Abstract
Fibrinolysis and pericellular proteolysis depend on molecular coassembly of plasminogen and its activator on cell, fibrin, or matrix surfaces. We report here the existence of a fibrinolytic cross-talk mechanism bypassing the requirement for their molecular coassembly on the same surface. First, we demonstrate that, despite impaired binding of Glu-plasminogen to the cell membrane by ϵ-aminocaproic acid (ϵ-ACA) or by a lysine-binding site–specific mAb, plasmin is unexpectedly formed by cell-associated urokinase (uPA). Second, we show that Glu-plasminogen bound to carboxy-terminal lysine residues in platelets, fibrin, or extracellular matrix components (fibronectin, laminin) is transformed into plasmin by uPA expressed on monocytes or endothelial cell–derived microparticles but not by tissue-type plasminogen activator (tPA) expressed on neurons. A 2-fold increase in plasmin formation was observed over activation on the same surface. Altogether, these data indicate that cellular uPA but not tPA expressed by distinct cells is specifically involved in the recognition of conformational changes and activation of Glu-plasminogen bound to other biologic surfaces via a lysine-dependent mechanism. This uPA-driven cross-talk mechanism generates plasmin in situ with a high efficiency, thus highlighting its potential physiologic relevance in fibrinolysis and matrix proteolysis induced by inflammatory cells or cell-derived microparticles.
Introduction
Fibrinolysis and pericellular proteolysis depend on plasminogen activation at fibrin and cell surfaces, respectively. Plasminogen is a 92-kDa protein composed of 791 amino acids (Glu1-Asn791; Glu-plasminogen) arranged in a serine protease region, 5 triple-loop structures (approximately 80 amino acid residues constrained by 3 disulfide bridges) called kringle (K) domains and an amino-terminal sequence (Glu1-Lys77/Val79).1 Domains K1 and K4 contain lysine-binding sites (LBSs)2 that allow binding of plasminogen to cells,3 fibrin,4 and extracellular matrix (ECM) components5 with a moderate affinity (overall dissociation constant, Kd = 0.5-1μM). This interaction is inhibited by carboxypeptidase B (CpB), an exopeptidase that targets the activation surface by cleaving exposed carboxy-terminal lysines (C-ter Lys), and by ϵ-aminocaproic acid (ϵ-ACA), a lysine analog that targets the LBS of plasminogen.
Domain K5 of plasminogen contains a LBS of weak affinity that interacts with the amino-terminal peptide of Glu-plasminogen and favors a predominant compact “closed” conformation6-8 over a very short-lived extended “open” form.8,9 Proteolytic removal of the amino-terminal peptide by plasmin (cleavage at either Lys62, Arg68, or Lys77) yields a stable truncated open form (Lys-plasminogen), unable to adopt the compact conformation.10-12 In a similar manner, saturation of the weak LBS in Glu-plasminogen with ϵ-ACA causes a transition from the compact closed form to the open extended conformation8,9 characteristic of Lys-plasminogen.12-14 Because ϵ-ACA is an analog of C-ter Lys, it has been accepted that Glu-plasminogen adopts an open Lys-like conformation on binding to C-ter Lys on surface biologic receptors.8,9 Plasminogen binding to these receptors is a prerequisite for its efficient transformation into plasmin by a plasminogen activator localized on the same cell surface (either the tissue-type, tPA, or the urokinase-type, uPA, plasminogen activator). Quiescent endothelial cells and neurons characteristically express tPA,15,16 whereas uPA is expressed by migrating capillary endothelial cells17 and inflammatory cells.18 It has recently been shown that a similar mechanism of plasmin formation is also functional at the surface of microparticles (EMPs) derived from a human microvascular endothelial cell line.19 Plasmin formed on these cellular membranes is implicated either directly or by activation of matrix metalloproteinases, in proteolytic processing of ECM proteins,20 cell migration, angiogenesis, and cell detachment-induced apoptosis.21,22
Previous studies have shown that uPA activates Lys-plasminogen and ϵ-ACA–liganded Glu-plasminogen faster than native Glu-plasminogen.10,11,23-25 Furthermore, single-chain uPA (scuPA) specifically activates plasminogen bound to C-ter Lys in fibrin,26 suggesting a relation between its molecular conformation and plasmin generation. We therefore hypothesized the existence of a new mechanism of plasmin formation bypassing the requirement for molecular coassembly on the same surface, by a proteolytic cross-talk. In this study, we show that conformational changes induced in Glu-plasminogen by either ϵ-ACA or its binding to fibrin, platelets, or matrix proteins are readily recognized by the uPA/uPA receptor (uPAR) system of either EMPs, monocytes or THP-1 cells but not by tPA-bearing cells. This cellular uPA-driven cross-talk mechanism results in a high efficiency in plasmin formation, thus highlighting its potential physiologic relevance in fibrinolysis and ECM degradation. This novel mechanism may be an intermediary pathway in pathophysiologically relevant inflammatory processes such as atherothrombosis, angiogenesis, and cell migration.
Methods
Reagents
Human Glu- and Lys-plasminogens were purified as described27 and were greater than 99% pure as assessed by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and by amino-terminal sequence analysis. Recombinant inactive human plasminogen with Ser741 mutagenized to Ala (r-Pg-Ala741) and recombinant scuPA with Ile159 mutagenized to Gly (r-scuPA-Gly159) were obtained and characterized as described.28,29 uPA and tPA (both greater than 99% single-chain form), a sheep antibody directed against uPA, and an immunoglobulin G1 mAb 34D3 were obtained as described.20,30 The mAb 34D3 reacts with plasminogen fragment K1 + 2 +3, blocks the LBS function of K1, and shows no cross-reaction with plasminogen K4.31 The chromogenic substrate selective for plasmin (methylmalonyl)-hydroxyprolylarginine-para-nitroaniline (CBS0065) was from Stago. The lysine analog ϵ-ACA, CpB (porcine pancreas), ZnCl2, amiloride (A7410), and poly-L-lysine (P-9155) were from Sigma. D-Valyl-L-phenylalanyl-L-lysine chloro-methyl ketone (VFK) was from Calbiochem. Surfactant-free white aliphatic amine latex beads were from Interfacial Dynamics Corp.
Preparation of fibrin surfaces and immobilization of matrix proteins
Fibrin surfaces were generated on polyglutaraldehyde-activated aliphatic amine latex beads or microtiter plates as described previously.27 The matrix proteins fibronectin and laminin (10 μg/mL) were immobilized onto polyglutaraldehyde-activated microtiter plates with a similar procedure.
To generate C-ter Lys residues on fibrin for the binding of plasminogen, the surfaces were treated with 10nM plasmin for 15 minutes at 37°C. Plasmin was discarded, and the surfaces were treated with a 0.1M phosphate buffer, pH 7.4, containing 1μM VFK and 0.1M ϵ-ACA to inhibit and elute the remaining bound plasmin. The surfaces were then stored at 4°C in 50mM phosphate buffer, pH 7.4, containing 80mM NaCl, 0.2% bovine serum albumin, 0.05% Tween-20, 0.01% Azide, and 1μM VFK.
Cell culture
The human monocytic cell line THP-1 (ATCC) was grown in RPMI-1640 medium supplemented with 10% fetal calf serum, 4mM glutamine, 0.5mM sodium pyruvate, 0.5% nonessential amino acids, and 1% antibiotics (penicillin, streptomycin). Cortical neurons were obtained from Swiss mice embryos at 16 days of gestation, seeded in 96-well plates coated with 25 μg/mL poly-L-lysine, cultured with the use of neurobasal medium (Invitrogen) supplemented with 2% B27 (Invitrogen) and 0.5mM L-glutamine as described.32 Neurons used for experiments 5 days after seeding contained less than 2.5% astrocytes.33 The human microvascular endothelial cell line (HMEC-1)34 (obtained from Dr Ades, Centers for Disease Control and Prevention) was cultured in MCDB 131 medium (Invitrogen) supplemented with 10% MP-free fetal calf serum, 10 ng/mL human recombinant epidermal growth factor (Upstate Cell Signaling Solutions), and 1 μg/mL hydrocortisone (Sigma). Cells were maintained at 37°C in a humidified atmosphere of 5% CO2.
Isolation of monocytes and platelets
Peripheral blood mononuclear cells were isolated from citrated blood of healthy donors by Lymphoprep (Axis-Shield PoC AS) separation at 600g for 20 minutes at room temperature. Monocytes were then purified by cell sorting with CD14-coupled magnetic beads (Miltenyi Biotec), following the supplier's instructions. The purified cell population contained more than 98% monocytes, as assessed by flow cytometry, with the use of CD14 monoclonal antibodies (Immunotech). Purified monocytes were allowed to adhere 2 hours onto 96-well plates (Techno Plastic Products AC).
Platelets were isolated from blood collected into acid-citrate-dextrose from healthy volunteers. Platelet-rich plasma was obtained by centrifugation at 200g for 15 minutes at room temperature, followed by a second centrifugation at 1200g for 12 minutes to pellet platelets. Platelets were then washed twice in 36mM citric acid buffer, pH 6.5, containing 100mM NaCl, 5mM KCl, 1mM MgCl2, and 5mM glucose.
Generation, harvesting, and flow cytometry of EMPs
EMPs were purified from culture medium conditioned by subconfluent HMEC-1 cells stimulated for 48 hours with 100 ng/mL tumor necrosis factor α (PeproTech Inc) as previously described with minor modifications.35 Culture supernatants from flasks were collected and cleared from detached cells or large cell fragments by centrifugation at 4300g for 5 minutes. The supernatants were then centrifuged at 20 000g for 90 minutes at 4°C. Pelleted EMPs were washed twice and resuspended in phosphate-buffered saline (PBS). Aliquots of 10 μL of EMP suspension, 1/100 diluted, were labeled with the use of fluorescein isothiocyanate–conjugated annexin V (Abcys), and EMPs were quantitated by flow cytometry as previously described.36
Characterization of plasminogen activators in cell and MP extracts
THP-1 cells, cortical neurons, HMEC-1 cells, pelleted EMPs, and platelets were lysed in 100mM Tris-HCl buffer, pH 8.1, containing 1% Triton X-100. Lysates were clarified by centrifugation, and their protein concentration was determined with the use of the BCA kit (Pierce Chemical). Fibrin autography-electrophoresis was performed as described previously.37 Briefly, 10 μg of proteins in cell lysates and 10 μL of reference proteins (tPA 1 IU/mL, uPA 3 IU/mL, and plasmin 200nM) were electrophoresed in an 8% polyacrylamide gel under nonreducing conditions. SDS was then exchanged with 2.5% Triton X-100. After washing off excess Triton X-100 with distilled water, the gel was carefully overlaid on a 1% agarose support containing 1 mg/mL bovine fibrinogen, 100nM bovine plasminogen, and 0.2 NIH unit/mL bovine thrombin. Zymograms were allowed to develop at 37°C during 24 hours and were photographed at regular intervals using dark-ground illumination. Active proteins in cell lysates were identified by reference to the migration of known markers (uPA, tPA, plasmin), and inhibition of their activity with specific antibodies incorporated into the fibrin-agarose gel. The presence of uPA and uPAR on EMPs was further identified both by electron microscopy and by flow cytometry as described.19
Effects of LBS ligands on plasmin formation by cells
Glu- or Lys-plasminogen at various concentrations (0-3μM) was incubated at 37°C with uPA-bearing THP-1 cells or tPA-bearing cortical neurons, in the presence of 0.75mM CBS0065, a plasmin-selective chromogenic substrate. The effect of various inhibitors (mAb anti–K1-LBS; amiloride, an uPA inhibitor; ϵ-ACA; PMSF-treated CpB) on plasminogen binding and activation was determined with the use of a fixed final plasminogen concentration. Kinetics of plasmin formation was followed by measuring the release of p-nitroaniline from the chromogenic substrate, detected as a change in absorbance (ΔA405nm/minute), using a multiwell plate reader (MX5000; Dynex) thermostated at 37°C. Rates of plasmin formation were calculated from the slopes of A405nm versus time. After the activation reaction, the cells were washed in PBS, and residual bound plasmin was detected by adding 0.75mM CBS0065.
Activation of platelet-bound plasminogen by monocytes or neurons
Isolated platelets were treated with 5nM plasmin for 30 minutes at room temperature to generate plasminogen-binding sites (C-ter Lys residues) at their surface as reported previously.38 Platelets were then washed, treated with 1μM (final concentration) aprotinin (Trasylol; Bayer) to inhibit residual plasmin, and were finally incubated with 2μM plasminogen for30 minutes at room temperature. Excess unbound plasminogen was removed by washing.
Isolated monocytes and neurons used as a source of distinct plasminogen activator, respectively uPA and tPA, were plated at 105 cells/well in 96-well plates and treated with 50 μg/mL CpB for 30 minutes at 37°C. The cells were then washed and incubated with various amounts of plasminogen-bearing platelets (0-15 × 106/well) in cell medium supplemented with 0.75mM of the plasmin substrate CBS0065. The kinetics of plasmin formation on platelets was followed during 12 hours by measuring the release of p-nitroaniline from the chromogenic substrate as a change in A405nm as a function of time. The cells were then carefully rinsed with PBS to discard platelets. A volume of 100 μL of culture medium containing 0.75mM CBS0065 was then added to determine whether plasmin was present at the surface of monocytes or neurons.
Activation on plasminogen bound to fibrin-coated beads by monocytes, neurons, or EMPs
Fibrin-coated beads were incubated with 1μM plasminogen for 30 minutes at 37°C. Excess unbound plasminogen was removed by washing. Isolated monocytes, neurons, and EMPs were treated with 50 μg/mL CpB for 30 minutes at 37°C. Cells were then washed and incubated with 2.5 × 105 beads/well in cell medium supplemented with 0.75mM of the plasmin substrate CBS0065. The kinetics of plasmin formation on fibrin-coated beads was followed during 12 hours at 37°C by measuring the release of p-nitroaniline.
In parallel experiments, fibrin-coated beads were incubated with 1μM native or r-Pg-Ala741 for 30 minutes at 37°C. Fibrin-coated beads with bound plasminogen were then incubated with 106 EMPs in a final volume of 200 μL. After overnight incubation at 22°C, the fibrin-coated beads were sedimented by centrifugation and resuspended in 10mM Tris-HCL, pH 6.8, containing 1% SDS to elute fibrin-bound plasminogen derivatives. The supernatant was electrophoresed under nonreducing conditions, proteins were transferred to PVDF membranes and revealed with a horseradish peroxidase–conjugated mAb (150 ng/mL) directed against plasminogen K1. Purified plasmin was used as reference.
Activation of plasminogen bound to fibrin surfaces and matrix proteins by monocytes or EMPs
Glu-plasminogen at 1μM was incubated with the fibrin surfaces or with immobilized fibronectin or laminin. In parallel experiments, fibronectin (50 μg/mL) and plasminogen (1μM) were simultaneously incubated and allowed to bind to the surface of fibrin. Unbound proteins were discarded by washing, and plasminogen bound to fibrin surfaces or to immobilized fibronectin and laminin was incubated with various concentrations of either THP-1 cells or EMPs in the presence of CBS0065. The transformation of bound plasminogen into plasmin was detected by measuring the release of p-nitroaniline as indicated in “Activation of platelet-bound plasminogen by monocytes or neurons.” After the activation reaction, the supernatant was collected, the plates were washed with PBS, and matrix-bound plasmin was detected by adding 0.75mM CBS0065. To quantitate degradation of the fibrin surface, the plates were again washed and then incubated with a mAb (FDP-14) that specifically recognizes fibrin fragmentation, as described.30 Degradation of fibronectin in the supernatant was detected by Western blot as described.22
Statistical analysis
Data are expressed as mean plus or minus SEM. The statistics were performed with the use of nonparametric tests of Kruskall-Wallis and of Mann-Whitney (StatView 5 software; SAS Institute). Statistical significance was set at P less than .05.
Results
Plasminogen activation on cells
To illustrate the accepted mechanism of plasmin formation by coassembly of plasminogen and its activators at the same cell surface, plasminogen activation experiments were performed on cultured cells. THP-1 and endothelial cells were shown to express active uPA at their membrane, identified by fibrin zymography of cell lysates (Figure 1A) on the basis of its molecular mass (Mr 54 000) and its inhibition with polyclonal anti-uPA immunoglobulin G added to the indicator fibrin gel (not shown). Similar results were obtained with human monocytes (not shown). A fibrinolytic band corresponding to tPA (Mr 70 000) was detected in lysates of mouse cortical neurons. In contrast, neither tPA nor uPA activity could be detected in the lysates of human platelets. The presence of uPA and uPAR on EMPs was shown by immunoelectron microscopy and flow cytometry as described previously.19 THP-1 and cortical neurons in culture were able to activate plasminogen at their surface in a specific and dose-dependent manner until saturation was reached (Figure 1B; THP-1, Km = 492nM; neurons, Km = 49 ± 9nM, not shown), in agreement with previous studies.33,39,40
Identification of activators and cellular activation of plasminogen. (A) Fibrin autography of platelets, cortical neurons, HMEC-1 cells, and THP-1 cells. The samples were electrophoresed on 8% (wt/vol) polyacrylamide, SDS was then exchanged with 2.5% (wt/vol) Triton X-100, and the gel was overlaid on a fibrin-agarose indicator gel. The picture was taken after 4 hours at 37°C. The position of purified controls (Pn indicates plasmin; tPA, and uPA) is indicated on top. The thin vertical line indicates assembly from the same gel. The thick vertical line separates 2 different gels. (B) THP-1 cells (105 cells/well) were incubated with various concentrations of plasminogen (0-3μM) and 0.75mM CBS0065. Kinetics of plasmin formation (mOD/minute) was followed by measuring the release of p-nitroaniline. Data were fitted according to the Michaelis-Menten equation (Km = 492nM).
Identification of activators and cellular activation of plasminogen. (A) Fibrin autography of platelets, cortical neurons, HMEC-1 cells, and THP-1 cells. The samples were electrophoresed on 8% (wt/vol) polyacrylamide, SDS was then exchanged with 2.5% (wt/vol) Triton X-100, and the gel was overlaid on a fibrin-agarose indicator gel. The picture was taken after 4 hours at 37°C. The position of purified controls (Pn indicates plasmin; tPA, and uPA) is indicated on top. The thin vertical line indicates assembly from the same gel. The thick vertical line separates 2 different gels. (B) THP-1 cells (105 cells/well) were incubated with various concentrations of plasminogen (0-3μM) and 0.75mM CBS0065. Kinetics of plasmin formation (mOD/minute) was followed by measuring the release of p-nitroaniline. Data were fitted according to the Michaelis-Menten equation (Km = 492nM).
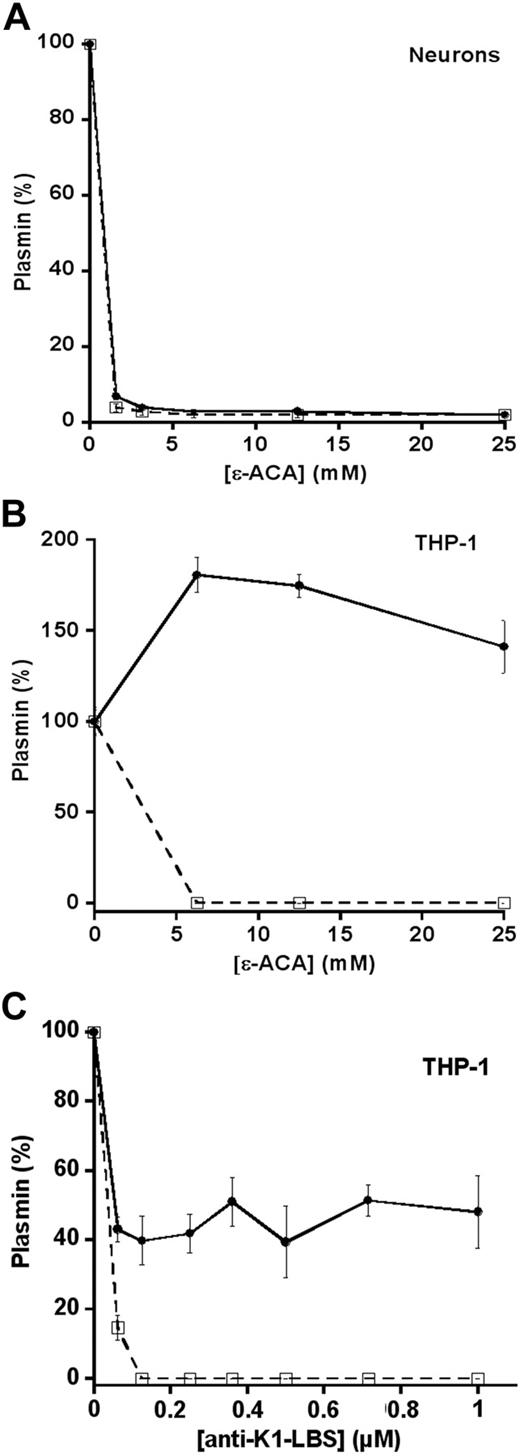
Contrasting effects of ϵ-ACA on plasmin formation by cell plasminogen activators
The activation of plasminogen by neuronal tPA was completely inhibited by the lysine-analog ϵ-ACA (Figure 2A), in agreement with a previous report.33 These data indicate that inhibition of plasminogen binding to the cell surface by ϵ-ACA prevents the formation of plasmin in situ by cells that express tPA.21,22 Surprisingly, cells that express uPA activate Glu-plasminogen despite the presence of ϵ-ACA (THP-1; Figure 2B). Because plasmin could not be shown at the cell surface (Figure 2B dotted line), our data suggest that plasminogen was activated on contact of ϵ-ACA–liganded plasminogen with uPA-bearing THP-1 cells. Because ϵ-ACA blocks both K1- and K4-LBS of plasminogen, we further explored the role of K1-LBS, known to be directly implicated in plasminogen binding, using the specific mAb anti–K1-LBS, 34D3. Neutralization of K1-LBS by mAb 34D3 impaired binding of plasminogen to the cell surface, and, as a consequence, cell-bound plasmin could not be detected on THP-1 cells (Figure 2C dotted line). However, and similarly to the effect of ϵ-ACA (Figure 2B), the formation of plasmin was apparent in the supernatant despite the inhibition of plasminogen binding (Figure 2C). These results indicate that Glu-plasminogen in complex with molecular probes that selectively block the LBS of K1 (ϵ-ACA or the anti–LBS-K1 mAb) adopts a conformation that could be recognized and activated by uPA-bearing cells.
Cellular activation of plasminogen: effect of LBS ligands. (A) Cortical neurons (105 cells/well) and (B) THP-1 cells (105/well) were incubated with 125nM Glu-plasminogen supplemented with various concentrations of ϵ-ACA (0-25mM) and 0.75mM CBS0065. Plasmin formation (●) was detected by measuring the release of p-nitroaniline. (C) THP-1 cells (105/well) were incubated with 125nM Glu-plasminogen supplemented with of 0 to 1μM anti–K1-LBS mAb 34D3 and 0.75mM CBS0065. (A-C) After detection of plasmin formation, the cells were washed twice with PBS and incubated with 0.75mM CBS0065 to detect cell-associated plasmin (□). Results are expressed as a percentage (mean ± SD; n = 3) of plasmin formation or of cell-associated plasmin activity in the absence of ϵ-ACA or mAb.
Cellular activation of plasminogen: effect of LBS ligands. (A) Cortical neurons (105 cells/well) and (B) THP-1 cells (105/well) were incubated with 125nM Glu-plasminogen supplemented with various concentrations of ϵ-ACA (0-25mM) and 0.75mM CBS0065. Plasmin formation (●) was detected by measuring the release of p-nitroaniline. (C) THP-1 cells (105/well) were incubated with 125nM Glu-plasminogen supplemented with of 0 to 1μM anti–K1-LBS mAb 34D3 and 0.75mM CBS0065. (A-C) After detection of plasmin formation, the cells were washed twice with PBS and incubated with 0.75mM CBS0065 to detect cell-associated plasmin (□). Results are expressed as a percentage (mean ± SD; n = 3) of plasmin formation or of cell-associated plasmin activity in the absence of ϵ-ACA or mAb.
Distinct effects of ϵ-ACA on Glu- and Lys-plasminogen activation by cellular uPA
To understand the role of conformational transitions of plasminogen (closed ⇌ open) on plasmin formation by cellular uPA, we compared the activation of equimolar amounts of either Glu- or Lys-plasminogen in the absence and presence of ϵ-ACA (Figure 3). In the absence of ϵ-ACA, Lys-plasminogen is activated 4.5-fold faster than native Glu-plasminogen (Figure 3A), and the amount of plasmin that remained bound to THP-1 cells shows a similar relationship (Figure 3B). The addition of ϵ-ACA has no stimulating effect on Lys-plasminogen. In contrast, ϵ-ACA produced a 2.3-fold increase in plasmin formation from Glu-plasminogen, in agreement with data shown in Figure 2B. The rate of Glu-plasminogen activation approaches the value obtained for Lys-plasminogen, whereas cell-bound plasmin was undetectable for both Glu- and Lys-plasminogens (Figure 3B).
Effect of ϵ-ACA and carboxipeptidase B on Lys- and Glu-plasminogen activation by cellular uPA. THP-1 cells (105/well) were incubated with 500nM Lys- ( ) or Glu-plasminogen (□) in medium alone or supplemented with ϵ-ACA (5 or 25mM) and 0.75mM CBS0065. Carboxypeptidase B, CpB (50 μg/mL), pretreated THP-1 cells were incubated with plasminogen and CBS0065. Rate of plasmin formation (A) and amount of cell-associated plasmin (B) were detected as indicated in Figure 2. Bars represent the amount of plasmin formed or associated to the cells (mOD/minute) versus the concentration of ϵ-ACA or after CpB treatment (mean ± SEM; n = 3). Significant changes compared with Lys-Pg (*P < .05)/Glu-Pg (§P < .05) alone.
) or Glu-plasminogen (□) in medium alone or supplemented with ϵ-ACA (5 or 25mM) and 0.75mM CBS0065. Carboxypeptidase B, CpB (50 μg/mL), pretreated THP-1 cells were incubated with plasminogen and CBS0065. Rate of plasmin formation (A) and amount of cell-associated plasmin (B) were detected as indicated in Figure 2. Bars represent the amount of plasmin formed or associated to the cells (mOD/minute) versus the concentration of ϵ-ACA or after CpB treatment (mean ± SEM; n = 3). Significant changes compared with Lys-Pg (*P < .05)/Glu-Pg (§P < .05) alone.
Effect of ϵ-ACA and carboxipeptidase B on Lys- and Glu-plasminogen activation by cellular uPA. THP-1 cells (105/well) were incubated with 500nM Lys- ( ) or Glu-plasminogen (□) in medium alone or supplemented with ϵ-ACA (5 or 25mM) and 0.75mM CBS0065. Carboxypeptidase B, CpB (50 μg/mL), pretreated THP-1 cells were incubated with plasminogen and CBS0065. Rate of plasmin formation (A) and amount of cell-associated plasmin (B) were detected as indicated in Figure 2. Bars represent the amount of plasmin formed or associated to the cells (mOD/minute) versus the concentration of ϵ-ACA or after CpB treatment (mean ± SEM; n = 3). Significant changes compared with Lys-Pg (*P < .05)/Glu-Pg (§P < .05) alone.
) or Glu-plasminogen (□) in medium alone or supplemented with ϵ-ACA (5 or 25mM) and 0.75mM CBS0065. Carboxypeptidase B, CpB (50 μg/mL), pretreated THP-1 cells were incubated with plasminogen and CBS0065. Rate of plasmin formation (A) and amount of cell-associated plasmin (B) were detected as indicated in Figure 2. Bars represent the amount of plasmin formed or associated to the cells (mOD/minute) versus the concentration of ϵ-ACA or after CpB treatment (mean ± SEM; n = 3). Significant changes compared with Lys-Pg (*P < .05)/Glu-Pg (§P < .05) alone.
The activation experiments were then performed in the presence of CpB to eliminate plasminogen binding and activation onto C-ter Lys residues. Our data (Figure 3) indicate that, at the concentration of CpB used (50 μg/mL), the activation of Glu-plasminogen (Figure 3A) at the cell surface and the amount of cell-bound plasmin (Figure 3B) were markedly decreased. In contrast, the activation of Lys-plasminogen (Figure 3A) was only moderately modified by CpB despite an important reduction in cell-bound plasmin (Figure 3B), suggesting that activation at the cell membrane was practically absent. These results indicate that cell-bound uPA is able to recognize and activate Lys-plasminogen and the Lys-like Glu-plasminogen conformation induced by ϵ-ACA without requirement for molecular coassembly on the same cell surface.
Plasmin generation via a proteolytic cell-to-matrix cross-talk
It has been generally accepted that the extended open conformation adopted by ϵ-ACA–liganded Glu-plasminogen mimics plasminogen bound to lysine residues in fibrin, ECM, or cells. We therefore hypothesized on the basis of the above data (Figures 2–3) that Glu-plasminogen bound to fibrin, matrix proteins, or cell membranes may be recognized and activated by other cells or cell-derived MPs present in the microenvironment and expressing uPA/uPAR complexes at their membrane. To verify this hypothesis, we analyzed the activation of r-Pg-Ala741 a recombinant active site–inactivated plasminogen, bound to fibrin-coated beads, by uPA-bearing EMPs. The r-Pg-Ala741 can bind to surface C-ter Lys and be cleaved into inactive Glu-plasmin by activators.26 Therefore, if its binding to fibrin-coated beads induces a conformational change, it should be recognized and activated by uPA present on EMPs. Functionality of the cross-talk was verified using native Glu-plasminogen and measuring plasmin activity. Figure 4A shows that the recognition and activation of fibrin-bound r-Pg-Ala741 by uPA-bearing EMPs results in the formation of inactive Glu-plasmin at the surface of fibrin. Formation of Lys-plasminogen was not an intermediary step in the cross-talk mechanism, supporting the view that Glu-plasminogen bound to fibrin adopts a conformation that is recognized by EMPs bearing uPA. To further verify this hypothesis, we determined the generation of plasmin activity during a matrix-to-cell cross-talk between matrix-bound native Glu-plasminogen and EMPs or monocytes. Fibrin-bound plasminogen was efficiently activated, proportionally to the number of EMPs incubated with the fibrin surface (Figure 4B). In a similar manner, plasminogen-bound to the ECM protein fibronectin (Figure 4C) and laminin (supplemental Figure 1, available on the Blood website; see the Supplemental Materials link at the top of the online article) was efficiently activated by EMPs. This activation was inhibited in the presence of amiloride or an anti-uPA specific antibody. Plasminogen bound to fibrin could also be specifically activated by uPA-bearing THP-1 cells in a cell concentration–dependent manner (Figure 4D main graph) and by adherent monocyte-borne uPA (Figure 4D inset). In contrast tPA-bearing adherent neurons failed to generate plasmin (Figure 4D inset). Plasmin formed on fibrin surfaces resulted in fibrinolysis as indicated by the specific binding of the mAb FDP-14 directed against fibrin degradation products, in agreement with previous published data.27 In a similar fashion, the fibronectin in complex with fibrin was degraded by plasmin as detected by Western blot (data not shown).
Fibrinolytic cross-talk: activation of fibrin- and fibronectin-bound plasminogen by cellular MPs. (A) Native or recombinant active site–inactivated Glu-plasminogen (Glu-Pg, r-Pg-Ala741) at 1μM was bound to fibrin-coated beads for 1 hour at 37°C. Fibrin-coated beads with bound plasminogen were then incubated with 106 EMPs in a final volume of 200 μL. After overnight incubation at 22°C, the fibrin-coated beads were sedimented by centrifugation and resuspended in 10mM Tris-HCl, pH 6.8, containing 10% SDS to elute fibrin-bound plasminogen derivatives. The supernatant was electrophoresed under nonreducing conditions, proteins were transferred to PVDF membranes and revealed with a horseradish peroxidase–conjugated mAb (150 ng/mL) directed against plasminogen K1. The Western blot shows Glu-plasmin formation by EMPs. Purified plasmin is shown as reference. (B-C) Glu-plasminogen (1μM) was bound to fibrin (B) or fibronectin (C) surfaces. After 3 washes with PBS, EMPs were added at various concentrations. (D) Glu-plasminogen (1μM) was bound to fibrin surfaces (main graph) or to fibrin-coated beads (inset). THP-1 cells were then added to fibrin surfaces at various concentrations (main graph) and 2.5 × 105 fibrin-coated beads were incubated with 105 adherent monocytes or neurons (inset). The formation of plasmin was detected by measuring the release of p-nitroaniline from the chromogenic substrate CBS0065 added at 0.75mM. Bars represent the amount of plasmin formed (mOD/minute; mean ± SEM; n = 3) by THP-1 cells on fibrin (B,D) and fibronectin (C), and by adherent monocytes or neurons on fibrin-coated beads (D inset). Amil indicates amiloride; IgG, antibody against uPA and its nonimmune IgG control. *Significant changes compared with activation without THP-1 (A) or EMPs (B-C) or activation on neurons (P < .05); §changes with inhibitors compared with activation at 5 × 105 EMPs (P < .05).
Fibrinolytic cross-talk: activation of fibrin- and fibronectin-bound plasminogen by cellular MPs. (A) Native or recombinant active site–inactivated Glu-plasminogen (Glu-Pg, r-Pg-Ala741) at 1μM was bound to fibrin-coated beads for 1 hour at 37°C. Fibrin-coated beads with bound plasminogen were then incubated with 106 EMPs in a final volume of 200 μL. After overnight incubation at 22°C, the fibrin-coated beads were sedimented by centrifugation and resuspended in 10mM Tris-HCl, pH 6.8, containing 10% SDS to elute fibrin-bound plasminogen derivatives. The supernatant was electrophoresed under nonreducing conditions, proteins were transferred to PVDF membranes and revealed with a horseradish peroxidase–conjugated mAb (150 ng/mL) directed against plasminogen K1. The Western blot shows Glu-plasmin formation by EMPs. Purified plasmin is shown as reference. (B-C) Glu-plasminogen (1μM) was bound to fibrin (B) or fibronectin (C) surfaces. After 3 washes with PBS, EMPs were added at various concentrations. (D) Glu-plasminogen (1μM) was bound to fibrin surfaces (main graph) or to fibrin-coated beads (inset). THP-1 cells were then added to fibrin surfaces at various concentrations (main graph) and 2.5 × 105 fibrin-coated beads were incubated with 105 adherent monocytes or neurons (inset). The formation of plasmin was detected by measuring the release of p-nitroaniline from the chromogenic substrate CBS0065 added at 0.75mM. Bars represent the amount of plasmin formed (mOD/minute; mean ± SEM; n = 3) by THP-1 cells on fibrin (B,D) and fibronectin (C), and by adherent monocytes or neurons on fibrin-coated beads (D inset). Amil indicates amiloride; IgG, antibody against uPA and its nonimmune IgG control. *Significant changes compared with activation without THP-1 (A) or EMPs (B-C) or activation on neurons (P < .05); §changes with inhibitors compared with activation at 5 × 105 EMPs (P < .05).
Plasmin generation via a proteolytic cell-to-cell cross-talk
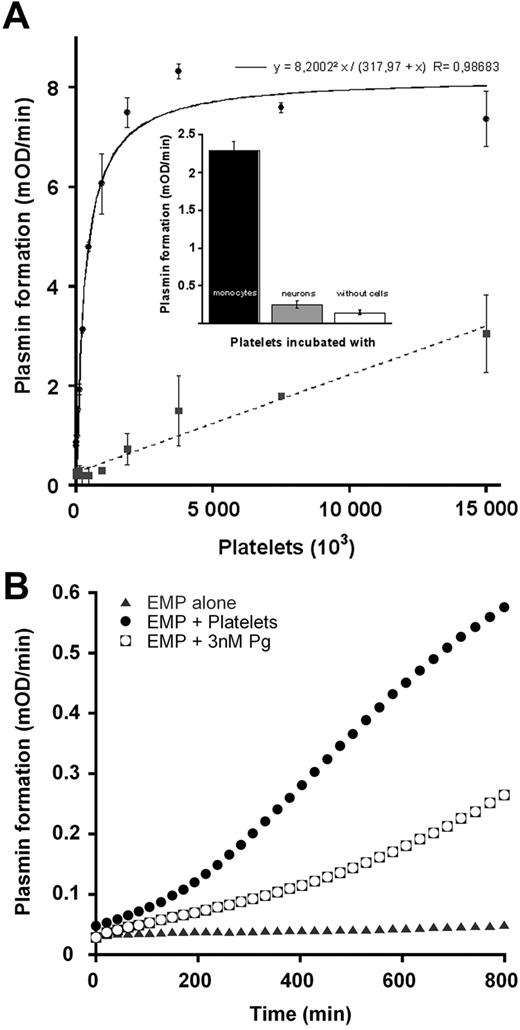
Cell-to-cell cross-talks were studied between platelets bearing plasminogen and cells bearing either uPA or tPA (monocytes and neurons, respectively) or EMPs. Platelets were pretreated with plasmin to enhance the number of plasminogen binding sites.38 After binding of plasminogen to platelets no conversion to plasmin was detected in agreement with a previous report.41 To avoid Glu-plasminogen transfer from platelets to cells bearing the plasminogen activators, monocytes and neurons were pretreated with CpB. Under these conditions, the extent of platelet-bound Glu-plasminogen transformation into plasmin by uPA-bearing monocytes is dependent on platelet number added until saturation (Figure 5A). In contrast to full plasminogen activation on 1.8 × 106 platelets by monocyte-bearing uPA, plasmin formation on platelets incubated with tPA-bearing neurons was approximately 10% (Figure 5A) and did not reach saturation at higher platelet concentrations. Moreover, Lys-plasminogen bound to platelets could not be activated by neuronal tPA as well (supplemental Figure 2). No plasmin was detected on monocytes or neurons surfaces, indicating that plasmin was indeed generated on the platelet surface and remained associated with the platelet (not shown). Plasmin formation on platelets in the absence of cells was undetectable at 1.8 × 106 plasminogen-bearing platelets (Figure 5A inset).
Proteolytic cross-talk: activation of platelet-bound plasminogen by cells bearing uPA (monocytes) or tPA (neurons). Glu-plasminogen (2μM) was bound to platelets as indicated in “Activation of platelet-bound plasminogen by monocytes or neurons.” (A) After treatment with 50 μg/mL CpB, monocytes (●) or neurons (■) were incubated with plasminogen-bearing platelets at various concentrations (0-1.5 106/well) in the presence of 0.75mM CBS0065. The formation of plasmin (mOD/minute) was detected by measuring the release of p-nitroaniline. (Inset) Detection of plasmin formation on platelets (1.8 × 106) in the absence of cells or incubated with monocytes or neurons (105 cells). Results are expressed as the rate of plasmin formation (mean ± SEM; n = 3). (B) After treatment with 50 μg/mL CpB, EMPs were incubated with 5 × 106 platelets bearing plasminogen or with 3nM plasminogen (concentration equivalent to that bound to platelets), or with buffer in the presence of 0.75mM CBS0065. The formation of plasmin (mOD/minute) was detected by measuring the release of p-nitroaniline. Results are expressed as rate of plasmin formation (n = 3). A representative experiment is shown.
Proteolytic cross-talk: activation of platelet-bound plasminogen by cells bearing uPA (monocytes) or tPA (neurons). Glu-plasminogen (2μM) was bound to platelets as indicated in “Activation of platelet-bound plasminogen by monocytes or neurons.” (A) After treatment with 50 μg/mL CpB, monocytes (●) or neurons (■) were incubated with plasminogen-bearing platelets at various concentrations (0-1.5 106/well) in the presence of 0.75mM CBS0065. The formation of plasmin (mOD/minute) was detected by measuring the release of p-nitroaniline. (Inset) Detection of plasmin formation on platelets (1.8 × 106) in the absence of cells or incubated with monocytes or neurons (105 cells). Results are expressed as the rate of plasmin formation (mean ± SEM; n = 3). (B) After treatment with 50 μg/mL CpB, EMPs were incubated with 5 × 106 platelets bearing plasminogen or with 3nM plasminogen (concentration equivalent to that bound to platelets), or with buffer in the presence of 0.75mM CBS0065. The formation of plasmin (mOD/minute) was detected by measuring the release of p-nitroaniline. Results are expressed as rate of plasmin formation (n = 3). A representative experiment is shown.
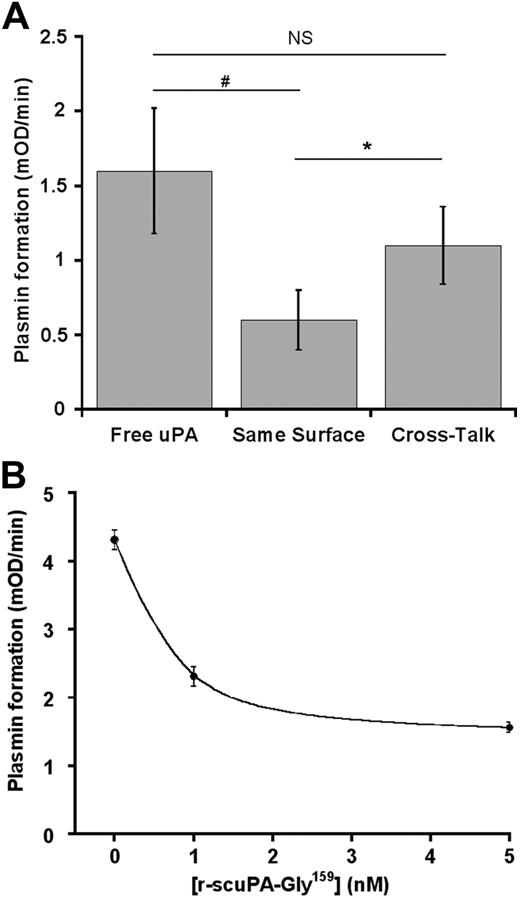
Platelet-bound plasminogen could also be activated by EMPs as indicated in Figure 5B. Because EMPs were pretreated with CpB, we exclude the possibility of plasminogen exchange during the cross-talk. Plasmin was therefore formed at the surface of platelets in contact with EMPs. The rate of plasmin formation during the platelet/EMP cross-talk (Vi = 0.54 ± 0.14 mOD/minute) was higher than the rate of plasminogen activation (Vi = 0.32 ± 0.06 mOD/minute) on EMPs supplemented with an amount of plasminogen equivalent to that present on platelets (Figure 5B). To further investigate the relevance of the intercellular cross-talk mechanism, the formation of plasmin by a platelet/monocyte cross-talk was compared with activation of plasminogen by uPA on the same cell surface (monocytes); for the purpose of demonstration, we also studied the activation of platelet-bound plasminogen by free uPA. Equivalent amounts of either plasminogen or uPA were used in all cases. The results are shown in Figure 6A. The cross-talk mechanism resulted in a higher rate (Vi = 1.1 ± 0.26 mOD/minute) of plasmin formed compared with activation on the same cell surface (Vi = 0.6 ± 0.2 mOD/minute; P = .005), thus qualifying the efficiency of the cross-talk mechanism. Addition of free uPA to platelets resulted in a nonsignificant (P = .078) increase in plasmin formation compared with the cross-talk. However, free uPA cannot be a relevant plasminogen activator, because most unbound uPA in physiologic fluids is complexed to plasminogen activator inhibitor-1. Specificity of the cross-talk between platelet-bound plasminogen and uPA-bearing monocytes was established by the ability of a recombinant inactive form of scuPA (r-scuPA-Gly159) to inhibit the formation of plasmin (64% inhibition at 5nM; Figure 6B).
Efficiency and specificity of the plasminogen cross-talk. Glu-plasminogen (2μM) was bound to platelets as indicated in “Activation of platelet-bound plasminogen by monocytes or neurons.” (A) After treatment with 50 μg/mL CpB, adherent monocytes were incubated with 5 × 106 platelets bearing plasminogen (cross-talk) or with plasminogen (concentration equivalent to plasminogen bound to platelets; same surface) in the presence of 0.75mM CBS0065. In a parallel experiment, uPA (concentration equivalent to uPA bound to monocytes) was incubated with 5 × 106 platelets bearing plasminogen (free uPA) in the presence of 0.75mM CBS0065. Results are expressed as the rate of plasmin formation (mean ± SEM; n = 3; triplicates). NS indicates nonsignificant (P = .078), *P < .005, #P < .014. (B) Monocytes were incubated with 1nM of native uPA and various concentrations of a nonactive mutant uPA (r-scuPA-Gly159). Monocytes were then incubated with 5 × 106 platelets bearing plasminogen, in the presence of 0.75mM CBS0065. The formation of plasmin (mOD/minute) was detected by measuring the release of p-nitroaniline (mean ± SEM; n = 2; triplicates). A representative experiment is shown (P = .023; 0 vs 5nM r-scuPA-Gly159).
Efficiency and specificity of the plasminogen cross-talk. Glu-plasminogen (2μM) was bound to platelets as indicated in “Activation of platelet-bound plasminogen by monocytes or neurons.” (A) After treatment with 50 μg/mL CpB, adherent monocytes were incubated with 5 × 106 platelets bearing plasminogen (cross-talk) or with plasminogen (concentration equivalent to plasminogen bound to platelets; same surface) in the presence of 0.75mM CBS0065. In a parallel experiment, uPA (concentration equivalent to uPA bound to monocytes) was incubated with 5 × 106 platelets bearing plasminogen (free uPA) in the presence of 0.75mM CBS0065. Results are expressed as the rate of plasmin formation (mean ± SEM; n = 3; triplicates). NS indicates nonsignificant (P = .078), *P < .005, #P < .014. (B) Monocytes were incubated with 1nM of native uPA and various concentrations of a nonactive mutant uPA (r-scuPA-Gly159). Monocytes were then incubated with 5 × 106 platelets bearing plasminogen, in the presence of 0.75mM CBS0065. The formation of plasmin (mOD/minute) was detected by measuring the release of p-nitroaniline (mean ± SEM; n = 2; triplicates). A representative experiment is shown (P = .023; 0 vs 5nM r-scuPA-Gly159).
Discussion
Specific fibrinolytic and pericellular proteolytic functions of plasmin are determined by in situ molecular coassembly of plasminogen and its activators on cell receptors or on binding sites present on macromolecular complexes (fibrin or matrix surfaces).18,42 The use of tPA as a thrombolytic agent and the pericellular proteolytic activity of uPA-bearing cells are based on this paradigm.18,42 It has also been suggested that monocyte-uPA reduces thrombus size,43 and we have recently shown that EMPs carrying uPA derived from the parent cell generate plasmin activity.19 We now show that uPA anchored on monocytes or EMPs recognizes and transforms into plasmin, Glu-plasminogen bound to platelets, fibrin, or ECM proteins (fibronectin, laminin). This new activation mechanism bypasses the requirement for molecular coassembly on the same surface, via a recognition and proteolytic cross-talk pathway. Because in this plasminogen activation cross-talk, plasmin is efficiently generated on platelets or on matrix surfaces by uPA-bearing cells or MPs, it may be of potential physiologic relevance in fibrinolysis or degradation of ECM components. Note that this mechanism complies with the prerequisite for activation of plasminogen on biologic surfaces, ie, plasminogen binding to C-ter Lys residues of platelets, fibrin, or ECM.18 However, it essentially differs in that the uPA is expressed on neighboring cells or MPs and in that cells bearing tPA do not reproduce this effect.
It is generally accepted that Glu-plasminogen bound to fibrin and cell surfaces adopts an open conformation similar to that induced by the C-ter Lys analog ϵ-ACA.6,9,13 This conformation is called Lys-like because it is similar to the conformation of truncated Lys-plasminogen as defined by electron microscopy and small-angle neutron scattering.8,13 The induction of an open conformation by this lysine analog has previously been shown and is well described in the literature (reviewed by Markus9 ). On the basis of this analogy and because Lys-plasminogen, the natural open form, is activated by uPA at a higher rate than Glu-plasminogen,10,23,24 we investigated whether ϵ-ACA–liganded Glu-plasminogen, which is unable to bind to C-ter Lys, could be activated by uPA- or tPA-bearing cells or EMPs. We demonstrate that, although ϵ-ACA–liganded Glu-plasminogen cannot bind to fibrin or cells, it was selectively activated by uPA-bearing cells. Furthermore, at equimolar concentrations, the rate of activation of Glu-plasminogen/ϵ-ACA complexes was higher than that of Glu-plasminogen alone, in the absence of both plasminogen binding to the cell surface and free uPA activity in the medium.
Glu-plasminogen/ϵ-ACA complexes were efficiently activated despite the cleavage of C-ter Lys on cells by CpB. Thus, ϵ-ACA–liganded Glu-plasminogen behaves as a surrogate that ensures surface-like binding conformational changes leading to its recognition and activation.
Because the LBSs of both K1 and K4 are simultaneously occupied by ϵ-ACA, we used the specific LBS-targeted 34D3 mAb to disclose the role of K1. This mAb completely inhibits plasminogen binding and activation by tPA, thus underlining the role of K1 in the initial phase of plasmin formation.31 In contrast, despite the inhibition of Glu-plasminogen binding and formation of plasmin at their surface, cells bearing uPA were able to activate Glu-plasminogen in the cellular microenvironment. These results indicate that uPA- but not tPA-bearing cells recognize the extended open conformation adopted by Glu-plasminogen in complex with ϵ-ACA or mAb 34D3. In a similar fashion, we demonstrated in a previous study26 that Glu-plasminogen bound to C-ter Lys residues of fibrin during ongoing fibrinolysis27,44 was specifically recognized and activated by scuPA.26 Furthermore, using a fibrin- or platelet-bound r-Pg-Ala741 in cross-talk with uPA-bearing EMPs, we demonstrate that the active site–inactivated recombinant plasminogen was cleaved into inactive Glu-plasmin in situ without being transformed into Lys-plasminogen. We therefore hypothesized that Glu-plasminogen bound to C-ter Lys of cell membranes, fibrin, or matrix proteins may be recognized and activated by uPA/uPAR complexes expressed on other cells or cell-derived MPs present in the microenvironment.
First, we demonstrated that human EMP- or monocyte-borne uPA, but not tPA-bearing cells, was able to specifically activate platelet-bound Glu-plasminogen in a dose- and saturating-dependent manner. The rate of plasmin formation on platelets by monocytes was increased 2-fold over the activation of plasminogen at the monocyte surfaces. The specificity of the uPA-driven proteolytic cross-talk was shown by its inhibition with a recombinant form of uPA having no activator activity. The fibrinolytic cross-talk mechanism bypasses the requirement for assembly of profibrinolytic proteins on the same surface, introduces a complementary and new dimension for enhancement of fibrinolysis by platelets,41,45 and its efficiency suggests a potential physiologic relevance. Indeed, occupancy of platelets by plasma Glu-plasminogen and a direct relation between platelet number and degree of clot lysis has been previously reported.46,47 Because human platelets have been shown not to express uPAR,48 these observations are in agreement with our data showing a direct relationship between platelet number and plasmin generation by uPA-bearing monocytes or its derived MPs. Thus, platelet-bound plasminogen activated by monocytes or MPs bearing uPA could be an additional source of plasmin in the fibrin clot as recently suggested.43 Our results also suggest that procedures that increase plasminogen binding to platelets may be a new direction in pharmacologic enhancement of platelet fibrinolytic functions.
Second, using a similar approach, we demonstrated that Glu-plasminogen bound to fibrin surfaces, to ECM proteins (fibronectin, laminin), or to fibrin/fibronectin complexes was selectively recognized and activated into plasmin by uPA expressed on cells or EMPs. This mechanism of cross-talk may be of physiologic relevance because it has recently been reported that monocytes may be involved in clot dissolution.43,49 Because activated monocytes and macrophages release MPs that may bear uPA, it is possible that these MPs may participate in activation of fibrin- or platelet-bound plasminogen. Indeed, leukocyte-derived MPs has been found in atherosclerotic plaques,50 where they can initiate fibrinolytic or proteolytic activities that may destabilize the atheroma plaque. A similar interaction may take place during inflammatory processes in which primed cells could initiate a proteolytic cross-talk with plasminogen bound to other cells or to the matrix as suggested in the proposed model (Figure 7).
Model of fibrinolytic and proteolytic cross-talk. In inflammatory processes of the vascular wall, activation of coagulation leads to fibrin deposits. Within this setting and according to data presented here, the fibrinolytic cross-talk mechanism could be an intermediary pathway for fibrinolysis and pericellular proteolysis. Fibrinolytic effects (top): plasminogen (Pg) bound to fibrin or to platelets could be activated by either monocyte- or MP-borne uPA (EMP or monocyte-derived microparticle [MoMP]). In ECM remodeling in the vascular wall (bottom), plasminogen bound to matrix components could be activated by MP-borne uPA (EMP or MoMP) or by macrophage-borne uPA.
Model of fibrinolytic and proteolytic cross-talk. In inflammatory processes of the vascular wall, activation of coagulation leads to fibrin deposits. Within this setting and according to data presented here, the fibrinolytic cross-talk mechanism could be an intermediary pathway for fibrinolysis and pericellular proteolysis. Fibrinolytic effects (top): plasminogen (Pg) bound to fibrin or to platelets could be activated by either monocyte- or MP-borne uPA (EMP or monocyte-derived microparticle [MoMP]). In ECM remodeling in the vascular wall (bottom), plasminogen bound to matrix components could be activated by MP-borne uPA (EMP or MoMP) or by macrophage-borne uPA.
Altogether, these data provide a mechanistic support to the proteolytic cross-talk mechanism described above and suggest that, in inflammatory states or in cancer, plasminogen bound to platelets, fibrin, extracellular matrices, or adherent cells could be efficiently activated by uPA expressed on migrating cells or cellular MPs. For instance, plasminogen bound to cells may be transported into not easily accessible tissues such as the brain and be activated in situ by resident or migrating glial cells expressing uPA.51
In conclusion, we propose a new mechanism for plasmin formation that not only does bypass the requirement for coassembly of plasminogen and uPA on the same surface, but it also makes Lys-plasminogen dispensable. These heterotypic cell-to-cell (platelets/monocytes; platelets/MPs), cell-to-matrix (THP-1/fibrin), or MPs-to-matrix (MPs/fibrin) proteolytic cross-talk represents an alternative pathway for localized plasmin formation that may be relevant to processes implicating cell migration and MP dissemination, ie, inflammation or angiogenesis. Finally, our data provide additional evidence for a novel role of MPs and platelets, as vectors that generate and propagate plasmin fibrinolytic and/or proteolytic activity, and could thereby constitute a pharmacologic tool.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by funds from Inserm and the Lower-Normandy Regional Council. E.A.-C., L.D., and L.P. are members of the European Community's Seventh Framework Program (FP7/2007-2013) under grant agreement no. 201024. The Center for Molecular and Vascular Biology is supported by the Excellentie financiering, KULeuven. T.D. is a recipient of a PhD fellowship awarded by the Nouvelle Societé Française d'Athérosclérose. L.D. is a recipient of a PhD fellowship from the Education and Research French Ministry.
Authorship
Contribution: T.D. and L.D. performed research, analyzed data, and wrote the manuscript; R.L. performed research and participated in manuscript drafting; L.P. provided experimental support and participated in manuscript drafting; F.D.-G. analyzed data and participated in manuscript drafting; H.R.L. contributed analytical tools and participated in manuscript drafting; and E.A-C. designed the research, analyzed data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for T.D. is Inserm, U698, Paris, France.
Correspondence: Eduardo Anglés-Cano, Inserm U919 Serine Proteases and Pathophysiology of the Neurovascular Unit, GIP Cyceron/Bd Henri Becquerel, 14074-cdx, Caen, France; e-mail: eduardo.angles-cano@inserm.fr or angles@cyceron.fr.
References
Author notes
T.D. and L.D. contributed equally to this study (alphabetical order).




![Figure 7. Model of fibrinolytic and proteolytic cross-talk. In inflammatory processes of the vascular wall, activation of coagulation leads to fibrin deposits. Within this setting and according to data presented here, the fibrinolytic cross-talk mechanism could be an intermediary pathway for fibrinolysis and pericellular proteolysis. Fibrinolytic effects (top): plasminogen (Pg) bound to fibrin or to platelets could be activated by either monocyte- or MP-borne uPA (EMP or monocyte-derived microparticle [MoMP]). In ECM remodeling in the vascular wall (bottom), plasminogen bound to matrix components could be activated by MP-borne uPA (EMP or MoMP) or by macrophage-borne uPA.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/115/10/10.1182_blood-2009-06-228817/4/m_zh89990948240007.jpeg?Expires=1769091126&Signature=oqYJDfLKemX33waSXMY~dizfSFyEK7HN~j5KDKgHQVhBGf3VUX~jTheuu1Ds~th03nd1FMh26TZOabsiUylhjnGCFB9Pq7~A-9aTUjP9TQ-bYx6aCHqfCh7x2csNgf-1ncnz0RnxZcd~jfTMTteoAtrZqZ-WkXrE9U75~LI4Qbt0~bMWKNUsuQkajfFFK9UBIWcRwpY-MlkDkso0KD5yRHas-W-Nbgn~-0GES9GxqSpumsdvr89zr0v2jMZijNpE14XUFhE2mDOQ3o8z4gKFKCRlBCZ5N1FpoRNZ39fBk6qqiD0QRu9wuwRNZYDCs8eRdL0n-SwPt0eEN8Yj8S0aTg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal