Abstract
The mitogen-activated protein kinases (MAPKs) extracellular signal-regulated kinase 1 (ERK1) and ERK2 are among the main signal transduction molecules, but little is known about their isoform-specific functions in vivo. We have examined the role of ERK1 in adult hematopoiesis with ERK1−/− mice. Loss of ERK1 resulted in an enhanced splenic erythropoiesis, characterized by an accumulation of erythroid progenitors in the spleen, without any effect on the other lineages or on bone marrow erythropoiesis. This result suggests that the ablation of ERK1 induces a splenic stress erythropoiesis phenotype. However, the mice display no anemia. Deletion of ERK1 did not affect erythropoietin (EPO) serum levels or EPO/EPO receptor signaling and was not compensated by ERK2. Splenic stress erythropoiesis response has been shown to require bone morphogenetic protein 4 (BMP4)–dependent signaling in vivo and to rely on the expansion of a resident specialized population of erythroid progenitors, termed stress erythroid burst-forming units (BFU-Es). A great expansion of stress BFU-Es and increased levels of BMP4 mRNA were found in ERK1−/− spleens. The ERK1−/− phenotype can be transferred by bone marrow cells. These findings show that ERK1 controls a BMP4-dependent step, regulating the steady state of splenic erythropoiesis.
Introduction
Erythropoiesis is a multistep process that involves the differentiation of pluripotent hematopoietic stem cells through the lineage-committed erythroid burst-forming unit (BFU-E) and erythroid colony-forming unit (CFU-E) progenitor cells, which give rise to a series of early and late erythroblasts, leading to the formation of reticulocytes and erythrocytes. The mouse spleen is a reserve erythropoietic organ, whereas bone marrow is an active site of erythropoiesis in the basal state. The modulation of progenitor cell amplification is thought to play a major role in increasing the erythroid output in response to the stress of tissue hypoxia.1-3 In response to stress, a splenic erythropoietic proliferative response occurs supported by the rapid expansion of erythroid progenitors. Two mechanisms have been proposed to date. The first mechanism involves the regulation of the erythropoietic rate through erythropoietin receptor-induced decreased apoptosis of early erythroblasts,4 whereas the second mechanism implicates amplification of specialized population of splenic resident erythroid progenitors, termed stress BFU-Es, through the activation of the Sonic Hedgehog/bone morphogenetic protein 4 (BMP4) pathway.5,6 In acute anemia, the stress BFU-Es are defined as immature progenitors that form large burst colonies in methylcellulose in 5 days when cultured in the presence of erythropoietin (EPO) alone. Basal level of stress BFU-Es is maintained low in steady-state conditions. An efficient stress erythropoiesis is crucial to survival in the face of hypoxic stress, whereas an inappropriate activation of this erythropoiesis may be associated to leukemic transformation.7
The mitogen-activated protein kinase (MAPK) extracellular signal-regulated kinase (ERK) signaling pathway represents the cooperation of different signal transducers in an activation cascade, linking receptors to gene activation.8,9 Despite detailed characterization of the molecular features and interactions of the proteins constituting the MAPK pathway, the resulting cellular readouts produced by ERK activation are essentially unpredictable, being highly dependent on the cell type or their state of proliferation or differentiation. Transient or sustained activation of the MAPK pathway can induce opposite cellular outputs such as proliferation or differentiation.10,11 In different culture models of erythroid progenitors, conflicting results have been obtained about the role of this pathway.12-16 For example, in the self-renewal of avian erythroid progenitors, the MAPK/ERK pathway was found to be required in early but not in late erythroid progenitors.17 Others studies proposed that the synergistic activation of ERK1/2 by EPO and stem cell factor is important for expansion of human primary erythroid progenitors.13 However, this pathway has been shown to be fully dispensable for the EPO receptor (EPOR)/c-kit–driven renewal of primary murine erythroid progenitors.16 It was also reported that the ERK/MAPK pathway has a negative regulatory role in erythroid differentiation in cell lines12,18,19 and in fetal liver cells infected with oncogenic H-Ras.20 Inhibition of MAPK activity has been shown to increase the number of erythroid colonies from human CD34+ hematopoietic progenitor cells and to promote erythroid differentiation while suppressing megakaryocyte differentiation.21,22
ERK activity is provided by 2 isoforms, named ERK1 and ERK2, that are highly similar, ubiquitously expressed, and share activators and substrates. Gene disruption has provided evidence for specific functions for each isoform. Ablation of ERK2 induces embryonic lethality with death of the animals in utero at day 7.5 because of defects in the mesoderm and placental development.23-25 Conversely, ERK1−/− mice are viable and fertile and present minor defects, showing that ERK1 is dispensable for mouse development and could be compensated by ERK2. So far, ERK1 has been shown to be specifically required for in vitro and in vivo adipogenesis,26 terminal differentiation of T lymphocytes,27 and long-term memory.28,29 However, in nearly all tissues examined so far, ERK2 is expressed at a higher level than ERK1. By combining single and double silencing of ERK1 and ERK2, the apparent dominant role of ERK2 was shown to be due to its higher expression rather than to a specific role.30 The contribution of ERK1 was shown when ERK2 activation was clamped, avoiding compensating overactivation of ERK2. This suggests that each isoform of ERK provides a share of ERK activity required for the control of a specific cellular function. The minor change in the level of ERK activation when ERK1 was inactivated was found to alter ERK-dependent responses such as immediate early gene expression.28 Thus, the existence of 2 isoforms could allow a more precise activation of ERK in a given cell to fit its exact needs.
To determine the role of the ERK1 isoform in adult mouse hematopoiesis, the ERK1−/− mice model was used. We show here that specific ablation of ERK1 enhances splenic erythropoiesis, leading to an erythropoiesis stresslike phenotype. We also provide evidence that ERK1 plays a role in the murine splenic erythropoiesis through the regulation of BMP4-dependent pathway.
Methods
Mice
ERK1−/− mice, backcrossed on C57BL/6 (CD45.2) background,27 were obtained from Gilles Pages (Nice Sophia Antipolis University). The C57BL/6J (CD45.2 and CD45.1) mice were obtained from Charles River Laboratories. All the mice were housed in a specific pathogen-free environment. Experiments were carried out in accordance with the guidelines of the French Veterinary Department. Approval was obtained from the French Ministry of Agriculture Institutional Review Board for these studies.
Hematologic parameters
Blood cell counts were analyzed with a M-Sampler (Melet Schloesing). Hematocrit was assessed on a hematocrit centrifuge (Sigma 113). The reticulocyte counts were measured as previously described.31 A stock solution of thiazole orange (1 mg/mL) was diluted 1:10 000 in phosphate-buffered saline (PBS). Whole blood (0.5 μL) was added to the diluted dye solution (1 mL) and incubated at room temperature for 15 minutes. Blood sample diluted in PBS was used as an unstained control. All samples were analyzed on a FACSCalibur (Becton Dickinson).
Histopathology
Freshly isolated spleens were fixed in formalin acid buffer (30% formalin, 60% ethanol, 10% acetic acid). Paraffin sections were processed and stained with hematoxylin-eosin-safranin and Perl method for iron detection. The photomicrographs were obtained using an Axiophot microscope (Carl Zeiss) and a PCO sensicam 12 bit coded imaging camera with SensiControl Utility software.
Flow cytometry
Freshly isolated bone marrow or splenocytes were filtered and immunostained for 20 minutes at 4°C in PBS–0.4% bovine serum albumin, with phycoerythrin (PE)– or allophycocyanin-conjugated anti-Ter119 (BioLegend), fluorescein isothiocyanate–conjugated anti-CD71 (BD Biosciences), and PE-conjugated anti-B220 (BioLegend). Staining for Fas or FasL was done for 20 minutes with 15 μg/mL PE-conjugated anti-Fas (Jo2 clone; BD Biosciences) or 5 μg/mL biotin-conjugated anti-FasL (MFL3 clone; BD Biosciences) and allophycocyanin-conjugated streptavidin. Signal of Fas-PE was amplified by Faser Kit PE Amplification (Miltenyi Biotec). Annexin V staining was carried out according to the manufacturer's instructions (BioLegend). 7-Amino-actinomycin D (BD Biosciences) was used to exclude dead cells. Control samples included unstained cells, single-color controls, and “fluorescence minus one” controls. Cells were analyzed on FACSCalibur, LSRII, or Canto II (BD Biosciences) flow cytometers. Data were analyzed with the FlowJo software (TreeStar Inc).
Colony-forming assays
Methylcellulose colony-forming assays were performed in MethoCult M3434 complete medium with cytokines (StemCell Technologies). In brief, bone marrow was plated at a density of 35 × 103 cells/mL and spleen at 35 × 104 cells/mL. The cultures were incubated at 37°C in 5% CO2 for 2 days for CFU-E and 7 days for both colony-forming cell (CFC), BFU-E, and granulocyte-macrophage colony-forming unit (CFU-GM) colonies. All of the cultures were done in duplicate. To assess stress BFU-E, methylcellulose colony forming assays were performed in MethoCult M3234 medium in the presence of EPO alone or EPO and BMP4, as previously described.32 Splenocytes (2 × 106) were plated in methylcellulose media containing 3 U/mL EPO alone or EPO plus 15 ng/mL BMP4. The cultures were incubated at 37°C in 5% CO2 for 5 days. All of the cultures were done in duplicate.
Western blot analysis
Western blots were performed as previously described.33 The anti–phospho ERK1/2 (Thr202/Tyr204), anti–phospho signal transducer and activator of transcription 5 (STAT5; Tyr694), anti–phospho STAT1 (Tyr701), and anti-STAT1 antibodies were obtained from Cell Signaling Technology, and the anti-ERK1/2 (Sc-94, K23), anti-STAT5 (Sc-835, C17), and anti-EPOR (SC-697, M20) antibodies were obtained from Santa Cruz Biotechnology Inc.
Quantitative in vitro determination of EPO in plasma
Plasma concentrations of EPO were determined with the use of an enzyme-linked immunosorbent assay according to the manufacturer's instructions (R&D Systems).
RNA isolation and quantitative polymerase chain reaction analysis
Total RNA was isolated from spleens with Trizol reagent (Invitrogen) followed by an on-column purification step with the RNeasy Mini Kit (QIAGEN). RNA was reverse transcribed with oligo dT primers with the use of SuperScript II First-Strand Synthesis System (Invitrogen). The expression of BMP4 was assessed by real-time quantitative polymerase chain reaction analysis (Q-PCR) with the use of the Lightcycler instrument (Roche Diagnostics) and the following primers: BMP4-F, 5′-TGCTTTTCGTTTCCTCTTCAACC-3′; BMP4-R, 5′-AAGTTTCCCACCGTGTCACA-3′. The relative expression of BMP4 was evaluated by the Pfallf method,34 using the geometric mean of 2 reference genes: β-actin (ACT-F, 5′-GTGGCATCCATGAAACTACAT-3′; ACT-R, 5′-GGCATAGAGGTCTTTACGG-3′) and succinate dehydrogenase (succinate dehydrogenase-F, 5′-GGAAGATCTCTGCGATATGA-3′; succinate dehydrogenase-R, 5′-TTTGCTCTTATTCGGTGTATG-3′). Data are expressed as fold change relative to ERK+/+ mice.
Phenylhydrazine stress test
Mice with a baseline hematocrit of at least 40% were used. Mice were injected subcutaneously with 40 mg/kg phenylhydrazine hydrochloride (PHZ) solution in PBS as previously described.4 Blood was obtained by phlebotomy from the retro-orbital plexus on days 0, 3, 6, 9, and 13 for hematocrit and reticulocyte count measurements.
Bone marrow transplantation
Bone marrow cells (5 × 106) were isolated from wild-type or ERK1−/− (CD45.2) mice and injected into lethally irradiated (9 Gy) CD45.1 congenic recipient mice. Peripheral blood chimerism was analyzed 4 months after transplantation, and mice with greater than 90% CD45.2 donor contributions were considered.35 Erythroblast subsets in spleen and bone marrow of the recipients were analyzed by flow cytometry as previously described.36
Data analysis
Data were analyzed with the Student 2-tailed, paired t test. Data with P values less than .05 were considered significant.
Results
Altered blood cell composition in ERK1−/− mice
The analysis of peripheral blood of adult ERK1−/− mice showed significant erythrocyte abnormalities compared with litter-matched control mice (Table 1). Red blood cell (RBC) count, hematocrit level, hemoglobin concentration and the mean corpuscular volume were significantly higher in ERK1−/− mice than in control mice. The relative proportion of platelets, white blood cells, monocytes, and lymphocytes were similar in ERK1−/− and control mice, showing that the loss of ERK1 affects specifically the erythroid lineage.
Hematologic parameters of ERK1−/− mice
| . | ERK1+/+ (n = 20) . | ERK1−/− (n = 20) . | P . |
|---|---|---|---|
| RBC count, ×106/μL | 8.76 ± 0.22 | 9.27 ± 0.20 | .025* |
| HGB level, g/dL | 14.64 ± 0.33 | 15.65 ± 0.25 | .005* |
| HCT, % | 42.31 ± 1.04 | 45.5 ± 0.94 | .007* |
| MCV, fL | 48.37 ± 0.64 | 49.21 ± 0.43 | .046* |
| PLT count, ×103/μL | 636 ± 88.16 | 765 ± 73.96 | .256 |
| WBC count, ×103/μL | 7.86 ± 0.77 | 6.16 ± 0.34 | .079 |
| MONO count, ×103/μL | 2.63 ± 0.33 | 2.44 ± 0.24 | .633 |
| LYMP count, ×103/μL | 85.74 ± 1.78 | 89.07 ± 1.46 | .100 |
| . | ERK1+/+ (n = 20) . | ERK1−/− (n = 20) . | P . |
|---|---|---|---|
| RBC count, ×106/μL | 8.76 ± 0.22 | 9.27 ± 0.20 | .025* |
| HGB level, g/dL | 14.64 ± 0.33 | 15.65 ± 0.25 | .005* |
| HCT, % | 42.31 ± 1.04 | 45.5 ± 0.94 | .007* |
| MCV, fL | 48.37 ± 0.64 | 49.21 ± 0.43 | .046* |
| PLT count, ×103/μL | 636 ± 88.16 | 765 ± 73.96 | .256 |
| WBC count, ×103/μL | 7.86 ± 0.77 | 6.16 ± 0.34 | .079 |
| MONO count, ×103/μL | 2.63 ± 0.33 | 2.44 ± 0.24 | .633 |
| LYMP count, ×103/μL | 85.74 ± 1.78 | 89.07 ± 1.46 | .100 |
All measurements were carried out on ERK1−/− mice at 2 to 4 months of age and on wild-type controls (ERK1+/+) from the same mouse colony. Data are mean ± SEM.
RBC indicates red blood cell; HGB, hemoglobin; HCT, hematocrit; MCV, mean corpuscular volume; PLT, platelet; WBC, white blood cell; MONO, monocyte; and LYMP, lymphocyte.
Significantly different from ERK1+/+ value (P < .05).
Splenomegaly and erythroblast amplification in the spleen of ERK1−/− mice
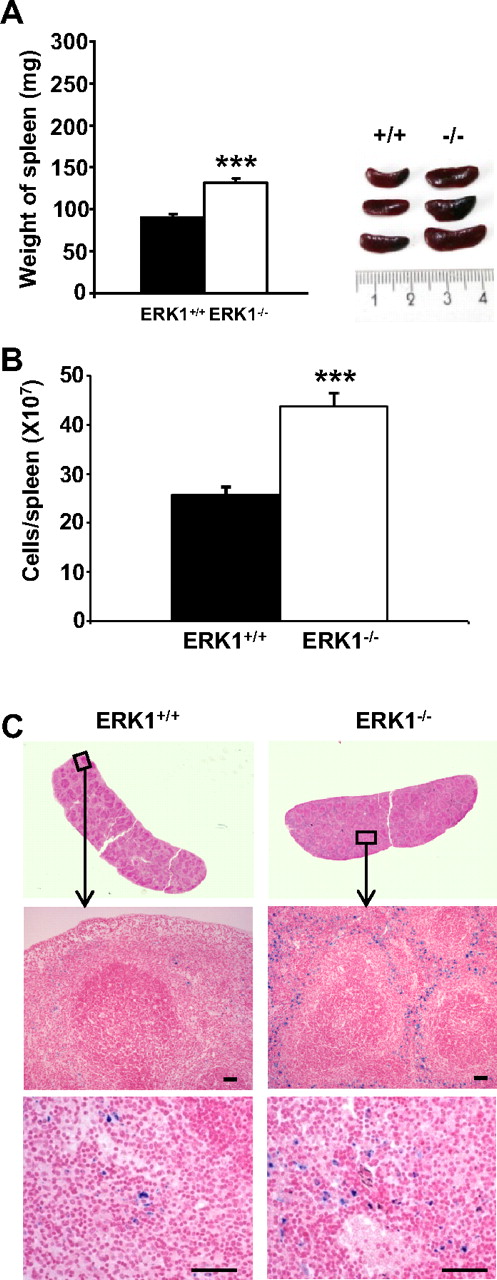
Spleens from 2- to 4-month-old ERK1−/− mice exhibited increase in weight (∼ 1.5 times the weight of age- and sex-matched ERK1+/+ mice; Figure 1A). A proportionate increase in cell number was observed in ERK1−/− mice compared with their ERK1+/+ counterparts (Figure 1B), indicating that the increase in spleen size is due to an increase in cellularity. Histologic examination of the enlarged spleens of ERK1−/− mice showed a marked expansion of the red pulp with predominantly erythropoiesis. Diffuse iron deposits confined to the red pulp were observed after Perl staining in ERK1−/− mice (Figure 1C).
Analysis of ERK1−/−-induced splenomegaly. (A) Age- and sex-matched ERK1−/− (□) and ERK1+/+ (■) mice at 2 to 4 months of age were killed, and spleen lengths and weights were measured. A representative comparison of ERK1+/+ and ERK1−/− spleens is shown in the inset (ruler increments are in millimeters). Data are means ± SEMs, n = 14 for ERK1+/+ and for ERK1−/− mice. (B) Splenocytes from ERK1+/+ and ERK1−/− mice at 2 to 4 months of age were counted on a hemocytometer. Data are means ± SEMs (n = 10) of cell number per spleen. (C) Perl Prussian blue staining for ferric iron in the spleen of 2-month-old ERK1+/+ and ERK1−/− mice (middle, original magnification ×100, with 10×/0.5 NA objective; bottom, original magnification ×200, with 20×/0.5 NA objective). Scale bars are for 50 μm. ***Significant differences between genotypes with P < .001.
Analysis of ERK1−/−-induced splenomegaly. (A) Age- and sex-matched ERK1−/− (□) and ERK1+/+ (■) mice at 2 to 4 months of age were killed, and spleen lengths and weights were measured. A representative comparison of ERK1+/+ and ERK1−/− spleens is shown in the inset (ruler increments are in millimeters). Data are means ± SEMs, n = 14 for ERK1+/+ and for ERK1−/− mice. (B) Splenocytes from ERK1+/+ and ERK1−/− mice at 2 to 4 months of age were counted on a hemocytometer. Data are means ± SEMs (n = 10) of cell number per spleen. (C) Perl Prussian blue staining for ferric iron in the spleen of 2-month-old ERK1+/+ and ERK1−/− mice (middle, original magnification ×100, with 10×/0.5 NA objective; bottom, original magnification ×200, with 20×/0.5 NA objective). Scale bars are for 50 μm. ***Significant differences between genotypes with P < .001.
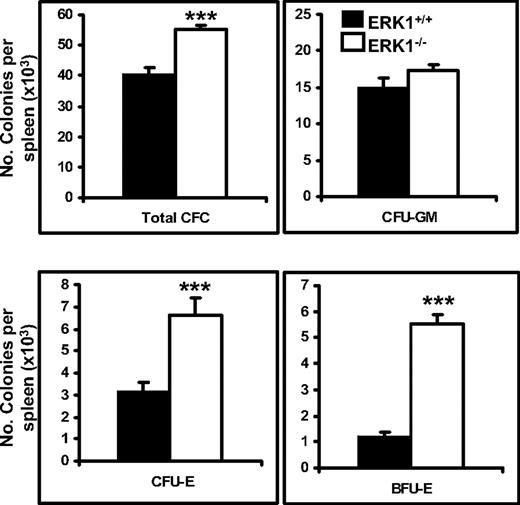
To determine whether the increase in total splenic cell number was due to a specific expansion of cells of the erythroid lineage, the number of total CFC, BFU-E, CFU-E, and CFU-GM colonies in the spleens of 2- to 4-month-old ERK1−/− and wild-type mice were determined (Figure 2). In the ERK1−/− mice, a 1.4-fold increase in total CFCs was observed. Two- and 4.46-fold, respectively, increases in CFU-Es and BFU-Es were also observed in these mice, whereas the CFU-GMs were unaffected. By contrast, in the bone marrow, there was no change in the number of either CFU-Es (mean ± SEM: 73 622 ± 8079 and 78 907 ± 7213 for wild-type and ERK1−/− mice, respectively, for 7 mice per genotype) or BFU-Es (10 154 ± 1267 and 10 770 ± 960 for wild-type and ERK1−/− mice, respectively, n = 7 per genotype).
Amplification of the erythroid lineage in the spleen of ERK1−/− mice. CFU-Es were measured after 2 days, and total CFCs, BFU-Es, and CFU-GMs were measured after 7 days in complete methylcellulose with cytokines. Data are mean colony numbers ± SEMs from 5 ERK1+/+ and 6 ERK1−/− (***P < .001).
Amplification of the erythroid lineage in the spleen of ERK1−/− mice. CFU-Es were measured after 2 days, and total CFCs, BFU-Es, and CFU-GMs were measured after 7 days in complete methylcellulose with cytokines. Data are mean colony numbers ± SEMs from 5 ERK1+/+ and 6 ERK1−/− (***P < .001).
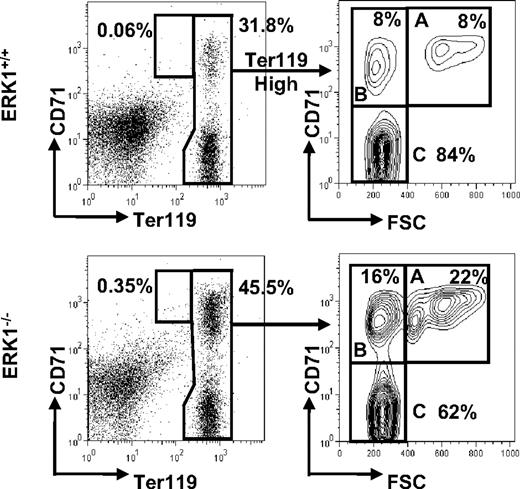
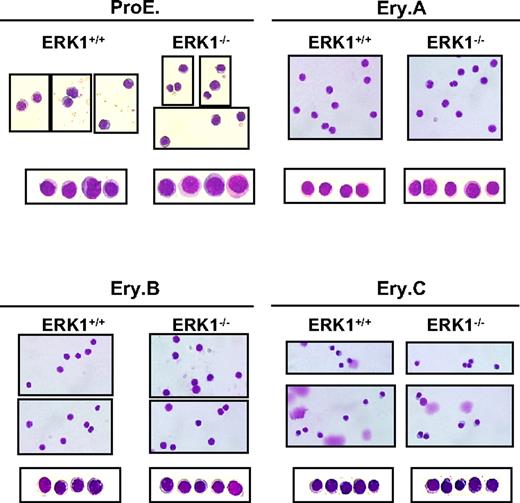
The splenocytes were then studied for their ability to stain for B (B220) and erythroid (Ter119 and CD71) markers. Phenotypic analysis of ERK1−/− spleen cells show that the B-cell compartment was not affected (36.1% ± 2.0%, n = 6 for ERK1−/−; 37.9% ± 3.0%, n = 5 for ERK1+/+), whereas the erythroid compartment was largely increased (Table 2). Because the early erythroid progenitors differentiate through the basophilic, polychromatic, and orthochromatic erythroblast stages, they lose the expression of CD71 while maintaining the expression of Ter119. Thus, these cell surface markers can be used to differentiate the various erythroblast stages. The different erythroblast subpopulations were distinguished by their respective expression level of both Ter119 and CD71 and the forward scatter (FSC) parameter, as previously described.37 Ter119+ (Ter119med and Ter119high) cells consistently resolved into 4 subpopulations, referred to as ProE for proerythroblasts (Ter119medCD71highFSChigh), Ery.A for basophilic (Ter119highCD71highFSChigh), Ery.B for late basophilic and polychromatic (Ter119highCD71highFSClow), and Ery.C for orthochromatic erythroblasts (Ter119highCD71lowFSClow; Figure 3). Cytospin analysis of sorted proE, Ery.A, Ery.B, and Ery.C confirmed the stage of differentiation of these subpopulations that were found similar in ERK1−/− and ERK1+/+ mice (Figure 4). The percentage of the overall Ter119+ cells increased 1.17-fold in ERK1−/− mice. In ERK1−/− mice, the analysis of the flow cytometric profiles shows that splenic ProE, Ery.A, and Ery.B subpopulations were increased by 4.2-, 2.7-, and 1.5-fold, respectively, compared with the wild-type mice, whereas the proportion of the most mature Ery.C subset declined by 1.4-fold (Table 2; Figure 3). When the data were normalized to correct for the higher cellularity in ERK1−/− spleens, proE, Ery.A, Ery.B, and Ery.C subpopulations were all enhanced, whereas the B-cell compartment remained unaltered (supplemental Table 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Percentages of cells in the different splenic and bone marrow erythroblast populations
| . | ERK1+/+ . | ERK1−/− . | P . |
|---|---|---|---|
| Spleen* | |||
| Ter119+ cells | 37.24 ± 2.24 | 43.58 ± 1.85 | .038† |
| ProE population | 0.12 ± 0.02 | 0.36 ± 0.05 | < .001† |
| Ery.A population | 7.01 ± 0.55 | 19.08 ± 1.18 | < .001† |
| Ery.B population | 9.42 ± 1.00 | 14.28 ± 1.03 | .005† |
| Ery.C population | 83.31 ± 1.32 | 66.27 ± 1.68 | < .001† |
| Bone marrow‡ | |||
| Ter119+ cells | 46.44 ± 1.68 | 46.56 ± 2.77 | NS |
| ProE population | 0.69 ± 0.04 | 0.70 ± 0.04 | NS |
| Ery.A population | 28.56 ± 1.90 | 27.78 ± 0.97 | NS |
| Ery.B population | 50.89 ± 1.84 | 50.11 ± 2.05 | NS |
| Ery.C population | 19.67 ± 1.01 | 21.44 ± 2.50 | NS |
| . | ERK1+/+ . | ERK1−/− . | P . |
|---|---|---|---|
| Spleen* | |||
| Ter119+ cells | 37.24 ± 2.24 | 43.58 ± 1.85 | .038† |
| ProE population | 0.12 ± 0.02 | 0.36 ± 0.05 | < .001† |
| Ery.A population | 7.01 ± 0.55 | 19.08 ± 1.18 | < .001† |
| Ery.B population | 9.42 ± 1.00 | 14.28 ± 1.03 | .005† |
| Ery.C population | 83.31 ± 1.32 | 66.27 ± 1.68 | < .001† |
| Bone marrow‡ | |||
| Ter119+ cells | 46.44 ± 1.68 | 46.56 ± 2.77 | NS |
| ProE population | 0.69 ± 0.04 | 0.70 ± 0.04 | NS |
| Ery.A population | 28.56 ± 1.90 | 27.78 ± 0.97 | NS |
| Ery.B population | 50.89 ± 1.84 | 50.11 ± 2.05 | NS |
| Ery.C population | 19.67 ± 1.01 | 21.44 ± 2.50 | NS |
Numbers of total Ter119+ cells and of each erythroblast subset populations are calculated as depicted in Figure 3, on freshly isolated splenocytes and bone marrow cells from ERK1+/+ and ERK1−/− mice. Data are mean percentage ± SEM.
NS indicates not significant.
ERK1+/+ (n = 14); ERK1−/− (n = 23).
Significantly different from ERK1+/+ value (P < .05).
ERK1+/+ (n = 9); ERK1−/− (n = 9).
Increased proportions of proE and early erythroblasts in the spleens of ERK1−/− mice. Representative flow cytometric analysis of freshly isolated splenocytes from ERK1+/+ and ERK1−/− mice. Ter119high cells were analyzed for their forward scatter (FSC) and CD71 expression. The number of proE cells is expressed as a percentage of all Ter119+ cells, whereas the number of cells in Ery.A (gate A), Ery.B (gate B), and Ery.C (gate C) subsets is expressed as percentages of Ter119high cells.
Increased proportions of proE and early erythroblasts in the spleens of ERK1−/− mice. Representative flow cytometric analysis of freshly isolated splenocytes from ERK1+/+ and ERK1−/− mice. Ter119high cells were analyzed for their forward scatter (FSC) and CD71 expression. The number of proE cells is expressed as a percentage of all Ter119+ cells, whereas the number of cells in Ery.A (gate A), Ery.B (gate B), and Ery.C (gate C) subsets is expressed as percentages of Ter119high cells.
Similar spleen erythroblast maturation between ERK1+/+ and ERK1−/− mice. May-Grünwald-Giemsa–stained cytospin preparations of sorted ProE, Ery.A, Ery.B, and Ery.C subsets. Representative fields of cells are shown for each region. The photographs were taken at an original magnification of ×400 with a Leica DXC 950P microscope and TRiBVN ICS image-acquisition software.
Similar spleen erythroblast maturation between ERK1+/+ and ERK1−/− mice. May-Grünwald-Giemsa–stained cytospin preparations of sorted ProE, Ery.A, Ery.B, and Ery.C subsets. Representative fields of cells are shown for each region. The photographs were taken at an original magnification of ×400 with a Leica DXC 950P microscope and TRiBVN ICS image-acquisition software.
Although some erythropoiesis occurs in the spleen under steady-state conditions, most of it normally occurs as a response to hematologic stress.4 We thus determined the status of the bone marrow erythropoiesis. The analysis of the percentages of erythroblasts in the various Ter119+ cell subsets in the bone marrow of ERK1−/− and ERK1+/+ mice showed no difference (Table 2). Altogether, these results indicate that the deletion of ERK1 specifically triggers a splenic erythropoiesis.
Apoptotic status of splenic erythroblasts in ERK1−/− mice
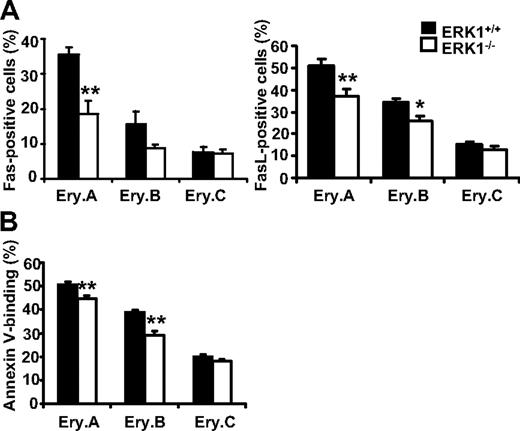
Previous studies have shown that, in the absence of stress, the low rate of splenic erythropoiesis is maintained by continuous apoptosis of the early erythroblasts through high coexpression of Fas and FasL at their surface.36 On stress, high level of EPO decreases Fas/FasL expression, leading to enhanced survival and an increased of the early erythroblast compartments. Flow cytometric analysis of splenocytes from ERK1−/− and wild-type mice were then performed to determine the Fas/FasL levels in the erythroblast subsets (Figure 5A). Early erythroblast expansion in ERK1−/− mice was associated with decreased cell surface Fas/FasL expression compared with wild-type controls. Figure 5A shows that there is a 1.91-fold reduction in Fas-positive splenic Ery.A in ERK1−/− mice. The percentages of FasL-positive Ery.A and Ery.B cells in ERK1−/− mice were also significantly decreased by 1.4- and 1.3-fold, respectively. This fall in Fas/FasL expression was correlated with a decrease in apoptosis, as shown by the lower Annexin V binding to splenic Ery.A and Ery.B cells in ERK1−/− mice (Figure 5B). The apoptotic status of the bone marrow erythroblasts were unaffected by the deletion of ERK1 (supplemental Table 2). These results show that the splenic erythropoiesis observed in ERK1−/− mice presents features of a stress erythropoiesis although these mice displayed no anemia.
Down-regulation of apoptosis in early splenic erythroblasts in ERK1−/− mice. (A) Changes in Fas (n = 6 ERK1+/+, n = 7 ERK1−/−; left) and FasL (n = 10 ERK1+/+, n = 11 ERK1−/−; right) cell-surface expression on splenic erythroblasts from ERK1−/− mice and ERK1+/+ mice. The results are expressed as percentages of Fas/FasL-positive cells within each erythroblast subset. (B) Annexin V expression on splenic erythroblasts from ERK1-deficient mice (n = 6) compared with ERK1+/+ mice (n = 6). The results are expressed as percentages of annexin V–positive cells within each erythroblast subset. Data are means ± SEMs (*P < .05; **P < .01)
Down-regulation of apoptosis in early splenic erythroblasts in ERK1−/− mice. (A) Changes in Fas (n = 6 ERK1+/+, n = 7 ERK1−/−; left) and FasL (n = 10 ERK1+/+, n = 11 ERK1−/−; right) cell-surface expression on splenic erythroblasts from ERK1−/− mice and ERK1+/+ mice. The results are expressed as percentages of Fas/FasL-positive cells within each erythroblast subset. (B) Annexin V expression on splenic erythroblasts from ERK1-deficient mice (n = 6) compared with ERK1+/+ mice (n = 6). The results are expressed as percentages of annexin V–positive cells within each erythroblast subset. Data are means ± SEMs (*P < .05; **P < .01)
Deletion of ERK1 has no effect on the EPO/EPOR signaling pathway and on the expression/activation level of ERK2
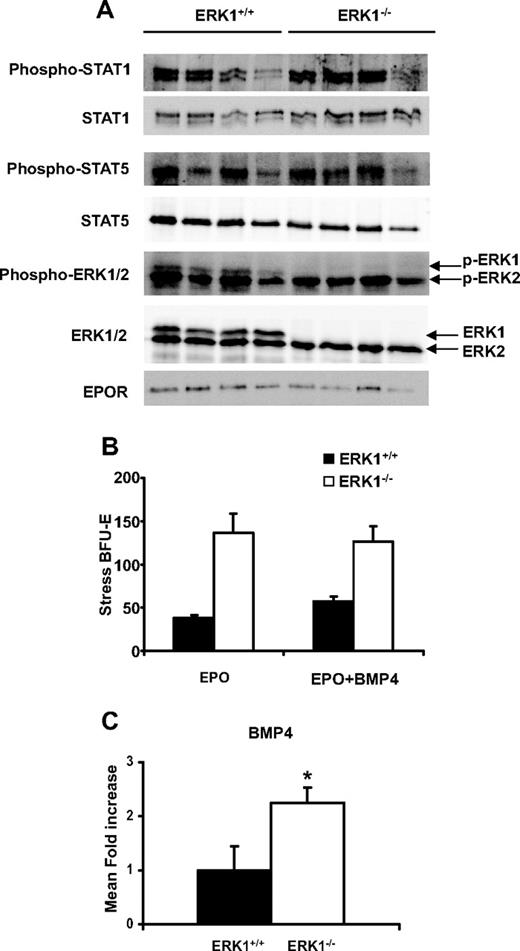
We then tested if the deletion of ERK1 increased the EPO concentrations in plasma and/or the expression of its receptor at the surface of the splenocytes. The results obtained showed that the EPO levels were slightly, but not significantly, lower in the ERK1−/− mice (185.7 ± 38 pg/mL, n = 7, for ERK1+/+; 135.6 ± 57 pg/mL, n = 11, for ERK1−/−). To evaluate if the deletion of ERK1 increased the EPOR expression and/or the EPOR-activated signaling pathways, Western blots were performed on splenocytes of both ERK1+/+ and ERK1−/− mice. The results shown in Figure 6A demonstrate that the deletion of ERK1 had no effect on the expression of EPOR. Recent studies have highlighted the role of both STAT1 and STAT5 in the regulation of erythropoiesis.37,38 Because STAT1-deficient erythroblasts display enhanced phosphorylation of STAT5 and ERK1/2,38 we thus studied the phosphorylation status of both STAT1 and STAT5 in the ERK1−/− mice. The deletion of ERK1 did not alter the phosphorylation of either STAT1 or STAT5 (Figure 6A). Altogether, these results suggest that the deletion of ERK1 does not affect the EPO/EPOR signaling. Previous studies have shown that the ablation of ERK1 could be compensated by an overexpression and/or overactivation of ERK2. The expression levels and the activation of the ERK kinases in splenic cells were thus analyzed by Western blot that discriminates the ERK1 and ERK2 isoforms. The results showed that the enhanced-splenic erythropoiesis in ERK1−/− mice is not due to an overexpression or to enhanced activation of the ERK2 isoform in ERK1−/− mice (Figure 6A) and is therefore specifically due to ERK1 ablation. Similar results were found in sorted purified populations of pooled Ery.A and Ery.B subpopulations (data not shown).
Enhanced BMP4 but not EPOR signaling in ERK1−/− spleen. (A) Lysates (100 μg) of ERK1+/+ and ERK1−/− splenocytes were resolved in sodium dodecyl sulfate–polyacrylamide gel electrophoresis and transferred to a nitrocellulose membrane. Expression and phosphorylation levels of STAT5, STAT1, and ERK1/2 and expression of EPOR were analyzed. (B) Spleen cells from wild-type or ERK1−/− mice were plated in methylcellulose media containing either EPO (3 U/mL) alone or EPO plus BMP4 (15 ng/mL), and stress BFU-E colonies were counted 5 days later. Data are mean colony numbers ± SEMs from 4 mice per genotype. (C) Q-PCR analysis of BMP4 expression in spleen of ERK1+/+ (n = 5) and ERK1−/− (n = 6) mice. Data are mean fold ± SEM (*P ≤ .05).
Enhanced BMP4 but not EPOR signaling in ERK1−/− spleen. (A) Lysates (100 μg) of ERK1+/+ and ERK1−/− splenocytes were resolved in sodium dodecyl sulfate–polyacrylamide gel electrophoresis and transferred to a nitrocellulose membrane. Expression and phosphorylation levels of STAT5, STAT1, and ERK1/2 and expression of EPOR were analyzed. (B) Spleen cells from wild-type or ERK1−/− mice were plated in methylcellulose media containing either EPO (3 U/mL) alone or EPO plus BMP4 (15 ng/mL), and stress BFU-E colonies were counted 5 days later. Data are mean colony numbers ± SEMs from 4 mice per genotype. (C) Q-PCR analysis of BMP4 expression in spleen of ERK1+/+ (n = 5) and ERK1−/− (n = 6) mice. Data are mean fold ± SEM (*P ≤ .05).
Deletion of ERK1 enhances stress BFU-Es and BMP4 expression
Recovery from acute anemia involved BMP4 expression in the spleen that drives expansion of a resident splenic-specialized population of stress erythroid progenitors, termed stress BFU-Es.6 In vitro, stress BFU-Es exhibit specific properties in that they form large colonies in methylcellulose in 5 days, rather than in 7 days for the classical-described BFU-Es, in the presence of EPO alone but which can be amplified in the presence of BMP4. The ERK1−/− splenic cells plated in methylcellulose in EPO alone gave rise to very numerous colonies, approximately 2-fold higher than those obtained with ERK1+/+ splenic cells plated in EPO and BMP4 (Figure 6B). The addition of BMP4 to the methylcellulose assays did not further increase colony formation with ERK1−/− cells, suggesting that in ERK1−/− spleens, expansion of stress BFU-Es had occurred in vivo, masking the effect of exogenously added BMP4. To examine whether ERK1 deletion was associated with activation of this pathway, real-time Q-PCR was performed for BMP4 (Figure 6C). The results showed that BMP4 mRNA was significantly up-regulated in ERK1−/− spleens compared with their ERK1+/+ counterparts. This result suggests that ERK1 may act as a negative regulator of BMP4 expression.
ERK1 deficiency enhances erythropoiesis during phenylhydrazine-induced anemia
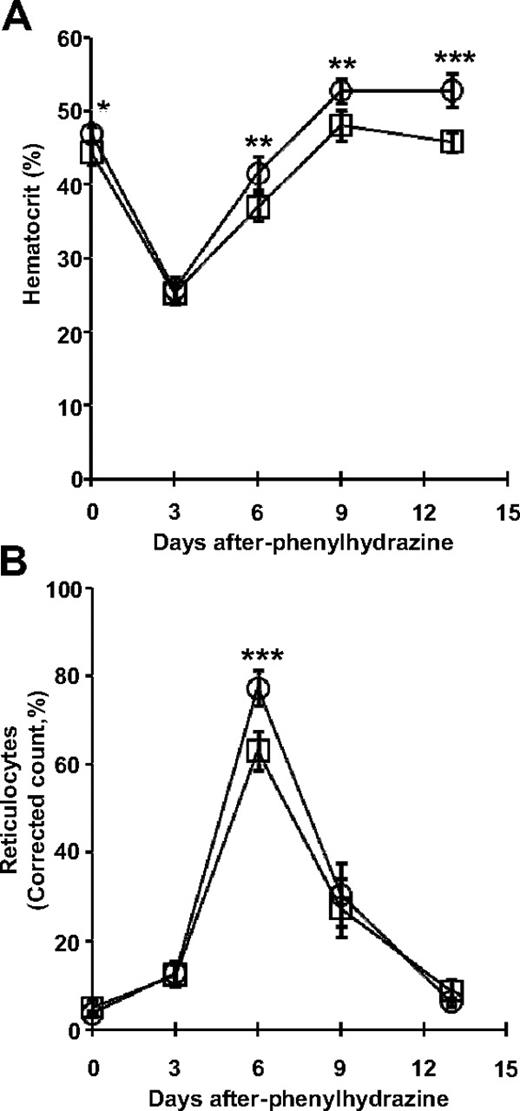
The erythroid response to acute anemia relies on the activation of the BMP4-dependent stress erythropoiesis. We therefore tested ERK1−/− mice for their ability to generate high erythropoietic rates under stress. The observed increases in stress BFU-Es and in early erythroblasts suggest that the ERK1−/− splenocytes may exhibit a stronger recovery from erythropoietic stress. This hypothesis was assessed during phenylhydrazine (PHZ)–induced hemolytic anemia. Mice were challenged with PHZ, and their hematocrit and reticulocyte counts were monitored over the next 13 days (Figure 7). The hematocrits of mice from both groups dropped sharply to 25% at day 3 (Figure 7A). By days 6 to 13, ERK1−/− mice exhibit higher hematocrits than wild-type mice. The proportion of reticulocytes in the peripheral blood is indicative of the erythropoietic rate. The basal reticulocyte count was less than 3% in both ERK1−/− and wild-type mice. Under PHZ-induced erythropoietic stress, the reticulocyte count in ERK1−/− mice reached a maximal level of approximately 80% at day 6, 1.2-fold above wild-type controls (Figure 7B). These results show that the ERK1−/− mice exhibited an enhanced recovery from erythropoietic stress.
Loss of ERK1 increases the response to erythropoietic stress. Five wild-type (□) and 6 ERK1−/− (○) mice were injected with PHZ on days 0, 1, and 3. Hematocrits (A) and reticulocyte counts (B) were assessed on days 0, 3, 6, 9, and 13. Data are mean ± SD (*P < .05; **P < .01; ***P < .001).
Loss of ERK1 increases the response to erythropoietic stress. Five wild-type (□) and 6 ERK1−/− (○) mice were injected with PHZ on days 0, 1, and 3. Hematocrits (A) and reticulocyte counts (B) were assessed on days 0, 3, 6, 9, and 13. Data are mean ± SD (*P < .05; **P < .01; ***P < .001).
Transplanted ERK1−/− bone marrow cells gave rise to enhanced erythropoiesis
The enhanced erythropoiesis observed in the ERK1−/− mice could result from a hematopoietic cell autonomous defect or from the loss of an extrinsic component of the spleen microenvironment or both. To distinguish these possibilities, hematopoietic chimaeras were performed by transplanting bone marrow cells from CD45.2+ ERK1−/− or ERK1+/+ mice into lethally irradiated congenic CD45.1+ recipients. After 4 months, greater than 96% of bone marrow cells were donor derived (CD45.2+CD45.1−), regardless of the transplantation with ERK1+/+ (97.12 ± 2.20; n = 10) or ERK1−/− (97.03 ± 4.67; n = 14) bone marrow cells. The spleens of the mice engrafted with ERK1−/− bone marrow cells exhibited the phenotype of the donor ERK1−/− mice regarding both the splenomegaly and the profile of erythroid cells. All the spleens of the engrafted mice (14 of 14) exhibited splenomegaly. There was no difference between ERK1+/+- and ERK1−/−-derived lymphoid B220pos cells within the spleens of engrafted mice (Table 3). Engrafted ERK1−/− bone marrow cells gave rise to a 3.8-fold increase in the frequency of proE, an approximately 2-fold increase of both Ery.A and Ery.B subsets, and a 1.5-fold decrease of Ery.C in the spleens of recipient mice. By contrast, bone marrows of wild-type mice recipients engrafted with ERK1−/− cells were found to display normal erythropoiesis (supplemental Table 3). These results show that the enhanced erythropoiesis in ERK1−/− mice can be transferred by bone marrow cells and suggest that the defect is carried by hematopoietic cells.
Percentages of the different erythroid subsets and B-cell compartment in the spleen after bone marrow transplantation
| . | Donor cells . | P . | |
|---|---|---|---|
| ERK1+/+ (n = 10) . | ERK1−/− (n = 14) . | ||
| Ter119+ cells | 31.18 ± 1.21 | 36.20 ± 1.67 | .032* |
| ProE population | 0.16 ± 0.02 | 0.60 ± 0.10 | < .001* |
| Ery.A population | 8.80 ± 1.40 | 20.29 ± 1.87 | < .001* |
| Ery.B population | 11.47 ± 1.26 | 25.55 ± 0.62 | < .001* |
| Ery.C population | 79.50 ± 2.59 | 53.86 ± 2.23 | < .001* |
| B220+ cells | 55.93 ± 1.05 | 52.76 ± 5.37 | .551 |
| . | Donor cells . | P . | |
|---|---|---|---|
| ERK1+/+ (n = 10) . | ERK1−/− (n = 14) . | ||
| Ter119+ cells | 31.18 ± 1.21 | 36.20 ± 1.67 | .032* |
| ProE population | 0.16 ± 0.02 | 0.60 ± 0.10 | < .001* |
| Ery.A population | 8.80 ± 1.40 | 20.29 ± 1.87 | < .001* |
| Ery.B population | 11.47 ± 1.26 | 25.55 ± 0.62 | < .001* |
| Ery.C population | 79.50 ± 2.59 | 53.86 ± 2.23 | < .001* |
| B220+ cells | 55.93 ± 1.05 | 52.76 ± 5.37 | .551 |
Bone marrow preparations from CD45.2 ERK1−/− or ERK1+/+ mice were used to repopulate irradiated CD45.1-marked wild-type recipients, in transplantation experiments. Four months after transplantation and for mice with greater than 90% CD45.2 engraftment, the percentage of total Ter119+ erythroblasts and those of the different erythroblast subpopulations in the spleen were determined as described in Figure 3. The percentage of B (B220+) cells was also determined. Data are mean percentage ± SEM.
Significantly different from ERK1+/+ value (P < .05).
Discussion
Previous studies have emphasized the role of the MAPK pathway in both proliferation and/or differentiation processes of the erythroid lineage in vitro.12,13,16-22 However, these studies did not decipher the specific role of each of the ERK kinases, that is, ERK1 and ERK2. Moreover, there are no data available on the role of these kinases in vivo on adult erythropoiesis. The present study shows that the loss of ERK1 alters the basal rate of splenic erythropoiesis. Indeed, adult ERK1−/− mice develop an increased splenic erythropoiesis mimicking a stresslike phenotype. This occurs in the absence of anemia, because adult mice have rather increased hematocrits and RBC counts. The loss of ERK1 resulted in an accumulation of the erythroid progenitor population in the spleens without any effect on the other lineages or on the bone marrow erythropoiesis. These results suggest that ERK1 is a negative regulator of the steady-state splenic erythropoiesis.
The absence of anemia and bone marrow abnormalities, even though both early progenitors and early erythroblasts accumulate in the spleens of ERK1−/− mice, suggests that the erythroid differentiation process is unaffected. Indeed, this was confirmed in the PHZ-induced erythropoietic stress experiment. ERK1−/− mice exhibited superior recovery after PHZ challenge, showing that terminal differentiation occurred. This suggests that ERK1 could play a role either in the regulation of the apoptosis status of the early progenitors and/or early erythroblasts or in the regulation of their proliferation rate. A normal homeostasis of the erythropoietic system requires an appropriate balance between the rate of erythroid cell production and that of RBC destruction. The spleen is a reserve erythropoietic organ, whereas bone marrow is an active site of erythropoiesis in steady state. It has been shown that the sensitivity to apoptosis of splenic and bone marrow early progenitors and early erythroblasts differs considerably; the splenic cells undergoing continuous apoptosis because of a higher level of coexpressed Fas and FasL at their surface.4,36 Fas is clearly up-regulated during the initial steps of erythroid differentiation from BFU-E to CFU-E, reaching elevated levels of expression at the stages of immature erythroblasts (proE and Ery.A). Our results clearly show a down-regulation of both Fas and FasL and an enhanced survival of the early erythroblast subsets in ERK1−/− mice. This result suggests that ERK1 might positively control the expression of Fas/FasL on these cells. However, it is generally admitted that activation of the MAPK/ERK pathway down-regulates the expression of Fas in most cellular models. With the use of oncogenic mutants of Ras, it has been shown that activity of the Ras/ERK pathway inhibits Fas-mediated apoptosis.39 Fas is selectively up-regulated at the level of mRNA during erythroid differentiation, and Raf-1 ablation selectively increases this up-regulation in fetal liver erythroblasts by inhibiting ERK activation.40 On the basis of these data, it seems unlikely that the down-regulation of Fas and FasL on the early erythroblasts observed in ERK1−/− mice is due to a direct effect of ERK1 on Fas/FasL expression. This defect may rather be the consequence of the induction of a stress erythropoiesis phenotype generated by the loss of ERK1.
The essential function of EPOR in red cell formation is limited to a narrow window of progenitors, between CFU-E and early erythroblasts (proE and Ery.A).3 In the basal state, when EPO levels are low, only a few EPORs are occupied,41 providing a weak signal. During stress, both the EPO concentration and the EPOR expression increase dramatically and EPOR signaling is fully activated. The ablation of ERK1 induced splenic erythropoiesis in the basal state, whereas EPO levels were not increased but rather slightly decreased in the ERK1−/− mice. In addition, this stress erythropoiesis phenotype of ERK1−/− mice could be reproduced upon transplantation of ERK1−/− bone marrow cells into wild-type recipients. This suggests that ERK1 is a negative regulator of splenic erythropoiesis.
One possibility is that ERK1 negatively regulates the EPOR expression on splenic cells. Indeed, mice expressing only one EPOR allele are deficient in their response to stress but show normal bone marrow erythropoiesis.42 Thus, a small increase of EPOR in ERK1−/− mice might conversely lead to enhanced splenic erythropoiesis without affecting that of bone marrow. We tested this hypothesis by performing Western blots and found no increase in the level of the EPOR in ERK1−/− mice. Because STAT1 and STAT5 have been shown to be involved in the erythroid development,37,38 ERK1 could be involved in the activation of this pathway independently of an action on the EPO level or the EPOR expression. Our results show that the deletion of ERK1 has no effect on the expression of STAT1 or STAT5 or on their phosphorylation status. These results suggest that ERK1 does not play a role in the regulation of the EPO/EPOR signaling pathway.
Previous work has shown that acute anemia leads to the expansion of splenic BMP4-responsive stress BFU-Es.5 Moreover, it has been shown that under Hedgehog signaling in the spleen microenvironment, BMP4 is expressed by bone marrow cells that have homed to the spleen and further differentiated into splenic BMP4-responsive stress erythroid progenitors.6 Our results show that the deletion of ERK1 is sufficient to induce splenic BMP4-responsive stress BFU-Es. The addition of BMP4 in vitro does not increase further the differentiation of stress BFU-Es from ERK1−/− mice while increasing that of ERK1+/+ mice, suggesting that ERK1 interplays with the BMP4 pathway. Supporting this possibility, the deletion of ERK1 induces the expression of BMP4 mRNA in the spleen that could account for the increase of the splenic stress BFU-Es observed in the ERK1−/− mice. The defect found in ERK1−/− mice can be reproduced by transplantation of ERK1−/− bone marrow cells to ERK1+/+ recipients. This result suggests that transplanted ERK1−/− bone marrow progenitors homing to the spleen gave rise to BMP4-responsive cells that differentiated into stress BFU-Es without any signal of stress erythropoiesis. BMP4 has been recently shown to be produced by both hematopoietic and nonhematopoietic stromal cells.6 Thus, we cannot rule out the hypothesis that the defect could result from transplanted ERK1−/− bone marrow accessory cells or macrophages to the recipient spleens.
ERK1 and ERK2 functions are largely redundant. Relatively little is known about the possible specific roles for each of these isoforms. These 2 proteins are coexpressed in virtually all tissues but with a remarkably different relative abundance, ERK2 being the predominant one. Generally, silencing of ERK1 has no effect, probably because the abundant level of ERK2 can compensate for the loss of ERK1 and because its invalidation has only a minor effect on total ERK activity.27,43 Interestingly, although ERK2 is also the major kinase expressed in splenic cells, deletion of ERK1 is not compensated by an increase in either the expression or the activation of ERK2. Thus, the increase in splenic erythropoiesis observed in ERK1−/− mice can be accounted for by the loss of ERK1, specifically.
The homeostatic mechanism of erythropoiesis in the spleen relies on negative autoregulation of cell numbers that would self-correct for small perturbations, maintaining a relatively constant erythroblast population size in the basal steady state. In this study, we demonstrate that ERK1 is a new player in the regulation of this pathway. Our results show that it is necessary to maintain ERK activation at a sufficient level to keep the BMP4 signal at a low level and to control steady-state erythropoiesis in the spleen. An alteration of ERK1 expression or activation could result in an inappropriate activation of stress erythropoiesis. Further investigations will give clues on how ERK1 regulates BMP4 expression and if this signal is specific for the ERK1 isoform or if it requires a threshold of ERK activation.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Dr Benoit Laurent for advice on Q-PCR.
This work was supported by Inserm and the Agence Nationale pour la Recherche (grant ANR-08-BLAN-0332), the Association pour la Recherche contre le Cancer (grant A06/1/4012), and the Institut National du Cancer (grant CSHLE 2008 INCa). S.G. is the recipient of a fellowship from the Ministère de l'Enseignement Supérieur et de la Recherche.
Authorship
Contribution: S.G. and M.G. designed and performed most of the experiments and analyzed data; D.C. designed and performed all of the cell-sorting experiments; L.C. and M.S. helped with the design of selected experiments; P.O. performed the histologic experiment and analyzed data; N.S. performed the Q-PCR experiments and analyzed data; G.P. and J.P. provided the ERK1−/− mice; and M.G. and F.P. conceived the project and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Murielle Gaudry, Institut Cochin, Bâtiment Gustave Roussy, 7ème étage, 22 rue Méchain, 75014 Paris, France; e-mail: murielle.gaudry@inserm.fr.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal