Abstract
Treatment of hemophilia B requires frequent infusions of factor IX (FIX) to prophylax against bleeding episodes. Hemophilia B management would benefit from a FIX protein with an extended half-life. A recombinant fusion protein (rFIXFc) containing a single FIX molecule attached to the Fc region of immunoglobulin G was administered intravenously and found to have an extended half-life, compared with recombinant FIX (rFIX) in normal mice, rats, monkeys, and FIX-deficient mice and dogs. Recombinant FIXFc protein concentration was determined in all species, and rFIXFc activity was measured in FIX-deficient animals. The half-life of rFIXFc was approximately 3- to 4-fold longer than that of rFIX in all species. In contrast, in mice in which the neonatal Fc receptor (FcRn) was deleted, the half-life of rFIXFc was similar to rFIX, confirming the increased circulatory time was due to protection of the rFIXFc via the Fc/FcRn interaction. Whole blood clotting time in FIX-deficient mice was corrected through 144 hours for rFIXFc, compared with 72 hours for rFIX; similar results were observed in FIX-deficient dogs. Taken together, these studies show the enhanced pharmacodynamic and pharmacokinetic properties of the rFIXFc fusion protein and provide the basis for evaluating rFIXFc in patients with hemophilia B.
Introduction
Hemophilia B is an X chromosome–linked bleeding disorder resulting from a deficiency in functional factor IX (FIX) and affects 1 in every 25 000 to 30 000 males.1,2 Patients with severe hemophilia B (FIX < 1 IU/dL) in particular have repeated bleeding episodes resulting in hemophilic arthropathy and other sequelae.2,3 Like hemophilia A (factor VIII deficiency), conventional treatment for hemophilia B has been on demand, that is, patients are administered concentrates of the deficient factor when hemorrhage occurs or before a surgical procedure.3 Alternatively, replacement therapy can be given in regular intervals prophylactically to maintain FIX levels greater than 1%, thereby converting a severe phenotype into one that is milder, and to potentially prevent life-threatening hemorrhages and musculo-articular bleeding events.3-6
When treating bleeding episodes on demand or in the surgical setting, several infusions of FIX may be required to avoid peaks and troughs and to maintain minimum coagulation factor levels for a sufficient duration. Similarly, in patients being treated on prophylactic regimens, 2 to 3 injections per week are usually required to achieve the necessary FIX plasma levels. This can be attributed, in part, to the relatively short half-lives of approximately 18 hours of currently available products.7 In hemophilia B, twice- or thrice-weekly dosing with FIX at 40 to 100 IU/kg body weight is recommended by the Medical and Scientific Advisory Council of the National Hemophilia Foundation.8 Because coagulation factors are administered by intravenous injection, repeat venous access is necessary, which is particularly difficult in a pediatric population, and may necessitate the use of ports for central venous access.
A recombinant FIX product with a longer half-life (t1/2) than currently available FIX products would be expected to require fewer injections, thus reducing the need for repeated venous access, potentially improving the acceptance of prophylactic regimens by pediatric patients and their parents, and possibly decreasing repeated dosing in the treatment of episodic bleeds or in surgical settings. Recombinant factor IX-Fc fusion protein (rFIXFc) contains a single molecule of FIX recombinantly attached to the constant region (Fc) of immunoglobulin G (IgG) and was developed to address the unmet medical need for a long-acting FIX product. The presence of the Fc domain enables the fusion protein to bind to the neonatal Fc receptor (FcRn), a heterodimer of a major histocompatibility complex (MHC) class I–like protein heavy chain with β2-microglobulin (β2m).9 FcRn serves a critical role in IgG homeostasis by protecting the Fc-containing molecules from catabolism.9,10 We report here the enhanced pharmacokinetics and pharmacodynamics of rFIXFc compared with commercially available recombinant FIX (rFIX).
Methods
Cloning, expression, and purification of rFIXFc
All molecular biology procedures were performed following standard techniques.11 The coding sequences for human FIX, the Fc region of the human IgG1 (hinge and CH2 and CH3 domains) and PC5 were obtained by polymerase chain reaction (PCR) from human liver mRNA, human lymphocyte cDNA, and human liver mRNA, respectively, and cloned into mammalian expression vectors. HEK-293H cells (Invitrogen) were stably transfected with expression cassettes for both FIXFc (native FIX coding sequence fused to the Fc region with no intervening linker) and Fc alone, leading to the secretion of 3 different protein products: FIXFc dimer, FIXFc monomer (1 FIXFc and 1 Fc chain), and Fc dimer. Cells were cotransfected with the expression cassette for PC5,12 a processing enzyme, to ensure full cleavage of the FIX propeptide. Cell lines were grown in serum-free suspension media in the presence of vitamin K. FIXFc monomer (hereinafter referred to as rFIXFc) was purified by column chromatography with the use of a Protein A capture step and 2 anion exchange steps, Fractogel DEAE and Q Sepharose. The last ion exchange step involved pseudo-affinity elution13 from a Q Sepharose resin with low ionic strength CaCl2 to obtain rFIXFc with highest specific activity.
Recombinant factor IX preparation
Lyophilized BeneFIX (coagulation FIX, recombinant; rFIX; Wyeth) was reconstituted in the formulation buffer supplied by the manufacturer.
γ-carboxylation analysis
Analysis of the γ-carboxylation (Gla) content of the rFIXFc was performed by total amino acid analysis after base hydrolysis, and the results were compared with those for plasma-derived FIX (pdFIX; Mononine; CSL Behring), which has been shown to contain 12 Gla/molecule FIX protein.14 Gla content was confirmed by mass spectroscopy after digestion with LysC and subsequent peptide mapping.
Additional posttranslational modification and other analyses
β-Hydroxylation, propeptide content, and O-glycosylation were assessed by LysC peptide mapping. Tyrosine sulfation was determined by AAA (base hydrolysis) relative to pdFIX. Serine phosphorylation was determined by Pro-Q Diamond staining quantitation of sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) gels relative to pdFIX. N-glycan content was determined by a normal-phase high-performance liquid chromatographic method profile of N-linked glycans released by PNGase F, followed by 2-aminobenzamide labeling.
Activated FIX was determined by enzyme-linked immunoabsorbent assay (ELISA) based on the binding of activated FIX to antithrombin III in the presence of heparin,15 modified for detection with an anti–human FIX antibody conjugated to horseradish peroxidase (HRP).
Activation by activated factor XI was assessed by reducing SDS-PAGE analysis after 5 minutes of incubation of either rFIXFc or rFIX with activated factor XI at 100:1 ratios and quantification by densitometry of the intact protein.
FIXFc and FIX ELISA
A sandwich ELISA designed to detect the rFIXFc protein concentration in animal plasma was developed with the use of a goat anti–human FIX IgG capture antibody (Enzyme Research Laboratories) and a goat anti–human IgG (Fc-specific)–HRP conjugate (Pierce Biotechnology).
The sandwich ELISA to detect rFIX used the same goat anti–human FIX capture antibody as for rFIXFc. The detection antibody for the FIX ELISA was a goat anti–human FIX-HRP conjugate (Enzyme Research Laboratories).
Protein concentrations determined by ELISA analysis are expressed as means (±SD) calculated from values obtained from multiple, individual animals assayed in triplicate, except in certain cases for rFIX (as indicated in figures) where, because of low levels of protein, samples were pooled to provide sufficient material for detection and are expressed as single data points. Data from the 2 individual FIX-deficient dogs are expressed as the mean of triplicate measurements for each data point.
Animals
A FIX knockout strain of mice, produced with the use of selective gene targeting,16 was acquired from Dr Darrel Stafford (University of North Carolina, Chapel Hill). Female Sprague Dawley rats and C57BL/6J mice were purchased from Charles River Laboratories. FcRn knockout (KO) mice were derived from C57BL/6J mice,17 and human FcRn transgenic (Tg32B) mice were originally obtained from Dr Derry Roopenian (The Jackson Laboratory). The FcRn KO mice do not express murine FcRn or murine β2m and are designated mFcRn−/−/mβ2m−/−. FcRn transgenic mice express human FcRn and human β2m on a murine FcRn and β2m KO background and are designated muFcRn−/−/muβ2m−/−/hFcRn+/+/hβ2m+/+.
Two dogs with hemophilia B18 (1 male and 1 female) from the closed colony at the Francis Owen Blood Research Laboratory at the University of North Carolina at Chapel Hill were infused with rFIXFc intravenously. These experiments were performed at the Francis Owen Blood Research Laboratory. All cynomolgus monkeys underwent physical examinations before the study; all animals were determined to be in good health before dosing.
All animal studies were conducted in compliance with approved protocols of the Institutional Animal Care and Use Committee at their respective institutions.
Administration of FIX preparations and blood sampling
Pharmacodynamic studies in FIX-deficient mice.
Single- and repeat-dose studies were performed in FIX-deficient mice to evaluate clotting activity of rFIXFc compared with rFIX. In both studies, FIX-deficient mice received rFIXFc at 219 IU/kg body weight or rFIX at 200 IU/kg body weight by intravenous administration. In the repeat-dose study, the same dose of each factor was given on days 0, 4, and 8. To obtain sufficient citrated plasma (0.32% final) free of tissue factor contamination (which has the potential to confound results of FIX activity assay), blood samples were obtained by cardiac puncture after humanely killing by CO2 at 0.25, 8, 24, 48, 72, and 96 hours after administration for the single-dose study, and at 0.25 hour and 96 hours after administration for the multiple-dose study, to evaluate clotting activity by a FIX activity assay. Citrated plasma was also obtained at 8, 24, 48, and 72 hours after each dose by tail bleeds in the multiple-dose study to evaluate FIX concentration by ELISA. Calculated rFIXFc/rFIX clotting activity was determined by converting rFIXFc/rFIX concentrations from microgram per milliliter to international unit per milliliter values with the use of the specific activity of each protein.
Pharmacokinetic studies in mice.
Mice (normal C57BL/6J, FIX-deficient, FcRn KO, FcRn transgenic) were administered a single intravenous dose of rFIX or rFIXFc at 100 or 200 IU/kg body weight. Blood was collected from the tails at 0.25, 8, 24, 48, 72, 96, and 168 hours after dosing in each animal, and citrated plasma (0.32% final) was prepared.
Rats.
Single intravenous doses of rFIXFc or rFIX at 200 IU/kg body weight were administered into the lateral tail vein in each of 2 groups of rats. Blood samples were collected at 0.25, 4, 8, 24, 48, 72, 96, and 168 hours after administration of rFIXFc or at 0.25, 8, 24, and 72 hours after administration of rFIX, and citrated plasma (0.32% final) was prepared.
FIX-deficient dogs.
Intravenous infusions into the cephalic vein were performed with rFIXFc at 140 IU/kg body weight. Blood samples were collected from the cephalic vein of the opposite leg before dosing and at 5, 15, and 30 minutes and at 1, 2, 4, 6, 8, 12, 24, 27, 30, 48, 51, 54, 72, 80, 96, 144, and 168 hours after dosing and analyzed directly for whole blood clotting time (WBCT), or citrated plasma (0.32% final) was prepared for clotting activity and protein concentration measurements with the use of the rFIXFc-specific ELISA.
Cynomolgus monkeys.
Animals were deprived of food a minimum of 12 hours and were anesthetized with ketamine at approximately 10 mg/kg body weight intramuscularly before administration of rFIXFc. Single intravenous doses of rFIXFc (0.5, 2, and 10 mg/kg body weight, corresponding to approximately 25, 100, or 500 IU/kg body weight, respectively) were administered through an indwelling cephalic vein catheter in a total infusion volume of 5 mL followed by a 3-mL saline flush. Blood samples were collected by femoral venipuncture into citrated evacuated tubes before dosing and at 0.25, 0.5, 1, 8, 24, 48, 72, 96, 120, 144, and 168 hours after dosing, and citrated plasma (0.32% final) was prepared.
Coagulation testing
FIX activity assay.
An automated FIX activity assay was performed with the use of the MLA Electra 1600C (Medical Laboratory Automation/Instrument Laboratories) to quantify the ability of the FIX component of the rFIXFc protein to restore the clotting activity of FIX-deficient plasma. This assay also detected the amount of FIX activity in citrated plasma from animals treated with rFIX or rFIXFc. Test samples were mixed with equal volumes of human FIX-deficient plasma (Diagnostica Stago; catalog no. 00724) and cephalin-containing ellagic acid activator (activated partial thromboplastin time [aPTT]–soluble activator; Helena Laboratories; catalog no. 5389), and, after 4 minutes of incubation, 5mM calcium chloride (25mM stock; Instrumentation Laboratory; catalog no. 020006910) was added, and the time to clot was measured. Activity was calculated on the basis of a calibration curve of clotting times versus activity unit concentration (in IU/mL) of serial dilutions of a World Health Organization FIX standard for purified proteins, dilutions of a calibrated human plasma standard for the mouse experiments, and dilutions of rFIXFc spiked into FIX-deficient dog plasma for the FIX-deficient dog experiments.
Whole blood clotting time.
FIX-deficient mice were dosed intravenously with rFIXFc or rFIX at 50 IU/kg body weight. Blood samples (30 μL) were collected before and after dosing at various times and incubated at 37°C. Samples were visually inspected for the presence of a blood clot once per minute, and the time for a clot to form was recorded. Sampling ceased when clotting time returned to baseline (144 hours for rFIXFc and 72 hours for rFIX).
Pharmacokinetic data analyses and statistical methods
Pharmacokinetic analysis was performed with the use of noncompartmental modeling with WinNonlin, Version 4.1 or 5.1 software (Pharsight Corporation). The pharmacokinetic parameter estimates derived from ELISA data included maximum concentration (Cmax), area under the time versus concentration curve (AUC), and elimination t1/2.
Results
Biochemical characterization of rFIXFc
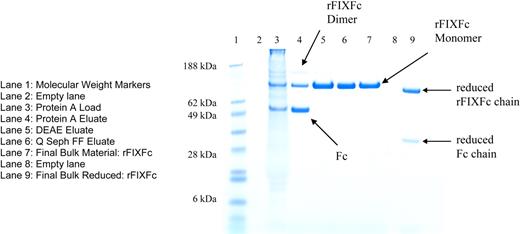
rFIXFc samples were analyzed by several biochemical methods (Table 1). Conditioned media from transfected cells contained 3 species, FIXFc dimer, FIXFc monomer, and free Fc dimer (Figure 1), and were purified to yield FIXFc monomer (hereinafter referred to as rFIXFc) with the use of a 3-column process. Purified rFIXFc was analyzed by both reducing and nonreducing SDS-PAGE. Nonreduced samples ran as single bands of the expected size (∼ 120 kDa), correlating to the intact rFIXFc, with no detectable Fc or FIXFc dimer (Figure 1). Reduced samples ran as 2 bands of expected sizes, corresponding to the rFIXFc (∼ 100 kDa) and Fc (∼ 30 kDa) chain. Protein was found to be greater than 95% pure by size exclusion chromatography (data not shown) and by reducing and nonreducing SDS-PAGE.
Biochemical characterization of recombinant FIXFc and recombinant and plasma-derived FIX
| Characteristic . | . | rFIXFc . | rFIX . | pdFIX . |
|---|---|---|---|---|
| γ-carboxylation | ||||
| aa 1-23 (K1K2 peptide) | % 6 Gla | 97.8 | 96.9 | 99.6 |
| % 5 Gla | 2.2 | 3.1 | 0.4 | |
| % 4 Gla | 0 | 0 | 0 | |
| aa 24-43 (K3 peptide) | %6 Gla | 61.3 | 63.7 | 98.9 |
| % 5 Gla | 26.3 | 30.9 | 1.1 | |
| % 4 Gla | 12.5 | 5.4 | 0 | |
| Total Gla/mol, peptide map | 11.5 | 11.6 | 12.0 | |
| Total Gla/mol, AAA | 11.2 ± 0.7 | 11.6 ± 0.5 | (12) | |
| Propeptide content | None detected | None detected | None detected | |
| β-hydroxylation Asp 64 | 70% | 49% | 37% | |
| Sulfation of Tyr 155 | 4% | 5% | (> 90%) | |
| Phosphorylation of Ser 158 | < 10% | < 10% | (> 90%) | |
| Ala 148/Thr 148 | Thr | Ala | 30% Ala/70%Thr | |
| Activated FIX | < 0.013% | 0.11% ± 0.0019% | 0.21% ± 0.010% | |
| FXIa activation | 94.8% ± 2.4% | 96.6% ± 1.8% | Not done |
| Characteristic . | . | rFIXFc . | rFIX . | pdFIX . |
|---|---|---|---|---|
| γ-carboxylation | ||||
| aa 1-23 (K1K2 peptide) | % 6 Gla | 97.8 | 96.9 | 99.6 |
| % 5 Gla | 2.2 | 3.1 | 0.4 | |
| % 4 Gla | 0 | 0 | 0 | |
| aa 24-43 (K3 peptide) | %6 Gla | 61.3 | 63.7 | 98.9 |
| % 5 Gla | 26.3 | 30.9 | 1.1 | |
| % 4 Gla | 12.5 | 5.4 | 0 | |
| Total Gla/mol, peptide map | 11.5 | 11.6 | 12.0 | |
| Total Gla/mol, AAA | 11.2 ± 0.7 | 11.6 ± 0.5 | (12) | |
| Propeptide content | None detected | None detected | None detected | |
| β-hydroxylation Asp 64 | 70% | 49% | 37% | |
| Sulfation of Tyr 155 | 4% | 5% | (> 90%) | |
| Phosphorylation of Ser 158 | < 10% | < 10% | (> 90%) | |
| Ala 148/Thr 148 | Thr | Ala | 30% Ala/70%Thr | |
| Activated FIX | < 0.013% | 0.11% ± 0.0019% | 0.21% ± 0.010% | |
| FXIa activation | 94.8% ± 2.4% | 96.6% ± 1.8% | Not done |
Posttranslational modifications and other analyses of rFIXFc, rFIX, and pdFIX were assessed in a variety of assays as described in “γ-carboxylation analysis” and “Additional posttranslational modification and other analyses.” Numbers in parentheses taken from published values.7
SDS-PAGE gel of purification intermediates and purified rFIXFc monomer. Samples from different steps in the purification of rFIXFc were analyzed by nonreducing SDS-PAGE. Lane 1: SeeBlue Plus Molecular Weight Markers (Invitrogen). Lane 2: Empty lane. Lane 3: Protein A Load. Lane 4: Protein A Eluate. Lane 5: Fractogel DEAE Eluate. Lane 6: Q Seph FF Eluate. Lane 7: Final Bulk Material: rFIXFc. Lane 8: Empty lane. The Final Bulk Material was also analyzed by SDS-PAGE after reduction with 2-mercaptoethanol in lane 9.
SDS-PAGE gel of purification intermediates and purified rFIXFc monomer. Samples from different steps in the purification of rFIXFc were analyzed by nonreducing SDS-PAGE. Lane 1: SeeBlue Plus Molecular Weight Markers (Invitrogen). Lane 2: Empty lane. Lane 3: Protein A Load. Lane 4: Protein A Eluate. Lane 5: Fractogel DEAE Eluate. Lane 6: Q Seph FF Eluate. Lane 7: Final Bulk Material: rFIXFc. Lane 8: Empty lane. The Final Bulk Material was also analyzed by SDS-PAGE after reduction with 2-mercaptoethanol in lane 9.
FIX Western blotting confirmed the identity of the FIX protein and the absence of the propeptide (data not shown). Proper propeptide processing was confirmed by Lys-C peptide mapping (data not shown). The rFIXFc chain contains 641 amino acids, and the Fc chain contains 227 amino acids. FIX is composed of several functional domains which undergo extensive posttranslational modification. Recombinant FIXFc was found to have 11.2 (±0.7) Gla/molecule, comparable with rFIX which was found to contain 11.6 (±0.52) Gla/molecule, consistent with literature values of 11.5 Gla/molecule.21 Peptide mapping confirmed the γ-carboxylation pattern to be similar to rFIX, with 10 of the 12 sites fully occupied, and the residues primarily at position 36 and 40 being under γ-carboxylated, which has been shown to have no effect on activity.14
In addition to γ-carboxylation and propeptide processing, several other posttranslational modifications were assessed for rFIXFc and compared with rFIX (BeneFIX) and pdFIX (Mononine). In general, rFIXFc was found to be comparable with rFIX, with respect to Ser 158 phosphorylation and Tyr 155 sulfation (Table 1). N-linked glycosylation fingerprints indicated that the FIX-derived glycans are not fully sialylated, similar to rFIX (data not shown). O-linked glycosylation isoforms in the first EGF domain were the same as rFIX but present at different relative ratios (data not shown). rFIXFc possessed a greater degree of β-hydroxylation of Asp 64 than did rFIX or pdFIX. The levels of activated FIX were measured in all 3 proteins and were found to be significantly lower in rFIXFc than in either rFIX or pdFIX.
The specific activity of rFIXFc ranged from 4.28 (±0.52) to 6.12 (±0.28) IU/nmol for protein lots that had undergone a 3-column purification process, corresponding to 43.8 (±5.4) to 62.7 (±2.87) IU/mg, based on the FIX activity assay with the use of a calibration standard from the World Health Organization. For some of the earlier in vivo experiments, the specific activity of rFIXFc was lower before optimization of the purification process. This, however, did not have an effect on pharmacokinetic properties of rFIXFc (data not shown).
Coagulation activity in FIX-deficient mice
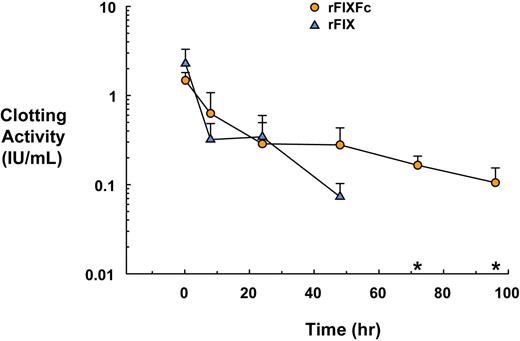
Fifteen minutes after administration, rFIXFc and rFIX corrected the circulating levels to 1.47 (±0.32) IU/mL and 2.28 (±1.01) IU/mL, respectively. At 48 hours, the rFIXFc clotting activity remained high at 0.27 (±0.15) IU/mL, whereas clotting activity with rFIX had decreased substantially to 0.07 (±0.03) IU/mL. Clotting activity for the rFIX-treated mice was undetectable at time points later than 48 hours after dosing, whereas rFIXFc-treated mice continued to retain measurable activity, remaining at 0.10 (±0.04) IU/mL at 96 hours (Figure 2).
Functional activity of rFIXFc and rFIX in FIX-deficient mice. FIX-deficient mice were dosed intravenously with rFIXFc at 219 IU/kg body weight (3 or 4 per group, 6 groups, n = 23) or rFIX at 200 IU/kg body weight (3 or 4 per group, 5 groups, n = 23) at time 0. Blood samples were collected at various times after dosing (0.25 hour to 96 hours) and analyzed for clotting activity with the use of FIX activity assay. *rFIX activity is undetectable in all of the mice at time points later than 48 hours after dosing.
Functional activity of rFIXFc and rFIX in FIX-deficient mice. FIX-deficient mice were dosed intravenously with rFIXFc at 219 IU/kg body weight (3 or 4 per group, 6 groups, n = 23) or rFIX at 200 IU/kg body weight (3 or 4 per group, 5 groups, n = 23) at time 0. Blood samples were collected at various times after dosing (0.25 hour to 96 hours) and analyzed for clotting activity with the use of FIX activity assay. *rFIX activity is undetectable in all of the mice at time points later than 48 hours after dosing.
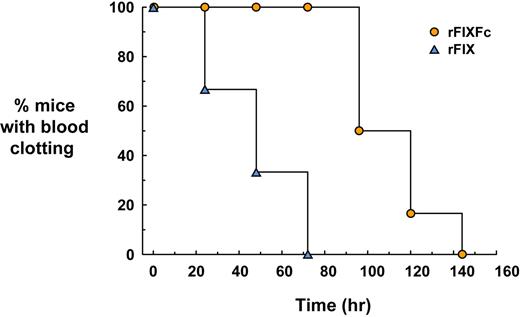
In whole blood samples, 15 minutes after administration, normal clotting was observed in all mice given rFIXFc or rFIX (Figure 3). Blood clotting times in all of the rFIX-treated animals returned to baseline by 72 hours, whereas blood from all of the rFIXFc-treated mice still clotted throughout this time. Clotting activity in blood from rFIXFc-treated mice returned to baseline by 144 hours in all animals, twice the time observed for the rFIX-treated mice.
WBCT of rFIXFc versus rFIX in FIX-deficient mice. FIX-deficient mice (6 per group) were dosed intravenously with rFIXFc at 50 IU/kg body weight or rFIX at 50 IU/kg body weight. Blood samples were collected before dosing and at various times after dosing. Blood samples were incubated at 37°C and were visually inspected for the presence of a blood clot once per minute. The time needed for a clot to form was recorded, and, once the clotting activity returned to baseline (ie, no clot formation), no additional samples were obtained (samples collected 15 minutes to 144 hours for rFIXFc or 15 minutes to 72 hours for rFIX). The graph indicates the percentage of animals able to clot blood at each time point.
WBCT of rFIXFc versus rFIX in FIX-deficient mice. FIX-deficient mice (6 per group) were dosed intravenously with rFIXFc at 50 IU/kg body weight or rFIX at 50 IU/kg body weight. Blood samples were collected before dosing and at various times after dosing. Blood samples were incubated at 37°C and were visually inspected for the presence of a blood clot once per minute. The time needed for a clot to form was recorded, and, once the clotting activity returned to baseline (ie, no clot formation), no additional samples were obtained (samples collected 15 minutes to 144 hours for rFIXFc or 15 minutes to 72 hours for rFIX). The graph indicates the percentage of animals able to clot blood at each time point.
In repeat-dose experiments in FIX-deficient mice comparing the activity of rFIXFc and rFIX, 15 minutes after the first dose, rFIXFc and rFIX showed comparable clotting activity, of 1.96 (±0.23) IU/mL and 1.09 (±0.14) IU/mL, respectively. By 96 hours, clotting activity was measurable in all 6 rFIXFc-treated mice (0.12 ± 0.08 IU/mL) but not in rFIX-treated mice (the limit of detection of the assay was ∼ 0.01 IU/mL). Similar results were obtained over the next 2 doses, measuring comparable peak activities 15 minutes after dosing for rFIXFc and rFIX (2.34 ± 0.73 vs 1.94 ± 0.35 IU/mL, respectively, after the second dose, and 2.12 ± 0.20 vs 1.69 ± 0.70 IU/mL, respectively, after the third dose) but only detecting clotting activity in the rFIXFc-treated mice after 96 hours (0.12 ± 0.017 and 0.12 ± 0.034 IU/mL after the second and third dose, respectively) with one exception, in that 1 of 5 animals dosed with rFIX exhibited detectable clotting activity (0.08 IU/mL) at 4 days after the second dose.
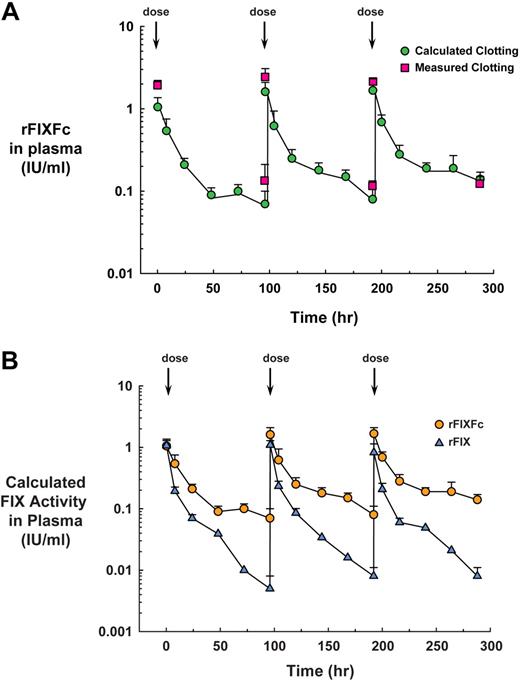
rFIXFc protein levels were also determined by ELISA analysis. In general, the pharmacokinetic profile appears to reflect the activity levels found in the single-dose experiment, possibly with a slight accumulation of drug because the peak levels were found to increase after the first, second, and third doses from 24.05 (±7.02) to 36.85 (±10.96) and to 38.19 (±9.49) μg/mL, respectively. Similarly, the trough levels increased from 1.60 (±0.60) to 1.88 (±0.72) to 3.22 (±0.74) μg/mL, respectively. On the basis of the specific activity of this lot of rFIXFc (43.8 IU/mg) and the ELISA data (in μg/mL), calculated plasma clotting activity level was determined (in IU/mL) and compared with measured clotting activity. The results of this comparison are shown in Figure 4A. Calculated clotting activity correlated well with empirical clotting activity data, indicating that activity of the rFIXFc molecule is maintained in circulation.
Pharmacodynamics of rFIXFc and rFIX in FIX-deficient mice. FIX-deficient mice were dosed with rFIXFc at 219 IU/kg body weight (5 per group, 6 groups, n = 30) or rFIX at 200 IU/kg body weight (4 or 5 per group, 6 groups, n = 28) on days 0, 4, and 8. Plasma samples were collected by cardiac puncture at 15 minutes and 96 hours after each dose, and clotting activity was measured with the use of a FIX activity assay. Plasma was also collected by tail bleeds at 8, 24, 48, and 72 hours after each dose. rFIXFc levels were measured in all of the samples with the use of an ELISA specific for rFIXFc. (A) Measured versus calculated activity. Clotting activity for rFIXFc was measured with the use of a FIX activity assay 15 minutes and 96 hours after 3 doses. The specific activity for this lot of rFIXFc was determined to be 43.8 (±5.4) IU/mg. On the basis of this activity (in IU/mg) and the measured protein levels, a calculated plasma clotting activity level was determined for time points at 15 minutes and for 8, 24, 48, 72, and 96 hours after each dose. (B) In FIX-deficient mice treated with up to 3 doses of rFIX at 200 IU/kg body weight, FIX levels were measured with FIX-specific ELISA. With the use of the measured specific activities of rFIXFc and rFIX, it was possible to compare calculated clotting activity for all samples analyzed by ELISA.
Pharmacodynamics of rFIXFc and rFIX in FIX-deficient mice. FIX-deficient mice were dosed with rFIXFc at 219 IU/kg body weight (5 per group, 6 groups, n = 30) or rFIX at 200 IU/kg body weight (4 or 5 per group, 6 groups, n = 28) on days 0, 4, and 8. Plasma samples were collected by cardiac puncture at 15 minutes and 96 hours after each dose, and clotting activity was measured with the use of a FIX activity assay. Plasma was also collected by tail bleeds at 8, 24, 48, and 72 hours after each dose. rFIXFc levels were measured in all of the samples with the use of an ELISA specific for rFIXFc. (A) Measured versus calculated activity. Clotting activity for rFIXFc was measured with the use of a FIX activity assay 15 minutes and 96 hours after 3 doses. The specific activity for this lot of rFIXFc was determined to be 43.8 (±5.4) IU/mg. On the basis of this activity (in IU/mg) and the measured protein levels, a calculated plasma clotting activity level was determined for time points at 15 minutes and for 8, 24, 48, 72, and 96 hours after each dose. (B) In FIX-deficient mice treated with up to 3 doses of rFIX at 200 IU/kg body weight, FIX levels were measured with FIX-specific ELISA. With the use of the measured specific activities of rFIXFc and rFIX, it was possible to compare calculated clotting activity for all samples analyzed by ELISA.
rFIX was detected at all time points for all 3 doses with each repeat dose resulting in peak and trough values of 3.2 to 4.2 μg/mL and 0.02 to 0.03 μg/mL, respectively. With the use of the measured specific activity for these lots of rFIXFc (43.8 IU/mg) and rFIX (259 IU/mg), it was possible to compare the calculated clotting activity for all plasma samples analyzed by ELISA (Figure 4B).
Pharmacokinetics in mice and rats
In rats, the t1/2 of rFIXFc was 34.8 (±5.3) hours compared with an elimination t1/2 for rFIX of 5.8 hours. The t1/2 of rFIXFc was 46.2 (±10.1) hours in FIX-deficient mice compared with 13.2 hours for rFIX, and 47.2 (±4.8) hours in normal mice compared with 12.3 hours for rFIX (Table 2).
Summary of terminal half-lives of rFIXFc and rFIX after a single intravenous dose
| Species . | rFIX, h . | rFIXFc, h . |
|---|---|---|
| Normal mice | 12.3 | 47.2 ± 4.8 |
| FIX-deficient mice | 13.2 | 46.2 ± 10.1 (47) |
| FcRn/β2m KO mice | 16.5 ± 3.0 | 16.9 ± 2.1 |
| hFcRn/hβ2m transgenic Tg32b mice | 14.2 ± 2.9 | 53.0 ± 6.6 |
| Rats | 5.8 | 34.8 ± 5.3 |
| FIX-deficient dogs | (17-18)* | 47.5 (38.3) |
| Monkey | 12.7† | 47.3 ± 9.1 |
| Species . | rFIX, h . | rFIXFc, h . |
|---|---|---|
| Normal mice | 12.3 | 47.2 ± 4.8 |
| FIX-deficient mice | 13.2 | 46.2 ± 10.1 (47) |
| FcRn/β2m KO mice | 16.5 ± 3.0 | 16.9 ± 2.1 |
| hFcRn/hβ2m transgenic Tg32b mice | 14.2 ± 2.9 | 53.0 ± 6.6 |
| Rats | 5.8 | 34.8 ± 5.3 |
| FIX-deficient dogs | (17-18)* | 47.5 (38.3) |
| Monkey | 12.7† | 47.3 ± 9.1 |
Animals were administered a single dose of rFIXFc (∼ 200 IU/kg in normal mice [n = 8], FIX-deficient mice [n = 11], FcRn KO mice [n = 4], FcRn Tg32B mice [n = 4], and rats [n = 9]; 140 IU/kg for FIX-deficient dogs [n = 2]; and 25, 100, or 500 IU/kg for monkeys [n = 2, 3, or 3, respectively]) or rFIX (100 IU/kg in normal mice [n = 5] and FIX-deficient mice [n = 5] and 200 IU/kg in FcRn KO mice [n = 4], FcRn Tg32B mice [n = 4], and rats [n = 5]). Blood samples were collected at various time points between 0.25 hours and 168 hours and plasma prepared for analysis of protein concentration by rFIXFc or FIX-specific ELISA. Data were then analyzed using WinNonLin to generate terminal half-life, utilizing the best fit of data from all time points. FIX activity data was also obtained from FIX-deficient mice (n = 4 / time point) and FIX-deficient dogs (n = 2) and terminal half-life based on activity calculated (listed in parentheses).
Published values of t1/2 for recombinant FIX, based on ELISA data.23
In FcRn KO mice, rFIXFc showed a t1/2 of 16.9 (±2.1) hours, similar to the t1/2 of rFIX in these animals of 16.5 (±3.0) hours. In human FcRn/human β2m transgenic mice made in FcRn KO background strain, the t1/2 of rFIXFc was 53.0 (±6.6) hours in contrast to rFIX, which had a t1/2 of 14.2 (±2.9) hours in these same mice.
Pharmacokinetics and pharmacodynamics in FIX-deficient dogs
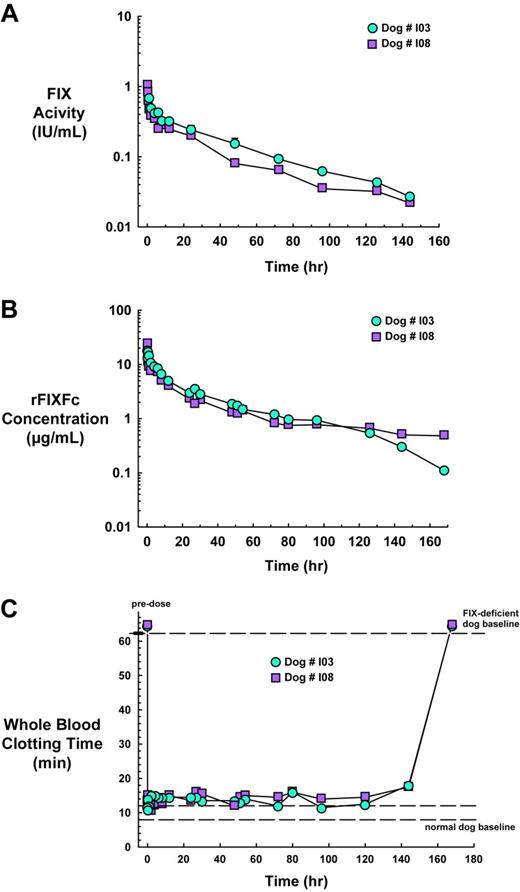
Clotting activity was measured for all time points and showed that the activity versus time curves are similar for the 2 animals (Figure 5A), declining in a biphasic manner characteristic of FIX.22 The Cmax at 15 minutes after infusion was found to be 1.028 IU/mL in dog I08; the earliest time point for which clotting activity was determined for dog I03 was 1 hour, at which time the level was 0.681 IU/mL compared with 0.466 IU/mL for I08. At 144 hours, clotting activity was still detectable and was found to be 0.027 and 0.022 IU/mL for dog I03 and I08, respectively, after which time the levels fell below the limit of detection of approximately 0.01 IU/mL at 168 hours. Terminal half-lives of 37.7 hours and 38.8 hours were calculated for dogs I03 and I08, respectively, using the best fit of the data from the 12- to 144-hour time points.
Pharmacokinetics and pharmacodynamics of rFIXFc in FIX-deficient dogs. Two dogs with hemophilia B were intravenously infused with rFIXFc at 140 IU/kg body weight. Blood samples were collected at 5, 15, and 30 minutes and at 1, 2, 4, 6, 8, 12, 24, 27, 30, 48, 51, 54, 72, 80, 96, 126, 144, and 168 hours. (A) FIX clotting activity was measured for all time points with respect to a standard curve generated with rFIXFc. (B) A sandwich ELISA that used a FIX capture antibody and Fc-HRP detection antibody was used to measure the concentration of intact rFIXFc in the plasma samples of the dog with hemophilia B. (C) Blood collected from animals was immediately analyzed for WBCT. Blood samples were incubated at 28°C and were visually inspected for the presence of a clot once per minute, and the time in which a clot formed was recorded.
Pharmacokinetics and pharmacodynamics of rFIXFc in FIX-deficient dogs. Two dogs with hemophilia B were intravenously infused with rFIXFc at 140 IU/kg body weight. Blood samples were collected at 5, 15, and 30 minutes and at 1, 2, 4, 6, 8, 12, 24, 27, 30, 48, 51, 54, 72, 80, 96, 126, 144, and 168 hours. (A) FIX clotting activity was measured for all time points with respect to a standard curve generated with rFIXFc. (B) A sandwich ELISA that used a FIX capture antibody and Fc-HRP detection antibody was used to measure the concentration of intact rFIXFc in the plasma samples of the dog with hemophilia B. (C) Blood collected from animals was immediately analyzed for WBCT. Blood samples were incubated at 28°C and were visually inspected for the presence of a clot once per minute, and the time in which a clot formed was recorded.
A sandwich ELISA was used to measure the concentration of intact rFIXFc in the plasma samples of the dog with hemophilic B (Figure 5B). The plasma concentration versus time curves were similar for the 2 animals, declining in a biphasic manner and consistent with the activity data (compare Figure 5A with 5B). The t1/2 of 37.5 hours and t1/2 of 57.4 hours were calculated for dogs I03 and I08, respectively, using the best fit of the data from the 24-hour to 144-hour time points.
Baseline WBCT is 8 to 12 minutes for normal dogs and longer than 60 minutes for FIX-deficient dogs. Immediately on administration of rFIXFc, clotting time was corrected from levels before dosing to approximately normal levels of 13.5 and 11.5 minutes for dog I03 and I08, respectively. Whole blood clotting activity remained corrected to approximately normal levels through 144 hours after dosing before returning to levels before dosing of longer than 60 minutes at 168 hours after dosing (Figure 5C).
Pharmacokinetics in nonhuman primates
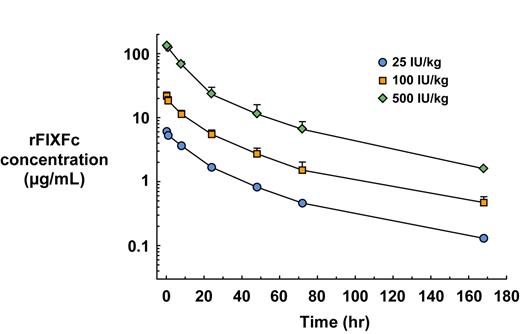
The pharmacokinetics of rFIXFc were evaluated at 3 dose levels (0.5, 2, and 10 mg/kg body weight, approximately 25, 100, and 500 IU/kg body weight), showing consistent levels within each group and showing dose proportionality between groups with average Cmax of 6.05 μg/mL, 24.54 (±1.59) μg/mL, and 129.82 (±3.45) μg/mL, respectively (Figure 6). At 168 hours, rFIXFc protein levels were 0.13, 0.37 (±0.03), and 1.59 (±0.07) μg/mL, respectively, for the 3 doses. The area under the time versus concentration curve also showed dose proportionality, with AUC of 164, 530 (±79), and 2654 (±415) μg-h per mL, at the levels of 0.5, 2, and 10 mg/kg body weight, respectively. Pharmacokinetic analysis determined the t1/2 to be 47.0 hours (n = 2), 47.8 (±2) hours (n = 3), and 47.0 (±13.3) hours (n = 3), respectively, for the 3 dose groups.
Pharmacokinetics of rFIXFc in cynomolgus monkeys. Monkeys were administered a single dose (0.5, 2, and 10 mg/kg body weight, corresponding to approximately 25, 100, or 500 IU/kg body weight) of rFIXFc (n = 2, 3, and 3, respectively). Blood samples were collected at 0.25, 0.5, 1, 8, 24, 48, 72, 96, 120, 144, and 168 hours after dosing, and plasma was prepared for analysis of protein concentration by FIXFc-specific ELISA.
Pharmacokinetics of rFIXFc in cynomolgus monkeys. Monkeys were administered a single dose (0.5, 2, and 10 mg/kg body weight, corresponding to approximately 25, 100, or 500 IU/kg body weight) of rFIXFc (n = 2, 3, and 3, respectively). Blood samples were collected at 0.25, 0.5, 1, 8, 24, 48, 72, 96, 120, 144, and 168 hours after dosing, and plasma was prepared for analysis of protein concentration by FIXFc-specific ELISA.
Discussion
rFIXFc, a fusion protein comprising a single molecule of FIX fused to the Fc domain of human IgG1, was developed to address the short t1/2 of currently available FIX products used to treat patients with hemophilia B. We have demonstrated that the elimination t1/2 of rFIXFc was consistently 3- to 4-fold longer than rFIX across species (Table 2), including direct comparisons in normal and FIX-deficient mice and normal rats. Half-lives calculated in our studies are markedly longer than published values of 17.7 hours (range, 15.6-21.6 hours) or 17.3 hours that has been reported for rFIX in FIX-deficient dogs22,23 or 12.9 (±1.5) hours23 and 13.2 (±1.6) hours24 found in normal dogs. Similarly, the t1/2 of rFIXFc of approximately 47 hours in cynomolgus monkeys is considerably longer than the previously reported 12.7-hour average for rFIX.23
The mechanism for the extension of t1/2 is based on the ability of the Fc domain of the fusion protein to bind to the FcRn at acidic pH (< 6.5) but not at physiologic pH (7.4).9,10 FcRn is a heterodimer comprising a MHC class I–related heavy chain and the β2m light chain that is found complexed to all MHC class I molecules.9,10,25 Both components of FcRn have been shown to have a role in IgG homeostasis, because mice deficient in either chain have lower levels of IgG antibodies in circulation, with significantly shortened half-lives.17,26-28 Plasma proteins are thought to be internalized by endothelial cells and targeted for degradation by the lysosome; however, Fc-containing proteins bind to FcRn present in the acidified endosome in a pH-dependent manner and are then recycled back to the cell surface where the Fc-containing proteins are released from FcRn at physiologic pH, thus protected from catabolism.9,10 The data presented herein show that rFIXFc has a 3- to 4-fold longer terminal t1/2 in mice expressing human FcRn and β2m compared with rFIX, whereas both proteins have similar short terminal half-lives in mice lacking FcRn (FcRn/β2m KO). These data confirm that FcRn mediates the longer t1/2 of rFIXFc, thus using the natural pathway responsible for protecting IgG antibodies from degradation.9,10 Consequently, rFIXFc has a longer circulating t1/2 than unconjugated FIX molecules such as rFIX in several species.
In addition to the pharmacokinetics of rFIXFc, we have examined the pharmacodynamics in 2 FIX-deficient species. In FIX-deficient mice, rFIXFc and rFIX, administered at approximately the same dose, corrected the clotting deficiency to comparable levels; however, the effect of rFIXFc was significantly prolonged to at least 96 hours after infusion. This trough activity of rFIXFc corresponds to approximately 10% of its normal clotting activity. Current prophylactic FIX replacement therapy for patients with hemophilia B aims to maintain plasma levels of 1% to 2% normal clotting activity.29
The WBCT assay is an ex vivo test that measures the ability of whole blood to form a clot and is an alternate confirmatory test for the restoration of clotting activity. In response to infusion with rFIXFc, the WBCT of the hemophilic dogs was corrected from longer than 60 minutes to approximately the normal range of 10 to 12 minutes and remained in this range for 144 hours before returning to levels before dosing at 168 hours. In a direct comparison in FIX-deficient mice, although both rFIXFc and rFIX led to whole blood clotting immediately on infusion, only rFIXFc-treated animals showed sustained clotting activity.
FIX is a complex protein that contains several functional domains which undergo extensive posttranslational modifications.21,30 One of the essential posttranslational modifications for FIX activity is γ-carboxylation of the first 12 glutamic acids in the Gla domain by vitamin K–dependent γ-glutamyl carboxylase. This modification facilitates the binding of FIX to phospholipid membranes and, thus, is critical to its function. FIX that is not γ-carboxylated is not functional; hence, γ-carboxylation is a rate-limiting step during FIX production.31,32 HEK-293 cells have proven to be an efficient expression system because they are capable of high-level expression and produce posttranslational modifications, in particular, for γ-carboxylation superior to Chinese hamster ovary or baby hamster kidney cells.33 HEK-293 cells are of human, rather than rodent, origin; therefore, they may result in posttranslational modifications more representative of human proteins. For these reasons, we chose to express rFIXFc in HEK-293 cells. The Gla content of rFIXFc was found to be comparable with that of rFIX by both amino acid analysis and peptide mapping. The rFIXFc protein was extensively characterized and found to be of high purity and comparable with rFIX in several posttranslational modifications, including serine phosphorylation, tyrosine sulfation, and glycosylation (Table 1). Although one or more of these characteristics have been proposed to be linked to the lower recovery of rFIX compared with pdFIX, it is unclear from the current data if this will be the case for rFIXFc as well, or if fusion with Fc will alter the recovery. One difference we have found is in the level of activated FIX, which is 10-fold lower in rFIXFc preparations than in rFIX or pdFIX.
Another key difference observed between rFIXFc and other FIX products has been in the specific activity as assessed by aPTT-based assays. As noted in “Biochemical characterization of rFIXFc,” the specific activity of rFIXFc ranges from 4.41 (±.22) to 6.12 (±0.28) IU/nmol for protein lots that had undergone 3-column purification, corresponding to 45.2 (±2.25) to 62.7 (±2.87) IU/mg, with more recent lots having a specific activity of approximately 60 IU/mg (Ulrika Härndahl, unpublished data, June 2007). In comparison, rFIX has a specific activity in aPTT-based assays of approximately 260 IU/mg, or 12.1 IU/nmol. Because of the presence of the Fc domain in rFIXFc, the more appropriate comparison for specific activity is on a molar basis, whereby rFIXFc is approximately 2-fold lower than rFIX. Extensive biochemical characterization of rFIXFc indicated that the difference in specific activity was not due to any deficiency in critical posttranslational modifications, such as γ-carboxylation or propeptide processing.
Further studies have been performed to examine the interactions of rFIXFc in the context of the tenase complex on a variety of phospholipid sources, including cephalin, synthetic phospholipid vesicles, and nonactivated and activated platelets and have found them to be comparable with rFIX (Garabet Toby and R.T.P., unpublished data, June 2007). Although the exact mechanistic basis for the difference in specific activity remains under investigation, this difference is unlikely to be therapeutically relevant, because rFIXFc is dosed on an international unit basis, and such a dosing regimen has been found to result in similar initial blood levels of activity compared with rFIX in experiments performed in FIX-deficient mice.
Traditionally, Fc fusion proteins have been made as dimers, with respect to the effector molecule and the Fc region.34 Previously, we have generated a novel fusion protein that has a monomeric effector molecule conjugated to the Fc domain.35 We had also demonstrated that such a configuration of EpoFc enhanced its pharmacodynamic and pharmacokinetic properties. Similarly, we have found that the FIXFc monomer has enhanced pharmacokinetic properties compared with the FIXFc dimer, in particular, in significantly higher Cmax and AUC.36
In conclusion, data from these studies indicate that rFIXFc has enhanced pharmacokinetic parameters compared with rFIX, particularly in its terminal t1/2. Clinical studies with this long-acting rFIXFc coagulation factor are in progress to investigate its potential to reduce the frequency of injections in patients receiving routine (prophylactic) doses of factor replacement. Whether rFIXFc can confer prolonged protection from bleeding after each dose of factor, decrease the overall units of factor needed to treat bleeding episodes, or maintain adequate hemostasis during surgical procedures with fewer injections are of considerable clinical interest.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank all of our colleagues at Syntonix as well as former employees for their efforts to support this work, including cell culture, purification, in vivo pharmacology, and analysis. We thank Anu Santhanagopal, PhD, from DesignWrite LLC, for assistance with manuscript preparation.
This work was supported in part by Biovitrum AB (Stockholm, Sweden).
Authorship
Contribution: R.T.P. designed research, performed research, analyzed and interpreted data, and wrote the manuscript; S.C.L. designed research, performed research, and analyzed and interpreted data; J.A.D. and A.J.B. designed research and analyzed and interpreted data; G.D.K., J.V.A., and Q.L. contributed vital analytical tools, performed research, and analyzed and interpreted data; G.Z.-P. and T.J.R. developed purification method and performed research. E.P.M. performed research; and T.C.N. performed research and analyzed and interpreted data.
Conflict-of-interest disclosure: R.T.P., G.D.K., J.A.D., J.V.A., Q.L., T.J.R., and A.J.B. are employees of and have an ownership interest in Biogen Idec. S.C.L. has an ownership interest in Biogen Idec. G.Z.-P., E.P.M., and T.C.N. declare no competing financial interests.
The current affiliation for S.C.L. is BIND Biosciences (Cambridge, MA). The current affiliation for G.Z.-P. is Percivia LLC (Cambridge, MA).
Correspondence: Robert T. Peters, Molecular and Cell Biology, Biogen Idec Hemophilia, 9 Fourth Ave, Waltham, MA 02451; e-mail: robert.peters@biogenidec.com.