanically separated from stromal cells, could be differentiated into cells with a number of mast cell characteristics.24
Here we report 2 different approaches for the generation of mast cells from hES cells. We compare these methods both in terms of the yield and the morphologic, biochemical, and functional characteristics of the human mast cells generated. We show that hES cells can be differentiated into hematopoietic progenitors by direct differentiation of immobilized EBs in the absence of supporting cell lines. Subsequent differentiation of the progenitors resulted in hES-derived mast cells (ESMCs) expressing tryptase, chymase, and functional FcϵRI. Activation of those ESMCs by multiple ligands led to phosphorylation of intracellular substrates, intracellular calcium mobilization, and histamine release after antigen stimulation. Furthermore, we show that these cells can be generated, not only from the original hES cells lines but also from genetically modified hES populations and clonal isolates.
Methods
Cell cultures
hES cell lines H1 and H9 (WA01, WA09; National Stem Cell Bank) were maintained on irradiated mouse embryonic fibroblasts at 37°C, 5% CO2 in hES medium (Dulbecco modified Eagle medium nutrient mixture F-12, 20% KO-serum replacement, 2mM l-glutamine, 1% nonessential amino acids, 100 U/mL penicillin, and 100 μg/mL streptomycin [all from Invitrogen], and 0.055mM β-mercaptoethanol and 8 ng/mL basic fibroblast growth factor [PeproTech]). hES were passaged every 5 to 6 days. Culture conditions of OP9 cells and cord blood-derived mast cells are given in supplemental Methods (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Differentiation of hES cells to hematopoietic progenitors
EBs were obtained by dispase (Invitrogen) treatment of hES cells for 10 minutes, aggregates of hES cells were transferred to 60-mm antiadhesive-treated plates in differentiation medium (Iscove modified Dulbecco medium; Invitrogen), 15% fetal calf serum, 2mM l-glutamine, 4 × 10−4 methyl thioglycolate, 300 μg/mL transferrin (Roche Diagnostics), 50 μg/mL ascorbic acid (Sigma-Aldrich), and 5% protein-free hybridoma medium (Invitrogen). Four days later, 20 to 50 of the EBs were transferred to a Matrigel-treated (BD Biosciences) 60-mm plate with 3 mL of mast cell medium I (Stem Pro-34 SMF supplemented with 1% nonessential amino acids, 2mM l-glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin [all from Invitrogen], 100 ng/mL stem cell factor [SCF], 5 ng/mL interleukin-3 [IL-3], 100 ng/mL IL-6, and 25 ng/mL Fms-like tyrosine kinase 3 ligand [Amgen]). Medium was replaced every 5 to 7 days, and nonadherent cells were collected after 3 weeks. Differentiation of progenitors by OP9 coculture is given in supplemental Methods.
Differentiation of hematopoietic progenitors to mast cells
CD34+CD43+ cells were cultured in Iscove modified Dulbecco medium supplemented with 10% fetal bovine serum, 1% nonessential amino acids, 1mM sodium pyruvate, 10mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid, 2mM l-glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin (all from Invitrogen), SCF (100 ng/mL), and IL-6 (50 ng/mL; Amgen).
Mast cell characterization
Detailed methods of mast cell characterization, including histochemistry, flow cytometry, gene expression, and functional assays, are given in supplemental Methods.
Genetic modification of hES
Lentiviral transfection and electroporation are described in supplemental Methods.
Results
Differentiation of hES cells to hematopoietic progenitors
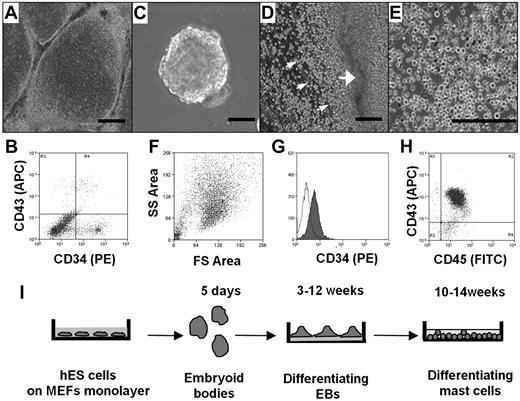
We first compared the ability of different culture conditions, namely, coculture with OP9 cells and differentiation to EBs, to direct the hES cell differentiation toward the hematopoietic lineage, as assessed by the appearance of CD34+ cells. In both cases, hES cell cultures that had achieved confluence were removed from mouse fibroblasts and basic fibroblast growth factor, factors essential for maintaining ES cell pluripotency. To test the ability of OP9 cells to stimulate differentiation to CD34+ cells, hES cells were transferred to monolayers of OP9 cells and cultured for 8 to 16 days (Figure 1A). CD34+ cells were recovered from each culture using magnetic beads labeled with anti-CD34 antibody. The number of CD34+ cells in the culture was dependent on the length of exposure to the OP9, with maximal yields observed at 12 days (data not shown). Interestingly, as shown in Figure 1B, only one-third of the CD34+ cells also expressed CD43, another marker for hematopoietic progenitors. Average yield of CD34+ CD43+ cells after 12 days of coculture with OP9 cells was 5 × 105 plus or minus 3 × 105 per 1 × 106 of hES cells (n = 4).
Differentiation of hES cells to hematopoietic progenitors. (A) hES cells were removed from mouse embryonic fibroblasts by treating with collagenase IV and cocultured for 10 days with a monolayer of OP9 in α-minimum essential medium containing 15% fetal calf serum. (B) FACS of a single-cell suspension after 12 days of hES cell coculture with OP9 cells; staining for CD34 and CD43. (C) EBs. hES cells were removed from mouse embryonic fibroblasts by treating with dispase and cultured in EB-differentiating medium for 4 days. (D) Phase-contrast image of adherent EBs differentiated in IL-6, IL-3, SCF, and Fms-like tyrosine kinase 3 ligand for 3 weeks with hematopoietic progenitors releasing to medium (small arrows indicate progenitors; and large arrow, differentiated EBs). (E) Phase-contrast image of homogeneous population of hematopoietic progenitors collected from medium 4 weeks after EB differentiation. (F) Forward-side scatter plot of hematopoietic progenitors. (G) CD34 surface expression on hematopoietic progenitors. Histogram shows antibody staining (in dark) relative to isotype-matched control (transparent). (H) CD45 and CD43 surface expression. Microscopic figures (A,C-E) were captured using an Olympus IX81 inverted fluorescence microscope with 4× (A,C,D) and 10× (E) objectives with a Hamamatsu ORCA RC camera, operated by Velocity software (PerkinElmer Life and Analytical Sciences). Bars in insets represent 100 μm. Data shown are representative from at least 3 experiments. (I) Schema showing coculture-free differentiation of human mast cells from hES cells.
Differentiation of hES cells to hematopoietic progenitors. (A) hES cells were removed from mouse embryonic fibroblasts by treating with collagenase IV and cocultured for 10 days with a monolayer of OP9 in α-minimum essential medium containing 15% fetal calf serum. (B) FACS of a single-cell suspension after 12 days of hES cell coculture with OP9 cells; staining for CD34 and CD43. (C) EBs. hES cells were removed from mouse embryonic fibroblasts by treating with dispase and cultured in EB-differentiating medium for 4 days. (D) Phase-contrast image of adherent EBs differentiated in IL-6, IL-3, SCF, and Fms-like tyrosine kinase 3 ligand for 3 weeks with hematopoietic progenitors releasing to medium (small arrows indicate progenitors; and large arrow, differentiated EBs). (E) Phase-contrast image of homogeneous population of hematopoietic progenitors collected from medium 4 weeks after EB differentiation. (F) Forward-side scatter plot of hematopoietic progenitors. (G) CD34 surface expression on hematopoietic progenitors. Histogram shows antibody staining (in dark) relative to isotype-matched control (transparent). (H) CD45 and CD43 surface expression. Microscopic figures (A,C-E) were captured using an Olympus IX81 inverted fluorescence microscope with 4× (A,C,D) and 10× (E) objectives with a Hamamatsu ORCA RC camera, operated by Velocity software (PerkinElmer Life and Analytical Sciences). Bars in insets represent 100 μm. Data shown are representative from at least 3 experiments. (I) Schema showing coculture-free differentiation of human mast cells from hES cells.
To induce differentiation of hematopoietic progenitors in coculture-free conditions through formation of EBs, large aggregates of hES cell colonies were cultured in suspension (Figure 1C). The EBs were then transferred to plates coated with laminin-rich Matrigel and cultured in serum-free medium supplemented with human recombinant (hr) IL-6, hrIL-3, hrSCF, and hrFms-like tyrosine kinase 3 ligand. Clusters of spherical cells appeared 12 days later. Four days after their appearance in culture, these cells transitioned into nonadherent populations that could be collected from the culture supernatant (Figure 1D). Cells continued to be released into the medium for more than 6 weeks. Morphologic analysis of these cells indicated a very homogeneous population (Figure 1E). This was consistent with fluorescence-activated cell sorting (FACS) analysis showing that the cells displayed uniform cell scatter properties (Figure 1F) and a uniform CD34 profile (Figure 1G). Unlike the CD34+ cells derived by coculture, the majority of these cells also expressed CD43. Consistent with their hematopoietic lineage, CD45 expression was abundant, whereas the cells did not express the monocyte marker CD14 (Figure 1H; and data not shown). The purity of these cells, determined by CD43 and CD45 double staining, was 89.8% plus or minus 2.1% (mean ± SEM, n = 6). Calculation of the number of CD34+ cells obtained via this route of differentiation showed that it was approximately 10-fold higher than that obtained by coculture with OP9 cells. Average hematopoietic progenitor yield was 4.5 × 106 plus or minus 2.0 × 106 cells per 1.0 × 106 hES cells (n = 6). Thus, although CD34+ cells can be obtained using both methods, the intermediate differentiation of the cells first to EBs had both qualitative and quantitative impact on the hematopoietic cells collected from the cultures. To determine whether coculture-independent differentiation into CD34+ cells was unique to the H1 ES cell line, we examined the ability of a second line, the H9 hES cell line, to form EBs and release hematopoietic progenitors. As can be seen in supplemental Figure 1, CD34+ cells were also obtained from the H9 hES cell line under these growth conditions.
Differentiation of human mast cells from hES cell–derived progenitors
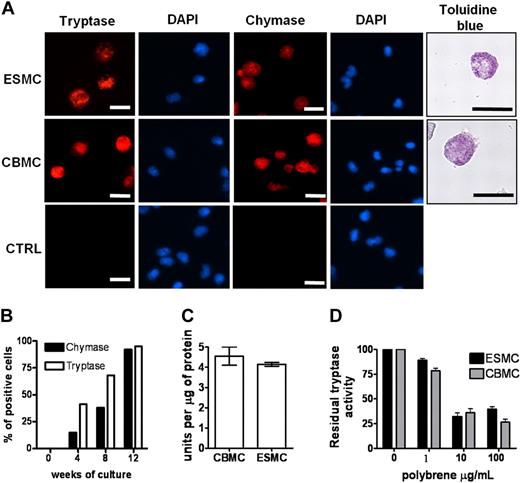
We next determined the ability of the hES H1-derived CD34+ cells to differentiate into mast cells by comparing the derived cells directly with those obtained from cord blood CD34+ cells. We included in this analysis both CD34+ populations isolated from hES/OP9 cocultures and those harvested from the supernatant of cultures derived from EBs. All CD34+ cells were placed in culture conditions previously defined and shown to differentiate CD34+ human cord blood cells to mast cells,11 essentially medium rich in IL-6 and SCF. Differentiation of these cells to mast cells was evaluated using a number of criteria. The presence of granules containing tryptase and chymase is unique to mast cells.25 Therefore, we first stained cells from both types of cultures with antitryptase and antichymase antibodies. As can be seen in Figure 2A, tryptase and chymase-positive cells were present in both cord blood mast cells (CBMCs) and ESMC cultures. In both cases, more than 80% of the cells were positive after 8 weeks in culture. Staining of cells with 4,6-diamidino-2-phenylindole revealed that, similar to CBMCs, ESMCs have a polylobed nucleus.26,27 To determine whether the method used to obtain CD34+ cells from hES cells impacted the subsequent differentiation into tryptase/chymase-positive cells, a more extensive comparison of the appearance of these cells was carried out. In both cultures, tryptase/chymase-positive cells became apparent 4 weeks after the initiation of differentiation, and by week 8 more than 90% of cells expressed both chymase and tryptase (Figure 2A-B; supplemental Figure 2). We did not find any qualitative differences in tryptase and chymase expression between mast cells differentiated from OP9 cell coculture or by differentiation from EBs, although the overall number of positive staining cells was higher from CD34+ cells differentiated from EBs: the average hES-derived mast cells (ESMCs) yield of chymase/tryptase staining was 1.2 × 106 plus or minus 0.8 × 106 per 1.0 × 106 of CD34+ progenitors isolated from OP9 coculture (n = 4), whereas 3.2 × 106 plus or minus 1.1 × 106 positively stained cells were obtained from 1.0 × 106 hematopoietic progenitors differentiated by EB formation (n = 6).
Phenotype of ES cell–derived mast cells. (A) Human mast cells differentiated from hES cells by EB formation were stained with antitryptase antibody, antichymase antibody, and Toluidine blue and compared with human mast cells derived from cord blood (CBMCs). Nuclei were stained with 4,6-diamidino-2-phenylindole. Figures were captured by an Olympus BX61 upright fluorescence microscope with 40× (antichymase, antitryptase staining) and 60× (Toluidine blue staining) objectives with a Hamamatsu ORCA RC camera, operated by Velocity software (PerkinElmer Life and Analytical Sciences). Bars in insets represent 10 μm. Controls represent cells stained without antitryptase, antichymase antibody. Figures represent 1 of at least 4 independent experiments. (B) Quantification of tryptase- and chymase-positive cells in culture during mast cell differentiation. (C) Activity of tryptase measured as a change in fluorescence activity of tosyl-Gly-Pro-Arg-pNA in mast cell lysates after 20-minute incubation at 37°C. Unit is defined as the amount of enzymatic activity that induces a 0.001 change in optical density at 405 nm/minute (n = 3). (D) Inhibition of tryptase activity by tryptase antagonist Polybrene, activity measured as in panel C (n = 3).
Phenotype of ES cell–derived mast cells. (A) Human mast cells differentiated from hES cells by EB formation were stained with antitryptase antibody, antichymase antibody, and Toluidine blue and compared with human mast cells derived from cord blood (CBMCs). Nuclei were stained with 4,6-diamidino-2-phenylindole. Figures were captured by an Olympus BX61 upright fluorescence microscope with 40× (antichymase, antitryptase staining) and 60× (Toluidine blue staining) objectives with a Hamamatsu ORCA RC camera, operated by Velocity software (PerkinElmer Life and Analytical Sciences). Bars in insets represent 10 μm. Controls represent cells stained without antitryptase, antichymase antibody. Figures represent 1 of at least 4 independent experiments. (B) Quantification of tryptase- and chymase-positive cells in culture during mast cell differentiation. (C) Activity of tryptase measured as a change in fluorescence activity of tosyl-Gly-Pro-Arg-pNA in mast cell lysates after 20-minute incubation at 37°C. Unit is defined as the amount of enzymatic activity that induces a 0.001 change in optical density at 405 nm/minute (n = 3). (D) Inhibition of tryptase activity by tryptase antagonist Polybrene, activity measured as in panel C (n = 3).
To further compare this aspect of mast cell differentiation, we evaluated the tryptase activity28 in lysates prepared from CBMCs and ESMCs. The level of activity did not differ between the 2 cell populations, indicating a similar level of enzyme in the CBMCs and ESMCs (Figure 2C). Tryptase activity is dependent on heparin-sensitive enzyme assembly.29-31 Inhibition of tryptase activity by the heparin antagonist Polybrene provides an indirect means of evaluation of heparin levels in mast cell populations. Dose-dependent inhibition of tryptase activity was observed in samples prepared from ESMCs and CBMCs (Figure 2D). Mast cell proteoglycans heparin and chondroitin sulfate stored in mast cell granules can be stained by Toluidine blue.32 As shown in Figure 2A and supplemental Figure 2, granules in mast cells derived by both methods from hES cells as well as CBMCs all stained with Toluidine blue. Taken together, these results show that ESMCs derived from hematopoietic progenitors have granules containing the mast cell-specific proteins tryptase and chymase and the proteoglycans heparin and chondroitin sulfate. However, similar to CBMCs, these cells do not develop the large granules and the high levels of proteolytic enzymes characteristic of tissue mast cells.
Expression and function of the high-affinity IgE receptor on hES-derived ES cells
The high-affinity IgE receptor FcϵRI is a heterotetramer or heterotrimer composed of 1 α-chain, 1 β-chain, and 2 γ-chains or 1 α-chain and 2 γ-chains, respectively. Cross-linking of surface expressed FcϵRI receptors leads to mast cell activation during allergic reactions. Although expression of the human α-chain and the γ-chain is not specific to mast cells and can be found at low levels on other hematopoietic cells, the expression of the β-chain, an amplifier of signaling through this receptor, is a unique marker for this population.4 To evaluate expression of FcϵRI in ESMCs, we first analyzed mRNA levels of the 3 FcϵRI chains by real-time polymerase chain reaction. The expression of the gene for each subunit was compared with expression of the genes in CBMCs. As shown in Table 1, ESMCs derived by either coculture with OP9 or by EB formation expressed a similar level of MS4A2 and FCERIG as CBMCs. Surprisingly, expression of mRNA encoding the FcϵRIα was not detected in ESMCs differentiated by OP9/hES cell coculture. In contrast, FCERIA transcripts were consistently detected in cultures derived from hES cells that were differentiated into EBs. The level of expression was, however, lower than that detected on mast cells derived from cord blood (Table 1).
Receptor expression in ESMCs measured by real time polymerase chain reaction
| Gene tested . | Differentiation by OP9 coculture . | Differentiation by EB formation . | CBMCs . |
|---|---|---|---|
| FCERIA | − | + | ++ |
| MS4A2 | ++ | ++ | ++ |
| FCERIG | +++ | +++ | +++ |
| PTGER1 | − | − | − |
| PTGER2 | + | ++ | + |
| PTGER3 | ++ | ++ | +++ |
| PTGER4 | + | + | + |
| Gene tested . | Differentiation by OP9 coculture . | Differentiation by EB formation . | CBMCs . |
|---|---|---|---|
| FCERIA | − | + | ++ |
| MS4A2 | ++ | ++ | ++ |
| FCERIG | +++ | +++ | +++ |
| PTGER1 | − | − | − |
| PTGER2 | + | ++ | + |
| PTGER3 | ++ | ++ | +++ |
| PTGER4 | + | + | + |
Expression pattern, relative to HPRT gene, of several receptors in mast cells derived by coculture with OP9 cells and from embryoid bodies (EBs) compared with cord blood mast cells (CBMCs) assayed by real-time polymerase chain reaction: − indicates expression < 0.1; +, expression 0.1-1.0; ++, expression 1.0-10.0; +++, expression > 10.0.
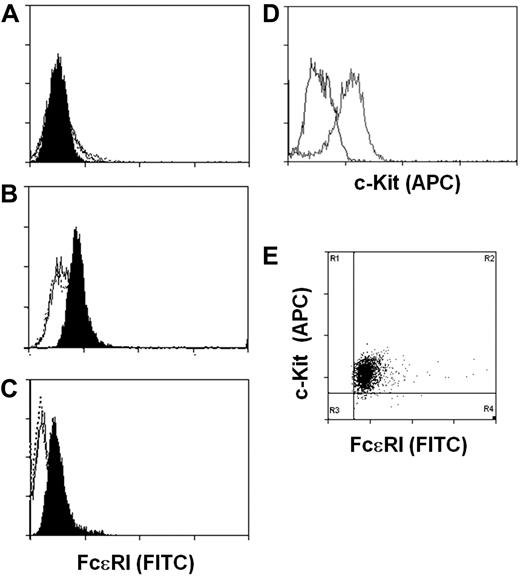
We next determined whether the expression of the FcϵRIα by the hES-derived mast cells was sufficient to direct expression of the high-affinity IgE receptor to the cell surface. Cell surface expression of FcϵRI was evaluated by staining of human IgE-loaded ESMCs with fluorescein isothiocyanate–labeled anti-IgE antibody and FACS analysis. To assess uniformity of mast cell populations, cells were also stained with anti–c-Kit antibody. Consistent with our failure to detect FCERIA transcripts, we did not detect FcϵRI on the surface of ESMCs derived by coculture with OP9 cells (Figure 3A). However, ESMCs derived from EBs and CBMCs do express FcϵRI on the cell surface. Surprisingly, similar levels of FcϵRI were observed on the ESMCs and CBMCs (Figure 3B-C). As shown in Figure 3D and E, more than 95% of ESMCs differentiated by EB formation expressed both FcϵRI and c-Kit.
Expression of FcϵRI and c-Kit on ESMCs. FACS analysis of ESMCs. Staining of cells with anti-IgE antibody after exposure of cells with human IgE for 5 days (black), without IgE pretreatment (empty), and isotype control (empty dotted line). (A) ESMCs differentiated by coculture with OP9. (B) ESMCs differentiated by EB formation. (C) CBMCs. (D) Expression of c-Kit in ESMCs differentiated by EB formation. Isotype control (black), and anti–c-Kit antibody (gray). (E) Double staining of ESMCs by anti-IgE and anti–c-Kit antibody. Results shown are representative of 6 experiments.
Expression of FcϵRI and c-Kit on ESMCs. FACS analysis of ESMCs. Staining of cells with anti-IgE antibody after exposure of cells with human IgE for 5 days (black), without IgE pretreatment (empty), and isotype control (empty dotted line). (A) ESMCs differentiated by coculture with OP9. (B) ESMCs differentiated by EB formation. (C) CBMCs. (D) Expression of c-Kit in ESMCs differentiated by EB formation. Isotype control (black), and anti–c-Kit antibody (gray). (E) Double staining of ESMCs by anti-IgE and anti–c-Kit antibody. Results shown are representative of 6 experiments.
To determine whether the FcϵRI receptor expressed by the ESMCs was functional, we stimulated ESMCs exposed to 5 μg/mL hIgE by cross-linking of FcϵRI receptor with anti-IgE antibody and assessed 2 well-characterized consequences of activation of mast cells by FcϵRI cross-linking: changes in mitogen-activated protein kinase (MAPK) phosphorylation and intracellular Ca2+ mobilization.33 Because ESMCs differentiated by OP9 coculture do not express FcϵRI, they were not included in this analysis.
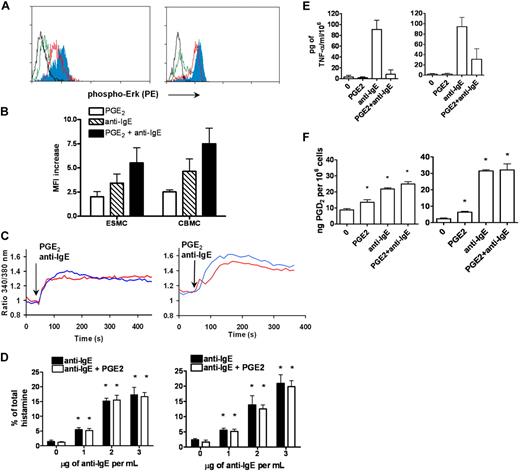
p42/44 ERK1/2 MAPK kinase was shown to be phosphorylated after activation of FcϵRI by receptor cross-linking. The extent of ERK1/2 phosphorylation was assessed by phosphospecific staining with antibody recognizing the phosphorylated form of Erk1/2 at T202 and Y204, followed by FACS analysis. Activation of ESMCs by FcϵRI receptor cross-linking resulted in increased binding of phosphospecific antibody compared with unstimulated control (Figure 4A). The increase was similar to that observed in the CBMC population (Figure 4B).
Activation of ESMCs by cross-linking of FcϵRI and PGE2. (A) Phosphorylation of p42/44 ERK assayed by FACS using a phosphospecific antibody that recognizes phosphorylation of T202 and Y204. ESMCs (left panel) or CBMCs (right panel) were activated by FcϵRI cross-linking or by PGE2 activation (black represents unactivated; green, PGE2; red, anti-IgE; and blue, PGE2 and anti-IgE). (B) Quantitative analysis (n = 6) of T202/Y204 phosphorylation in ESMCs stimulated by PGE2 (white bar), FcϵRI (hatched bar), and FcϵRI and PGE2 together (dark bar). (C) Intracellular calcium release in ESMCs (left panel) or CBMCs (right panel) and activated by FcϵRI cross-linking 3 μg/mL anti-IgE antibody (blue) or 1μM PGE2 (red). Point of stimulation is shown by arrow. Calcium mobilization is expressed as the ratio of fluorescence of Fura-2 measured at 340 and 380 nm. Typical results from at least 2 experiments are shown. (D) Histamine release in ESMCs (left panel) or CBMCs (right panel) preincubated with hIgE and cross-linked with various concentrations of anti-IgE antibody alone or together with 10−6M PGE2. Histamine release is presented as a percentage of total histamine assayed by lysis of cells by Triton X-100 (n = 3). *Significant difference from 0 μg/mL anti-IgE (P < .05). (E) Release of TNF-α was evaluated in ESMCs (left panel) and CBMCs (right panel) preincubated with hIgE and cross-linked with 3 μg/mL anti-IgE antibody alone or together with 10−6M PGE2 (n = 3). (F) PGD2 release from ESMCs (left panel) or CBMCs (right panel) measured using PGD2 methoxime enzyme immunoassay kit (n = 3). *Significantly different from untreated cells (P < .05). Results shown in right panels in panels C and E were obtained under identical conditions to those in the left panels but on different days.
Activation of ESMCs by cross-linking of FcϵRI and PGE2. (A) Phosphorylation of p42/44 ERK assayed by FACS using a phosphospecific antibody that recognizes phosphorylation of T202 and Y204. ESMCs (left panel) or CBMCs (right panel) were activated by FcϵRI cross-linking or by PGE2 activation (black represents unactivated; green, PGE2; red, anti-IgE; and blue, PGE2 and anti-IgE). (B) Quantitative analysis (n = 6) of T202/Y204 phosphorylation in ESMCs stimulated by PGE2 (white bar), FcϵRI (hatched bar), and FcϵRI and PGE2 together (dark bar). (C) Intracellular calcium release in ESMCs (left panel) or CBMCs (right panel) and activated by FcϵRI cross-linking 3 μg/mL anti-IgE antibody (blue) or 1μM PGE2 (red). Point of stimulation is shown by arrow. Calcium mobilization is expressed as the ratio of fluorescence of Fura-2 measured at 340 and 380 nm. Typical results from at least 2 experiments are shown. (D) Histamine release in ESMCs (left panel) or CBMCs (right panel) preincubated with hIgE and cross-linked with various concentrations of anti-IgE antibody alone or together with 10−6M PGE2. Histamine release is presented as a percentage of total histamine assayed by lysis of cells by Triton X-100 (n = 3). *Significant difference from 0 μg/mL anti-IgE (P < .05). (E) Release of TNF-α was evaluated in ESMCs (left panel) and CBMCs (right panel) preincubated with hIgE and cross-linked with 3 μg/mL anti-IgE antibody alone or together with 10−6M PGE2 (n = 3). (F) PGD2 release from ESMCs (left panel) or CBMCs (right panel) measured using PGD2 methoxime enzyme immunoassay kit (n = 3). *Significantly different from untreated cells (P < .05). Results shown in right panels in panels C and E were obtained under identical conditions to those in the left panels but on different days.
Changes in intracellular Ca2+ are an essential event in mast cell activation, with many downstream signaling events dependent on the sufficient release of Ca2+ from intracellular stores. Therefore, we next evaluated the intracellular Ca2+ release by ratiometric measurement of fluorescence at 340 and 380 nm after stimulation of ESMCs loaded with the Ca2+ selective fluorescent indicator Fura-2. As shown in Figure 4C, ESMCs exposed to 5 μg/mL hIgE for 5 days and cross-linked with 2 μg/mL anti-IgE antibody resulted in the mobilization of intracellular Ca2+ similar to CBMCs. Taken together, these results show that hES cell-derived mast cells express functional FcϵRI, which is able to stimulate signaling pathways after activation by cross-linking.
Expression and function of PGE2 pathways in hES cell–derived mast cells
The activity of the FcϵRI receptor can be modulated by numerous pathways, including those activated by G-protein–coupled receptors found on these cells. Among these are the pathways activated by prostaglandin E2 (PGE2), a lipid mediator released at the site of inflammation. The role of this lipid mediator in modulating mast cell function has been particularly difficult to study because of reported differences between species in the effects of PGE2 on mast cells.5,6 We therefore asked whether hES-derived mast cells express these receptors, and if so, whether these cells could provide a model system for studying the impact of PGE2 signaling on mast cell function. PGE2 stimulates 4 subtypes of E series of prostaglandin (EP) receptors: EP1, which couples to intracellular Ca2+ mobilization; EP2 and EP4, which couple to Gs; and EP3, which couples primarily to Gi/o proteins. As shown in Table 1, ESMCs as well as CBMCs expressed EP2, EP3, and EP4 receptors. We did not observe differences in the level of expression of these receptors between ESMCs and CBMCs. To determine whether these receptors were functional and have the potential to regulate mast cell activation, we activated ESMCs via EP receptors by PGE2.5 Similar to FcϵRI cross-linking, activation of mast cells by PGE2 resulted in both phosphorylation of MAPK kinase Erk1/2 and intracellular Ca2+ mobilization (Figure 4A,C). PGE2 phosphorylation of MAPK kinase ERK1/2, driven by ESMC activation by 1μM PGE2 and assessed by phosphospecific antibody, showed similar levels of phosphorylation compared with CBMCs. Stimulation by FcϵRI cross-linking, together with PGE2 stimulation, led to a further increase in Erk1/2 phosphorylation (Figure 4A-B). Elevations of intracellular calcium are one of the most significant responses after activation of human mast cells by PGE2. Stimulation of Fura-2–loaded ESMCs with 1μM PGE2 resulted in an increase in intracellular calcium release as measured by ratios of fluorescence at 340 and 380 nm (Figure 4C). The increase and kinetics of intracellular calcium mobilization induced by PGE2 were similar to the calcium response in CBMCs.
Evaluation of effector functions in hES cell–derived mast cells
Activation of mast cells by FcϵRI results in the release of granules, which contain chemical mediators, such as histamine, and also leads to increases in cytokine synthesis.2 We next asked whether the pathways and biochemical machinery necessary for this response were present in the ES cell-derived mast cells. To assess the ability of ESMCs to degranulate after stimulation, we measured histamine release after activation by either FcϵRI cross-linking or PGE2 treatment. ESMCs were primed with hIgE and cross-linked with various concentration of anti-IgE antibody. As shown in Figure 4D, histamine release was dose dependent and reached a maximum at a concentration 3 μg of anti-IgE antibody per milliliter. In contrast, PGE2 alone was unable to degranulate ESMCs at concentrations up to 10−5M (data not shown). Similar to what has been reported for CBMCs, PGE2 (10−6M) did not augment or inhibit IgE-stimulated histamine release. Total histamine levels for ESMCs and CBMCs are shown in Table 2.
Intracellular histamine level in human mast cells
| Type of mast cells . | Histamine content, μg/106 cells . |
|---|---|
| ESMCs | 9.32 ± 2.6 |
| CBMCs | 8.8 ± 1.9 |
| Type of mast cells . | Histamine content, μg/106 cells . |
|---|---|
| ESMCs | 9.32 ± 2.6 |
| CBMCs | 8.8 ± 1.9 |
A total of 103 hES-derived mast cells (ESMCs) or cord blood mast cells (CBMCs) 12 weeks in culture were lysed, and the cell supernatant was analyzed by an enzyme immunoassay histamine assay kit (n = 3).
One of the major cytokines released from human mast cells is tumor necrosis factor-α (TNF-α). To assess its release from ESMCs, we stimulated cells sensitized with hIgE with anti-IgE antibody. TNF-α was synthesized and released by ESMCs after activation by anti-IgE but not after stimulation with PGE2 (Figure 4E). PGE2 was, however, capable of completely inhibiting FcϵRI-mediated TNF-α production by these cells. Similar levels of TNF-α release were seen in CBMCs.
One of the major arachidonic acid metabolites produced by FcϵRI-activated human mast cells is prostaglandin D2 (PGD2). PGD2 was produced by both ESMCs and CBMCs after stimulation with PGE2 and activation of the IgE receptor (Figure 4F). However, some differences were observed. ESMCs displayed slightly higher constitutive release of this prostaglandin, whereas maximal release by CBMCs after stimulation was slightly higher than that observed in ESMC cultures. Similar to histamine release, simultaneous activation of human mast cells by PGE2 and anti-IgE was not additive in either population.
Derivation of mast cells from genetically modified hES
Genetic modification of mast cells is difficult.34 In contrast, genetic modification of mouse ES cells is common, and methods are becoming established for carrying out similar experimental maneuvers with hES cells. In theory, genetic changes desirable in the mast cell could be introduced in hES cells before their differentiation. It was therefore of interest to determine whether procedures required for such genetic manipulation of hES cells, including growth in selective media and colony isolation, would compromise the ability of these cells to produce CD34+ progenitors or to differentiate into the mast cell populations.
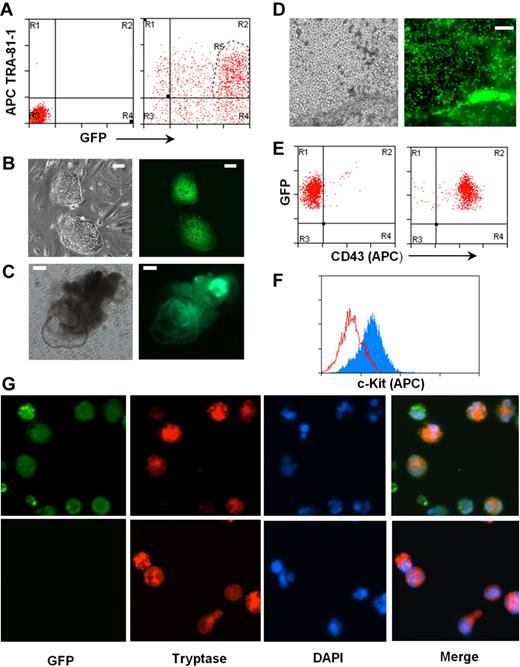
To test this, we introduced a GFP gene under the control of the EF-1P α promoter into the H1 hES cells using a lentiviral expression system. Two days after transfection, more than 85% of cells in culture expressed GFP, and the majority of them remained positive for pluripotent marker TRA-1-81 (Figure 5A). Double-positive cells were sorted and used for establishment of hES cells expressing GFP (Figure 5B). EBs were generated and further differentiated to CD43+ hematopoietic progenitors (Figure 5D-E), which were subsequently differentiated into mast cells. Differentiated mast cells expressed c-Kit and mast cell–specific tryptase together with GFP (Figure 5F-G).
Human mast cells differentiated from genetically modified hES cells. (A) A reporter gene expressing GFP under EF-1 α promoter was expressed in hES cell clone H1 by lentiviral transfection. hES cells double-positive for GFP and pluripotent marker TRA-1-81 (region R5) were sorted by MoFlow sorter. Left panel: Nontransfected cells, isotype control. Right panel: Transfected cells stained with TRA-1-81. (B) hES cell lines expressing GFP were established. (C) Differentiating EBs formed from GFP-hES cells. (D) GFP-expressing mast cell progenitors releasing from differentiated EBs to medium. Images were captured using an Olympus IX81 inverted fluorescence microscope with a 4× objective. (E) Mast cell progenitors express GFP and CD43. Left panel: Isotype control. Right panel: CD43 antibody. (F) Mast cell progenitors differentiated to mast cells expressing c-Kit, anti–c-kit-APC (gray), and isotype control (empty). (G) Mast cells derived from GFP-hES express tryptase. Top panel: GFP-hES cells. Bottom panels: Mast cells derived from nontransfected H1 clone. Figures were captured by an Olympus BX61 upright fluorescence microscope with a 40× objective with a Hamamatsu ORCA RC camera, operated by Velocity software (PerkinElmer Life and Analytical Sciences).
Human mast cells differentiated from genetically modified hES cells. (A) A reporter gene expressing GFP under EF-1 α promoter was expressed in hES cell clone H1 by lentiviral transfection. hES cells double-positive for GFP and pluripotent marker TRA-1-81 (region R5) were sorted by MoFlow sorter. Left panel: Nontransfected cells, isotype control. Right panel: Transfected cells stained with TRA-1-81. (B) hES cell lines expressing GFP were established. (C) Differentiating EBs formed from GFP-hES cells. (D) GFP-expressing mast cell progenitors releasing from differentiated EBs to medium. Images were captured using an Olympus IX81 inverted fluorescence microscope with a 4× objective. (E) Mast cell progenitors express GFP and CD43. Left panel: Isotype control. Right panel: CD43 antibody. (F) Mast cell progenitors differentiated to mast cells expressing c-Kit, anti–c-kit-APC (gray), and isotype control (empty). (G) Mast cells derived from GFP-hES express tryptase. Top panel: GFP-hES cells. Bottom panels: Mast cells derived from nontransfected H1 clone. Figures were captured by an Olympus BX61 upright fluorescence microscope with a 40× objective with a Hamamatsu ORCA RC camera, operated by Velocity software (PerkinElmer Life and Analytical Sciences).
The generation of GFP+ mast cells did not require isolation and expansion of individual hES cell colonies, a process that requires extensive time in tissue cultures and thus probably results in acquisition of mutations that could compromise the pluripotency of hES cells. However, many genetic maneuvers, including the introduction of mutations by homologous recombination, require expansion of clones after growth in selective media. To determine whether drug-resistant hES cell isolates would retain the ability to differentiate into mast cells, hES cells were transfected by electroporation with a neomycin gene driven by the PGK promoter. G418-resistant hES clones were individually isolated (supplemental Figure 3A) and expanded, and the ability of 2 of these clones to generate CD34+ hematopoietic precursors and mast cells was determined. As shown in supplemental Figure 3B, both clones retained the parental ability to differentiate into EBs and produce cultures that released CD34+ CD43+ cells. In both cases, these CD34+ cells, on exposure to IL-6 and SCF, differentiated into c-Kit+ populations (supplemental Figure 3C). These data demonstrate that individual hES cell clones carrying desirable genetic events can be selected, isolated, expanded, and a stock of this cell line maintained. As needed, mast cells carrying the same genetic modification can be generated for study.
Discussion
Here we report that hematopoietic precursors capable of giving rise to mast cells can be isolated from human ES cells and, furthermore, that they can be obtained without the coculture of the ES cells with stromal cells. Consistent with previous reports, we found that coculture of hES cells with OP9 cells yielded CD34+ cells. This protocol generally requires the mechanical separation of the progenitor cells from the stromal cells. To do this, we carried out positive selection of CD34+ cells. Further analysis showed that this yielded a population of cells that can be further divided based on CD43 expression. Previous studies have demonstrated that CD43 reliably separates CD34+ hematopoietic cells from CD34+CD43−KDR+CD31+ endothelial cells.35 This raises the possibility that only approximately one-third of the cells isolated were hematopoietic precursors, whereas the remaining cells represent precursors poised for differentiation into endothelial cells. We cannot rule out the possibility that, in part, the greater yield of CD34+ CD43+ precursor cells by direct culture reflects the ease with which these cells could be collected from the EB-derived cultures. The release of these cells into the culture media had 2 important consequences. First, loss of cells during mechanical separations was avoided. Second, the cells could be harvested for an extended period of time, presumably reflecting the continuous self-renewal and differentiation of an even earlier progenitor cell present in the complex embryo-derived culture into the CD34+CD43+ cell.
To date, the successful generation of large numbers of mast cells from human ES cell-derived progenitors has not been described. We show that both CD34+ populations generated by coculture and those generated directly by formation of EBs can be differentiated into cells with the well-established characteristics of primary human mast cells, including morphology and expression of proteases and c-Kit. Furthermore, the time line and cytokine requirements were similar to derivation of mast cells from cord blood hematopoietic progenitors. However, similar to CBMCs, the ESMCs have smaller granules, less heparin, and lower levels of proteolytic enzymes than mature tissue mast cells. The number of cells obtained per 1 × 106 starting CD34+ cells was higher in direct differentiation compared with OP9 coculture. Both populations also yield cells similar in expression of c-kit, chymase, tryptase, and proteoglycans.
However, an important difference was noted in FcϵRI expression by the mast cells derived by the 2 methods. Mast cells differentiated by coculture with OP9 cells failed to express FcϵRI on the cell surface because of lack of expression of the α-chain of this receptor. Critical architectural changes at the FcϵRI locus may precede the expression of this gene in mature mast cells, dendritic cells, and macrophages, and these early events occur before differentiation of the CD34+ cells to mast cells. It is possible that the OP9 culture conditions failed to send the appropriate signal required for altering the structure of the locus in a manner amenable for the transcription factors that later would regulate gene expression. Our data suggest that OP9 may only be able to support lineage-restricted progenitors or be able to support hES-derived hematopoietic progenitors without conferring their stem cell function.36
Previous studies have shown that PGE2 is an important modulator of mast cell-dependent allergic responses. Although, in general, PGE2 blocks allergic responses in humans and up-regulates the response in mouse mast cells in vitro, PGE2 did not suppress exocytosis in CBMCs.6 Similar to BMMC and CBMC cells, PGE2 stimulated phosphorylation of Erk1/2 in ESMCs as well as intracellular calcium mobilization and PGD2 production. When both stimuli were combined together, histamine release and PGD2 production were not significantly changed, but production of TNF-α was almost totally inhibited. These findings suggest that signaling responses are similar between ESMCs and CBMCs. The demonstration that the hES-derived mast cells express EP receptors opens the door for further investigation of the function of each EP receptor on the mast cell using siRNA vectors. These vectors can be introduced into the hES cells, stable transfectants isolated, and then differentiated into mast cells for study. As a first step toward carrying out such studies, we demonstrated that genetically manipulated hES cells retain their pluripotency, specifically their ability to generate CD34+ progenitor cells and that this can be achieved using a simple coculture-free protocol. Furthermore, these CD34+ cells retain their capacity to differentiate into mast cell populations. This approach should provide genetic tools for human mast cells studies, tools that until now have been limited to the mouse.
In conclusion, we report here a simple and robust protocol for the generation of human mast cells from hES cells, defined both by the expression of lineage-specific markers and by the response of these cells to PGE2 and antigen. Human mast cells can be obtained by this method in quantities sufficient for biochemical and physiologic studies and therefore will provide a novel tool for both identification of pathways critical to the function of mast cells in immune responses as well as a uniform source of mast cells for testing novel therapeutic compounds for the treatment of asthma and other allergic diseases.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Amgen for providing cytokines, Robert Bagnell and Victoria J. Madden from the University of North Carolina at Chapel Hill Microscopy Service Laboratory for assistance with microscopy, and the University of North Carolina at Chapel Hill flow cytometry facility for help with cell sorting.
This work was supported by the National Institutes of Health (grants HL068141, HL071802, and U19AI077437) and the University of North Carolina at Chapel Hill Research Council (grant 3-12817).
National Institutes of Health
Authorship
Contribution: M.K. designed research, performed experiments, and wrote the manuscript; A.M.L. performed experiments; K.D.C. and S.L.T. provided CBMCs; and B.H.K. designed research and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Beverly H. Koller, University of North Carolina at Chapel Hill, CB#7264, 5067 Genetic Medicine Bldg, 120 Mason Farm Rd, Chapel Hill, NC 27599; e-mail: Treawouns@aol.com.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal