Abstract
We studied the actions of 2-phenylacetylenesulfonamide (PAS) on B-chronic lymphocytic leukemia (CLL) cells. PAS (5-20 μM) initiated apoptosis within 24 hours, with maximal death at 48 hours asassessed by morphology, cleavage of poly(ADP-ribose) polymerase (PARP), caspase 3 activation, and annexin V staining. PAS treatment induced Bax proapoptotic conformational change, Bax movement from the cytosol to the mitochondria, and cytochrome c release, indicating that PAS induced apoptosis via the mitochondrial pathway. PAS induced approximately 3-fold up-regulation of proapoptotic Noxa protein and mRNA levels. In addition, Noxa was found unexpectedly to be bound to Bcl-2 in PAS-treated cells. PAS treatment of CLL cells failed to up-regulate p53, suggesting that PAS induced apoptosis independently of p53. Furthermore, PAS induced apoptosis in CLL isolates with p53 gene deletion in more than 97% of cells. Normal B lymphocytes were as sensitive to PAS-induced Noxa up-regulation and apoptosis as were CLL cells. However, both T lymphocytes and bone marrow hematopoietic progenitor cells were relatively resistant to PAS. Our data suggest that PAS may represent a novel class of drug that induces apoptosis in CLL cells independently of p53 status by a mechanism involving Noxa up-regulation.
Introduction
Chronic lymphocytic leukemia (CLL) is no longer thought to be an indolent disease characterized by reduced apoptosis becauseB-CLL cells have now been shown to proliferate rapidly, with birth rates of between 0.1% and 1.8% of the entire clone per day.1,2 CLL is a heterogeneous disease in which several prognostic risk factors have been identified, such as immunoglobulin heavy chain gene mutational status and CD38 and ζ-chain–associated protein 70 (ZAP-70) expression.2,3 Treatment options for CLL to date include the standard chemotherapeutic agents chlorambucil and fludarabine, fludarabine plus cyclophosphamide, or antibody therapies including rituximab and alemtuzumab either as single agents or in combination with chemotherapy
Numerous chromosomal abnormalities occur in CLL including the deletion of chromosome arms 17p or 11q, which results in reduced expression of the p53 protein or its upstream regulator ataxia telangiectasia mutated (ATM) protein kinase, respectively. These deletions are indicative of a worse prognosis with a shorter time from first presentation to treatment.4 The ATM/p53 pathway plays a pivotal role in securing the apoptotic response after the induction of DNA damage.5 Therefore, patients with 17p or 11q deletions are difficult to treat with conventional agents, resulting in shorter overall survival.4,6,7 Deletion of p53 in CLL cells is detectable in 10% to 15%8-11 of CLL patients at diagnosis. However, this percentage increases during the progression of the disease.12 Treatment with p53-dependent cytotoxic agents results in selection and expansion of cells with ATM or p53 deletions in approximately 50% of patients.6 In the recent United Kingdom CLL4 trial, treatment with fludarabine plus cyclophosphamide was ineffective in the cohort of patients with more than 20% 17p deleted cells.12 Recent evidence suggests that, in a proportion of these patients, alemtuzumab alone13,14 or in combination with high dose methylprednisolone15 can overcome drug resistance resulting from the lack of p53.
Apoptosis is regulated by adversarial interactions between Bcl-2 family members, including the antiapoptotic proteins Bcl-2, Bcl-xL, and Mcl-1 and the proapoptotic proteins Bax and Bak.16 The proapoptotic BH3-only Bcl-2 family members Puma, Noxa, and Bim play key roles in coupling specific death stimuli to the core apoptotic machinery. The direct activation model of apoptosis suggests that BH3-only proteins belong to one of 2 groups, namely activator or sensitizer proteins.17 This model postulates that activator BH3-only proteins are sequestered by binding to antiapoptotic proteins including Bcl-2. Increases in cellular levels of sensitizer proteins, including Puma and Noxa, result in the displacement of activator proteins, including Bim.18 The displaced Bim then interacts with Bax or Bak, which then change their conformation and insert into the outer mitochondrial membrane. The resulting release of cytochrome c activates caspases to initiate apoptosis.16 In addition to the canonical p53-dependent mechanisms, Puma and Noxa can also be up-regulated by p53-independent pathways, including E2F1-, c-Myc-, and Foxo3a-mediated transcriptional pathways.19-21
The p53 protein is a transcription factor that induces apoptosis via up-regulation of Noxa and Puma.22 This pathway is thought to play an important role in the killing of malignant cells after treatment with genotoxic agents.23,24 Because this mechanism is compromised in CLL cells with deletions of the p53 or ATM genes,25-27 it is important to identify novel agents that bypass this block to cell killing. Here we show for the first time that 2-phenylacetylenesulfonamide (PAS; also known as pifithrin-μ28 ) rapidly induced apoptosis of CLL cells and normal B cells, whereas normal T lymphocytes and bone marrow hematopoietic cells were substantially more resistant to this agent. We also demonstrate that PAS treatment resulted in the p53-independent up-regulation of Noxa, the physical association of the latter protein with Bcl-2 at the mitochondrial surface, a proapoptotic conformation change in the Bax protein, and cytochrome c release. The data herein therefore suggest that PAS represents a novel class of cytotoxic drug that may be of value in the treatment of CLL patients who have become refractory to conventional therapeutic regimes through loss of p53.
Methods
Reagents
Tissue culture materials were from Invitrogen. Chlorambucil and 3-(4,5-dimethylthiazol-2-yl)-2,5-dimethyltetrazolium bromide (MTT) were from Sigma-Aldrich. PAS was from ChemBridge and from Calbiochem. Nutlin-3 was from Calbiochem. Benzyloxycarbonyl-Val-Ala-Asp (ZVAD) was from Biomol International. All other reagents were of the highest grade available.
Patients and cells
This investigation was approved by the Local Research Ethics Committee of the Royal Free Hospital. Written informed consent was obtained before phlebotomy, in accordance with the Declaration of Helsinki. Cell isolation, determination of purity, and culture were as described.29 All CLL isolates studied contained less than 5% CD2+ cells as determined by flow cytometric analysis.30 A total of 35 CLL isolates were studied (supplemental Table 1, available on the Blood website; see the Supplemental Materials link at the top of the online article). The median lymphocyte count was 53.8 million/mL. IgVH genes were unmutated in 9 of 35 of the samples and mutated in 16 of 35, while the remaining 10 samples were not analyzed or were not quantifiable for technical reasons. The p53 gene was deleted, as shown by fluorescence in situ hybridization, in more than 97% of the malignant cells of 3 patients (AS32, AS33, AS34).
Peripheral blood mononuclear cells from normal subjects were isolated on Lymphoprep gradients. Monocytes were depleted by adherence on plastic tissue culture surfaces.29 B and T lymphocytes were isolated by negative selection procedures using the appropriate magnetic-activated cell sorting (MACS) immunomagnetic cell isolation kits (Miltenyi Biotec). B and T cells were more than 95% pure as judged by FACScan analysis of CD19 and CD2 expression.
Cell culture and protein extraction
Cells were cultured in RPMI 1640 medium supplemented with 10% fetal calf serum and antibiotics.29 Proteins were extracted for Western blot analysis as previously described.29 Extracts for immunoprecipitation were prepared using the buffer system described by Liu et al.31 Differential detergent fractionation was carried out by a modification32 of the procedure of Ramsby and Makowski.33 Analysis of distribution of cytosolic, mitochondrial, and nuclear markers has established that this procedure yielded essentially pure fractions.32 Protein extraction for analysis of histone acetylation was as described.34
Determination of cell killing
Cytotoxicity was assessed by carrying out triplicate assays for the reduction of MTT30 after 24 to 72 hours of incubation of cells with serial 2-fold dilutions of drugs. Fifty percent inhibitory concentration (IC50) values were computed using CalcuSyn software (Biosoft).
Hematopoietic colony assays
Hematopoietic colony formation was assessed by suspending 105 bone marrow mononuclear cells in 1 mL of MethoCult containing stem cell factor, granulocyte-macrophage colony-stimulating factor, interleukin-3, and erythropoietin (StemCell Technologies). Colony-forming unit-granulocyte/macrophage colonies (CFU-GM) and erythroid burst forming units (BFU-E) were quantified after an additional 14 days of incubation.
Quantitation of annexin V–positive CLL cells or T lymphocyte subsets
FACScan analysis of CLL cells labeled with fluorescein isothiocyanate (FITC)-annexin V and propidium iodide (PI) was as previously described.35 Agent-specific apoptosis was computed using a published equation36 : agent-specific death = (% annexin-positive cells induced by agent − % annexin-positive cells in untreated control)/(100 − % annexin-positive cells in untreated control).
Quantitation of annexin V–positive T lymphocyte subsets was by a modification of the above procedure. Peripheral blood mononuclear cells were isolated from normal donors by sedimentation on a Lymphoprep gradient and incubated for 18 hours with increasing concentrations of PAS. The cells were then incubated with FITC-annexin V (Miltenyi Biotec), phycoerythrin–anti-CD3, To-Pro, and either peridinin chlorophyll protein (PerCP)–anti-CD4 or PerCP–anti-CD8 (all from BD Pharmingen), followed by FACScan analysis. To-Pro was used in place of PI because of the requirements imposed by simultaneous analysis of 3 colors in these experiments. T lymphocytes were gated on the basis of CD3 positivity. CD4+ or CD8+ cells were then gated within the T cell population. The intensity of staining with To-Pro and annexin V within the CD3+/CD4+ or CD3+/CD8+ gates was then determined.
Western blotting
Proteins were separated on a 12% polyacrylamide gel (Invitrogen) and transferred to nitrocellulose membranes.29 Antibodies against the following proteins were used in Western blot experiments: PARP, Bax, caspase 3 (Alexis Biochemicals); actin (Sigma-Aldrich); Bcl-2 and secondary horseradish peroxidase (HRP)–conjugated antibodies (Dako); p53 DO-1 (Santa Cruz Biotechnology); Bim, cytochrome c oxidase IV, cytochrome c, acetylated histone 3a and histone 4A, histone 3a and histone 4A (Cell Signaling Technologies); Puma, Noxa (Merck); and heat shock protein 60 (Hsp60; Stressgen). Band intensities were quantified using the GS-700 imaging densitometer and Quantity 1 software (Bio-Rad).
Immunoprecipitation protocols
Bcl-2 was immunoprecipitated using a hamster anti–Bcl-2 antibody (BD Pharmingen) by a modification32 of a published procedure.18 The activated conformation of the Bax protein was immunoprecipitated using the conformation-specific Bax 6A7 antibody (BD Pharmingen) essentially as described.32 Precipitated Bax was then quantified by Western blotting using a pan-reactive antibody (Cell Signaling Technologies).
qRT-PCR
Briefly, RNA was extracted from 30 × 106 cells in 1 mL of TRIzol (Invitrogen). cDNA was generated by reverse transcription and subjected to quantitative reverse transcriptase–polymerase chain reaction (qRT-PCR) on a LightCycler 2.0 (Roche Applied Science). The LightCycler was run for 1 cycle at 95°C for 12 minutes followed by 40 cycles at 95°C for 15 seconds, 66°C for 10 seconds, and 72°C for 15 seconds. Noxa primer sequences were: Noxa forward, AGA GCT GGA AGT CGA GTG T; Noxa reverse, GCA CCT TCA CAT TCC TCT C. A panel of 2 primer pairs, actin, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) QuantiTect primer sets (Qiagen) were used as controls to normalize differences between samples.
Results
PAS induces apoptotic killing of CLL cells
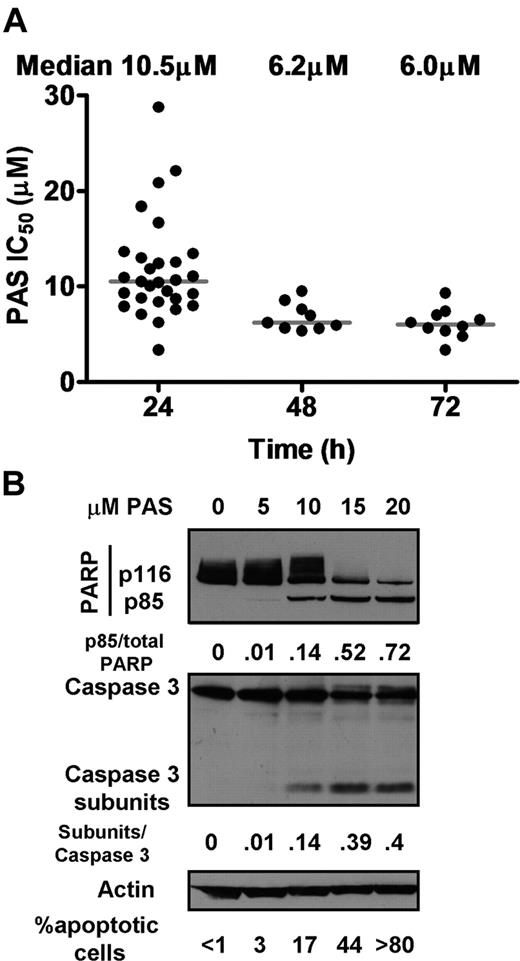
Cells from 30 CLL patients (supplemental Table 1) were treated for 24 to 72 hours with 2-fold doubling dilutions of PAS and their viability assessed by MTT dye reduction assay. Substantial loss of viability occurred between 24 and 48 hours of incubation (Figure 1A). The concentrations of PAS required to induce 50% loss of viability at 24 hours (IC50) were computed using CalcuSyn software. The cells from 25 patients (83%) required less than 15 μM PAS to induce 50% loss of viability, while isolates from 5 patients (17%) yielded IC50 values exceeding15 μM. The median PAS IC50 at 24 hours was 10.5 μM. CLL cells treated with PAS for longer periods yielded median IC50 values of 6.2 (n = 9) and 6.0 μM (n = 10) at 48 and 72 hours, respectively (Figure 1A), indicating that maximal killing in response to a single dose of PAS was essentially achieved by 48 hours.
Effect of PAS on CLL cells. (A) Cells from CLL patients were treated with 0 to 160 μM PAS for 24 hours (n = 30), 48 hours (n = 9), or 72 hours (n = 10). Viability was assessed by MTT analysis. IC50 values were determined using CalcuSyn software as previously described.30 (B) Proteins were extracted from cells of patient AS15 incubated with no addition or the indicated concentration of PAS and analyzed by Western blotting. PARP cleavage data are typical of 23 and the caspase 3 data of 3 experiments. The percentages of apoptotic cells observed by morphologic analysis (see supplemental Figure 2) is indicated below the Western blots and are typical of 33 experiments.
Effect of PAS on CLL cells. (A) Cells from CLL patients were treated with 0 to 160 μM PAS for 24 hours (n = 30), 48 hours (n = 9), or 72 hours (n = 10). Viability was assessed by MTT analysis. IC50 values were determined using CalcuSyn software as previously described.30 (B) Proteins were extracted from cells of patient AS15 incubated with no addition or the indicated concentration of PAS and analyzed by Western blotting. PARP cleavage data are typical of 23 and the caspase 3 data of 3 experiments. The percentages of apoptotic cells observed by morphologic analysis (see supplemental Figure 2) is indicated below the Western blots and are typical of 33 experiments.
Cleavage of the 116-kDa caspase 3 substrate PARP to an 85-kDa subfragment is an established molecular marker for apoptosis.37 PARP cleavage was therefore assessed by Western blot analysis of lysates from 23 CLL isolates after 24 hours of treatment with PAS. PARP cleavage was detectable in cells incubated with 10 μM PAS, with substantial cleavage evident at 15 and 20 μM PAS (Figure 1B). The dose-dependent cleavage of inactive pro-caspase 3 and the consequent generation of an active 17-kDa subunit was also clearly evident (Figure 1B).
The induction of apoptosis by PAS was also quantified by assessing the proportion of cells with nuclear condensation, a morphologic characteristic of apoptosis38,39 (supplemental Figure 1). The dose-dependent increase in morphologically identifiable cells, which approximately paralleled the increase in PARP and caspase 3 cleavage, is also annotated in Figure 1B. A similar dose-dependent induction of apoptosis was seen in isolates from an additional 34 patients. Cells from 3 patients that yielded PAS IC50 values greater than 20 μM showed some evidence of morphologic apoptosis, although significantly less than did more sensitive cells (data not shown).
Incubation with PAS in the presence of the pan-caspase inhibitor ZVAD completely inhibited cleavage of PARP and of caspase 3 and also blocked morphologic apoptosis, further confirming that PAS-induced killing of CLL cells was by a caspase-dependent apoptotic mechanism (supplemental Figure 2). Washing of cells pulsed with PAS for 10 hours followed by further incubation in drug-free medium for 14 hours established that maximal commitment to apoptosis was achieved within 10 hours of incubation with the drug (supplemental Figure 3).
Apoptosis is preceded by a caspase 3–dependent externalization of phosphatidylserine at the plasma membrane, which is readily detectable by FACScan analysis after the binding of FITC-labeled annexin V.35 PAS induced a clear dose-dependent increase in annexin V staining in cells from 5 CLL patients. A representative example of FACScan analysis of PAS-induced apoptosis in cells from CLL patient AS31 is shown in Figure 2A. Dose response data for AS31 and 3 additional patients with functional p53 (AS8, AS27, and AS30) are summarized in Table 1.
Annexin V/PI analysis of CLL cells treated with PAS. Cells from patient AS31 with functional p53 (A) or patient AS32 with more than 97% p53 deletion (B) were treated with 0, 5, 10, 15, and 20 μM PAS for 24 hours. Apoptosis was quantified by annexin V/PI FACScan analysis. Values in each quandrant represent the percentage of gated cells in each quarter.
Annexin V/PI analysis of CLL cells treated with PAS. Cells from patient AS31 with functional p53 (A) or patient AS32 with more than 97% p53 deletion (B) were treated with 0, 5, 10, 15, and 20 μM PAS for 24 hours. Apoptosis was quantified by annexin V/PI FACScan analysis. Values in each quandrant represent the percentage of gated cells in each quarter.
Annexin V positivity of CLL cells following incubation with PAS
| Patient no. . | PAS concentration (μM) . | |||
|---|---|---|---|---|
| 5 . | 10 . | 15 . | 20 . | |
| AS8 | 0 | 0 | 0 | 18.2 |
| AS27 | 2.5 | 4.9 | 20.7 | 55.0 |
| AS30 | 0 | ND | 15.3 | 43.1 |
| AS31 | 0.2 | 11.1 | 58.1 | 62.9 |
| AS32 | 0.4 | 12.5 | 57.8 | 63.0 |
| Patient no. . | PAS concentration (μM) . | |||
|---|---|---|---|---|
| 5 . | 10 . | 15 . | 20 . | |
| AS8 | 0 | 0 | 0 | 18.2 |
| AS27 | 2.5 | 4.9 | 20.7 | 55.0 |
| AS30 | 0 | ND | 15.3 | 43.1 |
| AS31 | 0.2 | 11.1 | 58.1 | 62.9 |
| AS32 | 0.4 | 12.5 | 57.8 | 63.0 |
CLL cells from 5 patients were incubated with increasing concentrations of PAS for 24 hours. The percentage of annexin V–positive cells was determined by FACScan analysis. The PAS-specific increase in the percentage of annexin V–positive cells was computed using an established equation.36 ND indicates not determined.
The cytotoxicity of PAS toward CLL cells was established by 5 independent criteria. Although we consistently observed that a 50% loss in MTT dye reduction was achieved by PAS concentrations between 7.5 and 15 μM in the majority of CLL isolates, the induction of approximately 50% PARP cleavage, annexin V positivity, or morphologic apoptosis occurred in the 15 to 20 μM concentration range. It is plausible that this modest discrepancy is accounted for by the fact that MTT reduction is dependent on a functional mitochondrial electron transport apparatus and that PAS inhibits this process more rapidly than it induces the molecular and phenotypic features of apoptosis.
No correlation was observed between PAS sensitivity and Binet/Rai staging, IGVH mutational status, or ZAP-70 expression (data not shown).
PAS treatment causes Bax activation and cytochrome c release
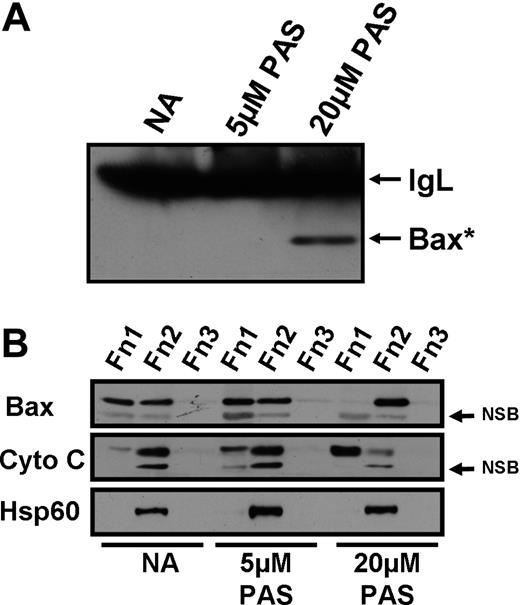
The Bax protein undergoes a proapoptotic conformational change in response to death stimuli.16 This conformational change enables Bax to insert into the outer mitochondrial membrane, forming pores that allow the release of cytochrome c into the cytosol. Cytochrome c release leads to the formation of the apoptosome, caspase activation, and subsequent apoptosis.16 Bax conformational change was quantified by immunoprecipitation using an antibody specific for the proapoptotic form of Bax. Treatment of CLL cells with 20 μM PAS clearly induced the conformational change (Figure 3A). In contrast, Bax conformational change was not detected in cells treated with 5 μM PAS.
Effect of PAS on Bax conformational change, cytochrome c release, and Bax insertion into the mitochondria. CLL cells were incubated for 18 hours with no addition (NA) or 5 or 20 μM PAS in the presence of the pan-caspase inhibitor ZVAD. (A) Cell extracts were immunoprecipitated using an antibody specific for the proapoptotic conformation of Bax (Bax*). Precipitates were analyzed by Western blotting. IgL, antibody light chain, NSB, nonspecific band. (B) DDF was performed to determine the movement of cytochrome c and Bax within the cell after PAS treatment. Fractions (Fn) 1, 2, and 3 correspond to cytosolic, mitochondrial plus organellar, and nuclear fractions, respectively. Data shown are for patient AS9 and are typical of 5 experiments.
Effect of PAS on Bax conformational change, cytochrome c release, and Bax insertion into the mitochondria. CLL cells were incubated for 18 hours with no addition (NA) or 5 or 20 μM PAS in the presence of the pan-caspase inhibitor ZVAD. (A) Cell extracts were immunoprecipitated using an antibody specific for the proapoptotic conformation of Bax (Bax*). Precipitates were analyzed by Western blotting. IgL, antibody light chain, NSB, nonspecific band. (B) DDF was performed to determine the movement of cytochrome c and Bax within the cell after PAS treatment. Fractions (Fn) 1, 2, and 3 correspond to cytosolic, mitochondrial plus organellar, and nuclear fractions, respectively. Data shown are for patient AS9 and are typical of 5 experiments.
We then used differential detergent fractionation (DDF) to isolate proteins from the cytosol, the organellar fraction (including mitochondria), and the nucleus, designated as fractions 1, 2, and 3, respectively. Western blot analysis clearly showed that Bax was detectable in both the cytosolic and organellar fractions of untreated cells, with a dramatic movement of Bax from the cytosol into the organellar fraction after treatment with 20 μM PAS (Figure 3B). The release of cytochrome c from the organellar fraction into the cytosol was also clearly evident after treatment with 20 μM PAS (Figure 3B). Treatment with 5 μM PAS resulted in a lower level of cytochrome c release, whereas Bax movement was not clearly evident. Therefore, the data presented in Figure 3 confirm that PAS-induced killing of CLL cells was via the mitochondria-dependent intrinsic apoptotic pathway.
PAS induces Noxa via a p53-independent pathway
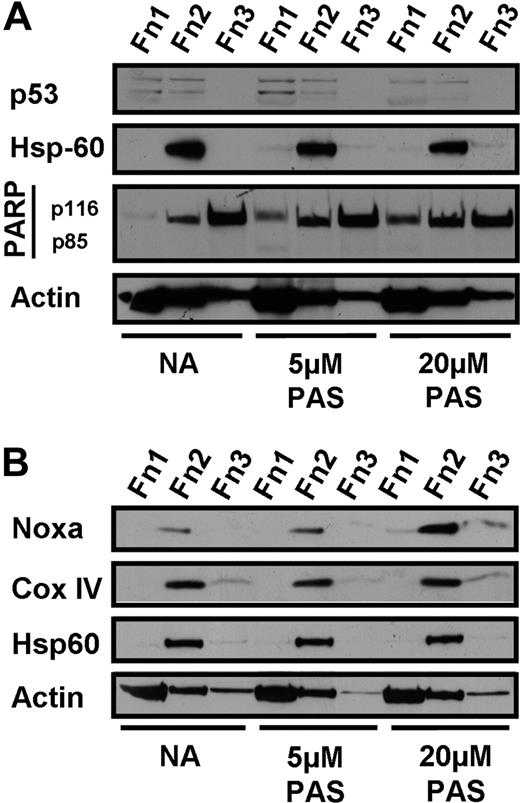
To further elucidate the mechanism of PAS-induced apoptosis, we fractionated untreated and PAS-treated CLL cells using the DDF protocol32,33 and determined the expression levels and subcellular distribution of p53 and several pro- and antiapoptotic members of the Bcl-2 family. These experiments were carried out in the presence of 100 μM of the pan-caspase inhibitor ZVAD, to determine molecular events that preceded caspase activation and consequent cleavage of cellular proteins. p53 was expressed at low levels in untreated cells and was not up-regulated after treatment with 5 or 20 μM PAS (Figure 4A). The efficacy of caspase inhibition by ZVAD is documented by the complete suppression of PARP cleavage in PAS-treated cells (Figure 4A). We failed to detect PAS-induced up-regulation of p53 in 26 of 29 isolates tested, while a modest level of induction was seen in the remaining3 patients. These observations suggest that PAS-induced killing of CLL cells was independent of p53 induction in most cases.
Effect of PAS on p53 and Noxa expression. Differential detergent fractionation was performed on CLL cells incubated for 18 hours in the presence of 100 μM ZVAD with no further addition (NA) or with 5 or 20 μM PAS. The effect of PAS on expression of p53 (A, patient AS9) and Noxa (B, patient AS25) is shown. Immunoblotting for Hsp60 and Cox IV documents successful fractionation and for actin to demonstrate equal loading. The data are typical of 29 (A) and 32 (B) experiments.
Effect of PAS on p53 and Noxa expression. Differential detergent fractionation was performed on CLL cells incubated for 18 hours in the presence of 100 μM ZVAD with no further addition (NA) or with 5 or 20 μM PAS. The effect of PAS on expression of p53 (A, patient AS9) and Noxa (B, patient AS25) is shown. Immunoblotting for Hsp60 and Cox IV documents successful fractionation and for actin to demonstrate equal loading. The data are typical of 29 (A) and 32 (B) experiments.
The proapoptotic BH3-only Noxa protein was induced after PAS treatment (Figure 4B). The augmentation of Noxa protein levels was 2- to 4-fold in the 32 CLL isolates investigated. The induced Noxa was almost exclusively localized to the organellar/ mitochondrial cell fraction, as documented by its colocalization with the mitochondrial markers Hsp60 and cytochrome c oxidase IV (Figure 4B).
RNA was extracted from untreated and PAS-treated CLL cells and reverse transcribed to generate cDNA, which was analyzed by quantitative PCR for Noxa expression. Our data show that Noxa mRNA was induced from 0.75- to 6.4-fold after PAS treatment (median, 4.7-fold; n = 10; Table 2), suggesting that the observed increases in Noxa levels were mediated at the transcriptional level. Taken together, these data suggest that PAS treatment resulted in augmentation of Noxa levels via a p53-independent transcriptional mechanism.
qRT-PCR for Noxa mRNA after treatment with PAS
| Patient no. . | Fold increase in Noxa mRNA . |
|---|---|
| AS2 | 0.85 |
| AS6 | 5.90 |
| AS7 | 4.66 |
| AS10 | 5.70 |
| AS19 | 6.45 |
| AS20 | 2.71 |
| AS21 | 0.75 |
| AS22 | 5.21 |
| AS27 | 4.92 |
| AS29 | 1.53 |
| Median | 4.79 |
| Mean | 3.87 |
| Patient no. . | Fold increase in Noxa mRNA . |
|---|---|
| AS2 | 0.85 |
| AS6 | 5.90 |
| AS7 | 4.66 |
| AS10 | 5.70 |
| AS19 | 6.45 |
| AS20 | 2.71 |
| AS21 | 0.75 |
| AS22 | 5.21 |
| AS27 | 4.92 |
| AS29 | 1.53 |
| Median | 4.79 |
| Mean | 3.87 |
Noxa expression was assessed on a LightCycler 2, with actin used as the control gene. A plot of the fold increase in Noxa transcripts versus the IC50 determined for the same isolate yielded a correlation coefficient of 0.66.
Treatment of CLL cells with inhibitors of histone deacetylase (HDAC) has been shown to induce Noxa expression and subsequent apoptosis.40 We therefore determined whether induction of Noxa by PAS was dependent on the inhibition of deacetylation of histones 3 and 4. Whereas the known HDAC inhibitor valproic acid caused a clear increase in the acetylation of both histones 3 and 4, PAS treatment resulted in a modest decrease in acetylation of both histones (supplemental Figure 4). We therefore conclude that PAS did not induce apoptosis of CLL cells via inhibition of HDAC. Treatment of CLL cells with HDAC inhibitors resulted in the coordinate up-regulation of Noxa and Bim.40 Therefore, the observations here that PAS caused an up-regulation of Noxa but not of Bim (see Figure 6C) provides additional evidence that PAS did not induce apoptosis via HDAC inhibition.
PAS-induced Noxa binds to Bcl-2
We have shown that PAS-induced Noxa associated almost exclusively with the mitochondrial fraction (Figure 4B). Since the antiapoptotic Bcl-2 protein is also localized to the same subcellular fraction, we used an immunoprecipitation technique to show that Noxa was bound to Bcl-2 in PAS-treated cells (Figure 5A). Noxa binding to Bcl-2 was also evident in CLL cells treated with 2 p53-dependent cytotoxic agents, chlorambucil and nutlin 3. The latter 2 agents additionally induced up-regulation of p53 and its association with Bcl-2, confirming our previous observations.32 In contrast, we did not detect p53 binding to Bcl-2 after PAS treatment (Figure 5A). The BH3-only protein Bim was constitutively associated with Bcl-2 in untreated CLL cells, in agreement with previous observations.18 The Bim-Bcl-2 association was decreased after PAS treatment (Figure 5). In contrast, treatment with chlorambucil or nutlin did not decrease binding of Bim to Bcl-2.
Coimmunoprecipitation of proapoptotic proteins with Bcl-2. CLL cells were incubated for 18 hours in the presence of 100 μM ZVAD with no further addition (NA) or with 15μM PAS, 60μM chlorambucil (CHL), or 20μM nutlin 3 (NUT). (A) Lysates were immunoprecipitated with anti–Bcl-2 antibody or an isotype control antibody (IC) and analyzed by Western blotting. The blots were probed with p53, Bim, Noxa, Bcl-2, and Hsp60 antibodies. Lysate from CHL-treated cells was run in the right-hand lane to provide migration markers for the proteins analyzed. (B) Unprecipitated lysates were also analyzed directly by Western blotting using anti-Hsp60 and anti–Bcl-2 antibodies.
Coimmunoprecipitation of proapoptotic proteins with Bcl-2. CLL cells were incubated for 18 hours in the presence of 100 μM ZVAD with no further addition (NA) or with 15μM PAS, 60μM chlorambucil (CHL), or 20μM nutlin 3 (NUT). (A) Lysates were immunoprecipitated with anti–Bcl-2 antibody or an isotype control antibody (IC) and analyzed by Western blotting. The blots were probed with p53, Bim, Noxa, Bcl-2, and Hsp60 antibodies. Lysate from CHL-treated cells was run in the right-hand lane to provide migration markers for the proteins analyzed. (B) Unprecipitated lysates were also analyzed directly by Western blotting using anti-Hsp60 and anti–Bcl-2 antibodies.
The abundant mitochondrial protein Hsp60 was clearly detectable in the unprecipitated lysates (Figure 5B) but was completely undetectable in the Bcl-2 immunoprecipitates (Figure 5A). Therefore, it is unlikely that the detection of Noxa, p53, Bim, and Puma in Bcl-2 immunoprecipitates was the result of inadequate washing. The specificity of immunoprecipitation reactions was further established by the inability of an isotype-matched control antibody to immunoprecipitate Bcl-2, p53, or Noxa (Figure 5A).
PAS induces cell killing independently of p53 status
More than 97% of the malignant cells from 3 CLL patients (AS32, AS33, AS34) were deleted for the p53 gene, as shown by fluorescence in situ hybridization analysis (not shown). The complete absence of functional p53 in these isolates was further emphasized by Western blot analysis showing that treatment with chlorambucil failed to up-regulate p53 (supplemental Figure 5). The ability of chlorambucil to elevate p53 in cells from control patient AS2 was established in the same experiment. Cells from patient AS32 were readily induced to apoptosis, evidenced by PARP cleavage, as efficiently (Figure 6A-B) as were cells with functional p53 (compare with Figure 1B). Importantly, the AS32 isolate was completely refractory to induction of PARP cleavage by the p53-dependent cytotoxic agents chlorambucil and nutlin 3 (Figure 1B). Similar results were obtained using cells from patient AS33, whereas the isolate from patient AS34 was relatively resistant (not shown), requiring more than 20 μM PAS to elicit PARP cleavage. The ability of PAS to induce dose-dependent apoptosis of cells from patient AS32 was also shown by FACScan analysis of annexin V/PI-stained cells (Figure 2B; Table 1). Morphologic analysis of Giemsa-stained cells additionally confirmed the ability of PAS to induce apoptosis of p53-deficient CLL cells (supplemental Figure 6). Therefore, these data suggest that PAS can induce apoptosis of CLL cells lacking functional p53, which are consequently resistant to conventional p53-dependent cytotoxic agents. The relative resistance of cells from one p53-deleted patient and also of 3 p53-functional patients (Figure 1A) additionally suggests that a minority of CLL isolates are refractory to PAS killing by a mechanism that is independent of p53 loss.
Effect of PAS on CLL cells with p53 deletion. CLL cells from patient AS32 with greater than 97% deletion of p53 were treated with 5 to 20 μM PAS, 60 μM chlorambucil, or 20 μM nutlin 3 for 6 hours (A) or 24 hours (B). Lysates were analyzed by Western blotting. Cells from the same patient were incubated for 6 hours with the indicated concentrations of PAS in the presence of 100 μM ZVAD and analyzed by Western blotting (C).
Effect of PAS on CLL cells with p53 deletion. CLL cells from patient AS32 with greater than 97% deletion of p53 were treated with 5 to 20 μM PAS, 60 μM chlorambucil, or 20 μM nutlin 3 for 6 hours (A) or 24 hours (B). Lysates were analyzed by Western blotting. Cells from the same patient were incubated for 6 hours with the indicated concentrations of PAS in the presence of 100 μM ZVAD and analyzed by Western blotting (C).
CLL cells lacking functional p53 retain the ability to up-regulate Noxa after PAS treatment
Cells from p53-deleted patient AS32 were incubated in the presence of ZVAD and the indicated concentrations of PAS (Figure 6C). The Puma protein was constitutively expressed by these cells (Figure 6C), confirming our earlier conclusions that basal Puma expression in CLL cells was mediated by a p53-independent mechanism32 We have also shown that Puma expression was not further augmented after treatment of p53-defective cells with chlorambucil or nutlin.32 In contrast, Noxa expression was undetectable in untreated p53-deleted cells and was strongly induced by PAS in a dose-dependent fashion (Figure 6A). These observations reinforce the conclusions that the elevation of Noxa expression by PAS occurs by a p53-independent mechanism and that elevated Noxa is able to induce apoptosis despite the inability of p53-deleted CLL cells to elevate Puma.
PAS actions on normal B and T lymphocytes and on bone marrow myeloid progenitors
We assessed the ability of PAS to induce PARP cleavage in B and T lymphocytes isolated from 3 normal donors. Normal B cells were at least as susceptible to PAS-induced apoptosis as were CLL cells, showing significant evidence of PARP cleavage at 10 μM PAS (Figure 7). Killing of these cells was also associated with a dramatic up-regulation of Noxa. In contrast, PARP cleavage was not evident in normal T cells except at 40 μM PAS, a level approximately 4 times greater than that required to elicit PARP cleavage in normal B cells (Figure 7). Noxa was only modestly elevated by 20 μM PAS, with substantial up-regulation evident at 40 μM of the drug. The data also support the hypothesis that susceptibility to apoptosis induction by PAS of different cell types may be dependent on the differential ability of this agent to elevate Noxa.
Effect of PAS on normal B and T cells. Normal B and T cells were purified by negative selection and treated with the indicated concentrations of PAS for 18 hours. Lysates were analyzed by Western blotting.
Effect of PAS on normal B and T cells. Normal B and T cells were purified by negative selection and treated with the indicated concentrations of PAS for 18 hours. Lysates were analyzed by Western blotting.
To assess the actions of PAS on T cell subsets, peripheral blood mononuclear cells from 3 additional donors were stained with annexin V, To-Pro, anti-CD3, and either anti-CD4 or anti-CD8. FACScan analysis was used to determine annexin V positivity within cells within the CD3+/CD8+ or CD3+/CD4+ gates (Table 3). The data show that the susceptibility of either subset to the induction of annexin V positivity by PAS was substantially less than the susceptibility of CLL cells at equivalent PAS concentrations. For example, the mean percentage of annexin-positive cells after incubation with 20 μM PAS was 13 plus or minus 1.1 (SEM) and 7.6 plus or minus 1.8 for CD4+ or CD8+ T cells, respectively (Table 3), whereas treatment of CLL cells with the same concentration of PAS resulted in a mean percentage of 48.4 plus or minus 8.4 annexin-positive cells (Table 1).
Annexin V positivity of T lymphocytes following incubation with PAS
| . | PAS concentration (μM) . | |||
|---|---|---|---|---|
| 5 . | 10 . | 20 . | 40 . | |
| CD3+/CD4+ cells | ||||
| Donor 1 | 0.2 | −0.3 | 10.9 | 23.3 |
| Donor 2 | −2.8 | −3.1 | 14.7 | 28.1 |
| Donor 3 | −2.2 | −0.2 | 13.3 | 39.7 |
| CD3+/CD8+ cells | ||||
| Donor 1 | −0.5 | −2.7 | 5.6 | 16.7 |
| Donor 2 | −2.7 | −1.8 | 8.8 | 19.7 |
| Donor 3 | 0.2 | 1.1 | 8.5 | 29.8 |
| . | PAS concentration (μM) . | |||
|---|---|---|---|---|
| 5 . | 10 . | 20 . | 40 . | |
| CD3+/CD4+ cells | ||||
| Donor 1 | 0.2 | −0.3 | 10.9 | 23.3 |
| Donor 2 | −2.8 | −3.1 | 14.7 | 28.1 |
| Donor 3 | −2.2 | −0.2 | 13.3 | 39.7 |
| CD3+/CD8+ cells | ||||
| Donor 1 | −0.5 | −2.7 | 5.6 | 16.7 |
| Donor 2 | −2.7 | −1.8 | 8.8 | 19.7 |
| Donor 3 | 0.2 | 1.1 | 8.5 | 29.8 |
Peripheral blood mononuclear cells from 3 normal donors were incubated with increasing concentrations of PAS. The percentage of annexin V–positive cells within the CD3+/CD4+ or CD3+/CD8+ populations was determined as described in “Quantitation of annexin V–positive CLL cells or T lymphocyte subsets.” The PAS-specific increase in annexin V positivity relative to control cells was computed using an established equation.32
We finally determined the actions of PAS on normal myeloid hematopoietic progenitor cells. Bone marrow mononuclear cells from 2 normal donors were incubated with 10 or 20 μM PAS, washed, and plated in assays for CFU-GM and BFU-E (Table 4). The data show that incubation with 20 μM PAS for 16 hours, an interval in excess of the 10 hours required for maximal commitment of CLL cells to PAS-induced apoptosis (supplemental Figure 2), resulted in only a modest impact on the ability of either type of progenitor to give rise to colonies.
Effect of PAS on hematopoietic colony formation
| . | 0 μM PAS . | 10 μM PAS . | 20 μM PAS . |
|---|---|---|---|
| Donor 1 | |||
| CFU-GM | 348 | 363 (104) | 272 (78) |
| BFU-E | 284 | 379 (133) | 280 (98) |
| Donor 2 | |||
| CFU-GM | 184 | 180 (98) | 154 (84) |
| BFU-E | 204 | 192 (94) | 178 (87) |
| . | 0 μM PAS . | 10 μM PAS . | 20 μM PAS . |
|---|---|---|---|
| Donor 1 | |||
| CFU-GM | 348 | 363 (104) | 272 (78) |
| BFU-E | 284 | 379 (133) | 280 (98) |
| Donor 2 | |||
| CFU-GM | 184 | 180 (98) | 154 (84) |
| BFU-E | 204 | 192 (94) | 178 (87) |
Bone marrow cells from 2 normal donors were incubated for 18 hours with the indicated concentrations of PAS. The cells were washed and seeded into hematopoietic colony assays as described in ″Methods.″ Numbers in parentheses represent the percentage yield of colonies relative to the control.
Discussion
The data herein show that PAS induced apoptosis of CLL cells within 24 hours, with maximum killing at 48 hours. PAS treatment resulted in a proapoptotic Bax conformational change, Bax movement from the cytosol to the mitochondria, cytochrome c release from mitochondria to cytosol, and subsequent PARP cleavage, confirming that cell killing occurred via the intrinsic apoptotic pathway. Apoptosis correlated with the transcriptionally mediated up-regulation of the proapoptotic BH3 protein Noxa, as assessed by qRT-PCR and Western blotting. PAS-induced Noxa was predominantly located in the mitochondrial/organellar fraction. We demonstrated additionally that Noxa was stably bound to the antiapoptotic protein Bcl-2, which is itself largely associated with the mitochondria.
Lymphocytes and thymocytes from Noxa knockout mice were not protected against etoposide and gamma radiation–induced apoptosis,44,45 suggesting that Noxa was a less potent inducer of apoptosis than were Puma and Bim. However, other data have suggested that Noxa is a significant inducer of apoptosis in diverse cell types,19,22,41,46-48 including CLL cells.40
Noxa was initially identified as a target of p53-mediated transcription.22 However, PAS treatment did not induce p53 in cells of CLL patients with functional p53 genes, suggesting that Noxa elevation by PAS occurred via a p53-independent pathway. Therefore, we investigated the effect of PAS on cells from CLL patient with greater than 97% p53 deletion. Two of 3 such isolates were as susceptible to PAS as were CLL cells with functional p53. Furthermore, Noxa was readily induced in p53-deleted cells, confirming that Noxa induction by PAS was not dependent on the p53 pathway.
Our observations suggest that PAS-induced Noxa elevation was not the result of inhibition of either histone deacetylases or of the proteasome by PAS. Noxa can also be induced by other p53-independent mechanisms, including transcriptional pathways mediated by E2F1,19 c-Myc,20 or FoxO3a.21 The signaling pathways involved in PAS-induced p53-independent Noxa expression warrant further investigation.
Noxa has been shown to preferentially bind to the antiapoptotic protein Mcl-1,49 but it also binds to other antiapoptotic proteins, including Bcl-xL and Bcl-2,23 although with lower affinity. We have shown in the experiments reported herein that Noxa interacts with Bcl-2, the predominant antiapoptotic protein of CLL cells.18 This observation adds weight to the hypothesis that induction of Noxa by PAS is a major component of the mechanism by which PAS induces apoptosis of CLL cells. In contrast to Noxa, the BH3-only protein Puma was constitutively expressed by CLL cells and was not further induced by PAS. We suggest that constitutive Puma is prevented from inducing apoptosis by the overexpression of the antiapoptotic Bcl-2 protein,18 which is known to sequester Puma.24
The proapoptotic BH3-only activator protein Bim was also shown to be constitutively expressed and bound to Bcl-2 in CLL cells.18 This observation, which was confirmed in the present study, may explain why CLL cells appear to be primed for death, requiring only a small shift in the ratio of pro- and antiapoptotic proteins to induce apoptosis. It is plausible that Noxa up-regulation by PAS may displace Bcl-2–bound Bim, which would then be able to interact with Bax, inducing a proapoptotic conformational change.16 Our immunoprecipitation studies on lysates from PAS-treated cells showed that Bcl-2–bound Bim was indeed decreased concomitant with the binding of Noxa to Bcl-2. However, we failed to detect the displaced Bim in the immunoprecipitation supernatants, but it is possible that Noxa displaces Bim from Bcl-2, allowing a transient proapoptotic interaction between Bim and Bax before subsequent rapid degradation of Bim. Alternatively, PAS treatment may result in an overall decrease in Bim protein levels, while concomitant induction of Noxa overrides the decrease in Bim and induces apoptosis. The latter hypothesis is consistent with observations that pre-B cells from Bim knockout mice undergo apoptosis after gamma irradiation.44 In addition, Bim was shown to be dispensible for UV-induced apoptosis of mouse embryo fibroblasts, which was primarily driven via Puma and Noxa.48
Recent studies have shown that p53 can induce apoptosis via a nontranscriptional pathway, in addition to its well-known transcription-dependent mechanism.50-52 Strom et al28 showed that PAS (referred to as pifithrin-μ by Strom et al28 ) apparently blocked the binding of p53 to mitochondrial antiapoptotic proteins Bcl-2 and Bcl-xL in Saos-2 cells ectopically expressing p53 via viral transduction. They also showed that PAS protected mice from doses of radiation that caused lethal hematopoietic syndrome,28 and suggested that this compound prevented apoptosis induction by blocking the nontranscriptional death-inducing action of p53. In contrast, we have shown for the first time that PAS induced p53-independent apoptosis in CLL cells and normal B lymphocytes. PAS also induced killing of leukemic cell lines, including Daudi, Raji, and K562 (A.J.S., unpublished observations, December 2007). These strikingly discordant observations may be accounted for by the use by Strom et al28 of a p53-negative Saos-2 cell line engineered to express p53 via lentiviral transduction, whereas the p53 system in the cell types studied by us was under normal cellular regulation.
In summary, we have shown herein that PAS selectively induces apoptosis of CLL cells in a manner that is apparently dependent ontranscriptional up-regulation of the proapoptotic BH3-only protein Noxa, but independent of p53. The data also emphasize the potent apoptosis-inducing action of Noxa in CLL cells. We suggest that PAS, a low-molecular-mass compound with a simple structure, may be a useful novel agent for the treatment of CLL and especially of patients who have become refractory to standard cytotoxic drugs due to the loss of p53.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgment
This study was supported by the Leukemia Research Fund, United Kingdom.
Authorship
Contribution: A.J.S., A.G.P., and R.G.W. designed studies, analyzed data, and wrote the paper; A.J.S., B.C.Y., A.C., S.H., J.N., M.W.L., E.R.S., E.N., and R.G.W. carried out research; and A.G.P., A.V.H., P.K., and K.C. recruited and obtained written consent from patients and collated clinical data used in this study.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: R. Gitendra Wickremasinghe, Department of Hematology, Royal Free and University College Medical School, Rowland Hill St, London NW3 2PF, United Kingdom; e-mail: r.wickremasinghe@medsch.ucl.ac.uk.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal