Abstract
Chronic lymphocytic leukemia (CLL) is characterized by cells that exhibit dysfunctional apoptosis. Here, we show that deacetylase inhibition led to the E2F1- and myc-mediated transcriptional activation of the microRNA miR106b in primary CLL cells. Induction of miR106b was associated with a down-regulation in the levels of the E3-ubiquitin ligase Itch. Decreases in Itch protein levels were associated with a reciprocal accumulation of its proapoptotic substrate, TAp73 (p73), and induction of p53 up-regulated modulator of apoptosis (PUMA) mRNA and protein. This event was accompanied by mitochondrial dysfunction, processing of caspase-9, and apoptosis of CLL cells. Ectopic expression of miR106b in CLL cells demonstrated that Itch was a direct target of miR106b such that miR106b-induced decreases in Itch resulted in an accumulation of p73. Thus, our results identify a novel regulatory mechanism wherein microRNA regulate cell survival by mediating the posttranscriptional down-regulation of an ubiquitin ligase, leading to the induction of a proapoptotic regulator in malignant cells. Silencing of miRNA expression in CLL may selectively suppress proapoptotic pathways, providing such tumors with a survival advantage. Consequently, chemotherapeutic drugs that activate miR106b could initiate a p53-independent mechanism that targets CLL cells.
Introduction
MicroRNAs (miRNAs) are a new class of evolutionarily conserved small RNAs that regulate gene expression at a posttranscriptional level by blocking translation or degrading target messenger RNAs (mRNAs).1,2 The precise biologic effects of miRNAs are yet to be elucidated in detail, partly because each miRNA is predicted to negatively regulate the expression of several target genes,3 depending on the tissue in which it is expressed.
Chronic lymphocytic leukemia (CLL) is characterized by a global underexpression of miRNA levels,2,4 raising the possibility that down-regulation of miRNA may be associated with not only the pathogenesis but also the resistance to apoptosis that is characteristic of this disease. The mechanisms underlying the down-regulation of miRNA in CLL are largely unknown; DNA methylation accounts for a small fraction of the aberrantly silenced genes in CLL.5 An alternative possibility is the silencing of miRNA expression by the adoption of an inactive chromatin configuration driven by the activity of deacetylases. If this is true, strategies that promote histone acetylation would be expected to induce miRNA expression.
Deacetylase inhibitors are a class of chromatin-modulating agents that function by promoting histone acetylation at gene promoters, thereby inducing a transcriptionally active chromatin configuration and epigenetically influencing gene expression.6,7 Deacetylase inhibitors have been shown to have potent antitumor activity in many preclinical models,7-9 including CLL.10,11 However, it is unknown whether deacetylase inhibition in CLL would alter miRNA expression so as to functionally influence cell survival. In clinical trials, this class of agents had activity against cutaneous T-cell lymphomas12 and acute myelogenous leukemia,13-15 but initial studies with depsipeptide were associated with toxicity in CLL.16 Systematic trials with newer generation deacetylase inhibitors in CLL would help in evaluating this class of agents for the therapy of CLL.
The p53 tumor suppressor family members, p53 and p73, orchestrate the cellular response to stimuli that induce apoptosis.17 In CLL, p53 mutations can accumulate as a consequence of DNA-directed therapy,18 a phenomenon associated with resistance to such chemotherapy and with aggressive disease.19 p73 protein, on the other hand, is rarely mutated in human cancer.20,21 p73 proteins exist as transactivation proficient (TAp73) or deficient (ΔN73) isoforms that are generated by differential splicing of the p73 transcript.22 TAp73 (referred to herein as p73), the proapoptotic form of p73, efficiently binds to p53 response elements on gene promoters, to transcriptionally induce the expression of p53-regulated genes.23
Under normal circumstances, the levels and activity of p73 are maintained at low levels because of the constitutive activity of Itch,24 a member of the HECT family of ubiquitin E3 ligases. Itch binds p73 via a C-terminal PPPY motif that is absent in the p53 protein24 and selectively targets p73 for poly-ubiquitylation and degradation via the ubiquitin proteosome pathway.24 Down-regulation of Itch has been associated with a decreased level of ubiquitylation on p73 and subsequent stabilization of protein levels.24 On stabilization, p73 associates with specific transcriptional coactivators25 that promote acetylation of the protein, further increase its stability by preventing access to Itch,26 and preferentially recruit p73 to a subset of p53-transactivated promoters, such as that of the proapoptotic genes CD95,22 p53AIP,25 and PUMA.23,27 Consequently, p73 can function in place of p53 to facilitate induction of apoptosis.23 Although Itch plays a critical role in regulation of p73 levels, the mechanisms by which Itch is down-regulated in response to cellular stress are unknown.
The purpose of this investigation was to determine whether deacetylase inhibition induces miRNAs that regulate p73-dependent apoptotic signaling and death in quiescent CLL cells.
Methods
Clinical samples
Forty-six samples from patients with CLL were used in this study. Samples were obtained after informed consent from patients at the CLL Research Consortium institutions, in accordance with the Declaration of Helsinki. This study was approved by the Institutional Review Board of CLL Research Consortium. Briefly, blood was obtained from CLL patients and isolated through Ficoll-Hypaque gradient centrifugation (GE Healthcare, Little Chalfont, United Kingdom) and processed for RNA extraction, protein lysates, determination of cell death (annexin positivity), and mitochondrial integrity (tetramethylrhodamine, methyl ester, perchlorate [TMRM]) or used for transfection. For the majority of samples, genetic information, such as fluorescent in situ hybridization (FISH) cytogenetics, to evaluate the p53 status was available (Table 1).
Genetic features and the individual response of the parameters investigated within each CLL sample to establish the miRNA106b-initiated signaling cascade, after exposure to LBH589 or MS275
| Patient no. . | Percentage of cells with del17p (p53)* . | AcH3 . | E2F1 . | MCM7 . | miRNA 106b . | Itch protein . | p73 protein . | PUMA mRNA . | PUMA protein . | Cleaved caspase-9 . | Annexin positivity . | ZAP70 . | p73 mRNA . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 0 | ↑ | —† | ↓ | ↑ | ↑ | ↑ | ↑ | ↑ | positive | ↓ | ||
| 2 | 0 | ↑ | — | ↓ | ↑ | — | ↑ | ↑ | ↑ | — | — | ||
| 3 | 0 | ↑ | — | ↓ | ↑ | — | ↑ | ↑ | ↑ | positive | — | ||
| 4 | —‖ | ↑ | — | ↓ | ↑ | ↑ | ↑ | ↑ | ↑ | — | ↓ | ||
| 5 | 69% | ↑ | — | ↓ | ↑ | — | ↑ | ↑ | ↑ | positive | — | ||
| 6 | 12 double deletion, 9 monosomy‖ | ↑ | — | ↓ | ↑ | ↑ | ↑ | ↑ | ↑ | positive | ↑ | ||
| 7 | 0 | ↑ | neg | ∼ | ∼ | ↓ | ↑ | — | ↑ | ↑ | ↑ | — | — |
| 8 | 36 | ↑ | + | ↑ | ↑ | ↓ | ↑ | — | not detectable | ↑ | ↑ | — | — |
| 9 | 89 | ↑ | + | ↑ | ↑ | ↓ | ↑ | — | ↑ | ↑ | ↑ | positive | — |
| 10 | 0 | ↑ | — | ↓ | ∼‡ | ∼‡ | not detectable | partial | < 20%§ | negative | ↓ | ||
| 11 | 0 | ↑ | — | ↓ | ↑ | ↑ | ↑ | ↑ | ↑ | positive | ↓ | ||
| 12 | 18 | ↑ | — | ↓ | ↑ | ↓ | ↑ | ↑ | ↑ | — | ↑ | ||
| 13 | 0 | ↑ | — | ↓ | ∼‡ | ↑ | ↑ | ↑ | ↑ | — | ↓ | ||
| 14 | 0 | ↑ | — | ↓ | ↑ | — | ↑ | ↑ | ↑ | positive | — | ||
| 15 | 0 | ↑ | — | ↓ | ↑ | ↑ | ∼ | ↑ | ↑ | negative | ↓ | ||
| 16 | 67.5 | ↑ | — | ↓ | ↑ | ↑ | ↑ | ↑ | ↑ | — | ↑ | ||
| 17 | 0 | ↑ | — | ↓ | ↑ | ∼ | ↑ | ↑ | ↑ | — | ↓ | ||
| 18 | 0 | ↑ | — | ↓ | ↑ | ↑ | ↑ | ↑ | ↑ | positive | ∼ | ||
| 19 | 0 | ↑ | — | ↓ | ↑ | ↑ | ↑ | ↑ | ↑ | positive | ↑ | ||
| 20 | 0 | ↑ | — | ↓ | ∼ | ↓ | ↑ | ↑ | ↑ | — | ↓ | ||
| 21 | 86 | ↑ | + | ↑ | ↑ | ↓ | ∼ | ↓ | ↓ | ↑ | < 20% | positive | ↓ |
| 22 | 9.5 | ↑ | + | ↑ | ↑ | ↓ | ↑ | ↑ | ↑ | ↑ | ↑ | positive | ↓ |
| 23 | 70 | ↑ | — | ↓ | ↑ | ↓ | ↓ | ↑ | ↑ | — | ↑ | ||
| 24 | 0 | ↑ | — | ↓ | ↑ | ↑ | ↑ | ↑ | ↑ | positive | ↓ | ||
| 25 | 82 | ↑ | neg | ∼ | ∼ | ↓ | ↑ | ↓ | ∼ | ↑ | ↑ | — | ↓ |
| 26 | 93 | ↑ | + | ↑ | ↑ | ↓ | ↑ | — | ↑ | ↑ | ↑ | positive | — |
| 27 | 0 | ↑ | + | ∼ | ∼ | ↓ | ↑ | — | ↑ | ↑ | ↑ | negative | — |
| 28 | 0 | ↑ | + | ↑ | ↑ | ↓ | ↑ | — | ↑ | ↑ | ↑ | negative | — |
| 29 | 0 | ↑ | neg | ∼ | ∼ | ↓ | ∼ | — | ↑ | ∼ | < 20% | positive | — |
| 30 | 0 | — | — | — | — | — | — | — | — | positive | — | ||
| 31 | 0 | ↑ | — | ↓ | ↑ | ↑ | ∼ | ↑ | ↑ | — | ↑ | ||
| 32 | 0 | ↑ | + | ↑ | ↑ | ↓ | ↑ | ↑ | ∼ | ↑ | ↑ | positive | ↓ |
| 33 | — | ↑ | + | ↑ | ↑ | ↓ | ↑ | ↑ | ↑ | ↑ | ↑ | — | — |
| 34 | 18 | — | — | — | — | ↑ | — | — | — | positive | — | ||
| 35 | 0 | ↑ | + | ∼ | ↑ | ↓ | ↑ | — | ↑ | ↑ | ↑ | positive | — |
| 36 | 0 | ↑ | neg | ∼ | ∼ | ↓ | ↑ | — | ↑ | ↑ | ↑ | positive | — |
| 37¶ | 0 | ↑ | — | — | ∼ | ∼ | ∼ | — | — | partial | < 20% | positive | — |
| 38¶ | 0 | ↑ | + | ↑ | ↑ | ↓ | ↑ | — | — | ↑ | ↑ | positive | — |
| 39** | 0 | ↑ | + | ↑ | ↑ | ↓ | ↑ | — | — | ↑ | ↑ | positive | — |
| 40 | 0 | ↑ | + | ↑ | ↑ | ↓ | ↑ | — | — | ↑ | ↑ | negative | — |
| 41 | 0 | ↑ | + | ↑ | ↑ | ↓ | ↑ | — | — | ↑ | ↑ | positive | — |
| 42 | 0 | ↑ | + | ↑ | ↑ | ↓ | ↑ | — | — | ↑ | ↑ | — | — |
| 43 | 67 | ↑ | + | ↑ | ↑ | positive | |||||||
| 44 | — | neg | ∼ | ∼ | — | ||||||||
| 46 | — | ↑ | + | ∼ | ∼ | — | |||||||
| 47 | — | ↑ | + | ↑ | ↑ | — |
| Patient no. . | Percentage of cells with del17p (p53)* . | AcH3 . | E2F1 . | MCM7 . | miRNA 106b . | Itch protein . | p73 protein . | PUMA mRNA . | PUMA protein . | Cleaved caspase-9 . | Annexin positivity . | ZAP70 . | p73 mRNA . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 0 | ↑ | —† | ↓ | ↑ | ↑ | ↑ | ↑ | ↑ | positive | ↓ | ||
| 2 | 0 | ↑ | — | ↓ | ↑ | — | ↑ | ↑ | ↑ | — | — | ||
| 3 | 0 | ↑ | — | ↓ | ↑ | — | ↑ | ↑ | ↑ | positive | — | ||
| 4 | —‖ | ↑ | — | ↓ | ↑ | ↑ | ↑ | ↑ | ↑ | — | ↓ | ||
| 5 | 69% | ↑ | — | ↓ | ↑ | — | ↑ | ↑ | ↑ | positive | — | ||
| 6 | 12 double deletion, 9 monosomy‖ | ↑ | — | ↓ | ↑ | ↑ | ↑ | ↑ | ↑ | positive | ↑ | ||
| 7 | 0 | ↑ | neg | ∼ | ∼ | ↓ | ↑ | — | ↑ | ↑ | ↑ | — | — |
| 8 | 36 | ↑ | + | ↑ | ↑ | ↓ | ↑ | — | not detectable | ↑ | ↑ | — | — |
| 9 | 89 | ↑ | + | ↑ | ↑ | ↓ | ↑ | — | ↑ | ↑ | ↑ | positive | — |
| 10 | 0 | ↑ | — | ↓ | ∼‡ | ∼‡ | not detectable | partial | < 20%§ | negative | ↓ | ||
| 11 | 0 | ↑ | — | ↓ | ↑ | ↑ | ↑ | ↑ | ↑ | positive | ↓ | ||
| 12 | 18 | ↑ | — | ↓ | ↑ | ↓ | ↑ | ↑ | ↑ | — | ↑ | ||
| 13 | 0 | ↑ | — | ↓ | ∼‡ | ↑ | ↑ | ↑ | ↑ | — | ↓ | ||
| 14 | 0 | ↑ | — | ↓ | ↑ | — | ↑ | ↑ | ↑ | positive | — | ||
| 15 | 0 | ↑ | — | ↓ | ↑ | ↑ | ∼ | ↑ | ↑ | negative | ↓ | ||
| 16 | 67.5 | ↑ | — | ↓ | ↑ | ↑ | ↑ | ↑ | ↑ | — | ↑ | ||
| 17 | 0 | ↑ | — | ↓ | ↑ | ∼ | ↑ | ↑ | ↑ | — | ↓ | ||
| 18 | 0 | ↑ | — | ↓ | ↑ | ↑ | ↑ | ↑ | ↑ | positive | ∼ | ||
| 19 | 0 | ↑ | — | ↓ | ↑ | ↑ | ↑ | ↑ | ↑ | positive | ↑ | ||
| 20 | 0 | ↑ | — | ↓ | ∼ | ↓ | ↑ | ↑ | ↑ | — | ↓ | ||
| 21 | 86 | ↑ | + | ↑ | ↑ | ↓ | ∼ | ↓ | ↓ | ↑ | < 20% | positive | ↓ |
| 22 | 9.5 | ↑ | + | ↑ | ↑ | ↓ | ↑ | ↑ | ↑ | ↑ | ↑ | positive | ↓ |
| 23 | 70 | ↑ | — | ↓ | ↑ | ↓ | ↓ | ↑ | ↑ | — | ↑ | ||
| 24 | 0 | ↑ | — | ↓ | ↑ | ↑ | ↑ | ↑ | ↑ | positive | ↓ | ||
| 25 | 82 | ↑ | neg | ∼ | ∼ | ↓ | ↑ | ↓ | ∼ | ↑ | ↑ | — | ↓ |
| 26 | 93 | ↑ | + | ↑ | ↑ | ↓ | ↑ | — | ↑ | ↑ | ↑ | positive | — |
| 27 | 0 | ↑ | + | ∼ | ∼ | ↓ | ↑ | — | ↑ | ↑ | ↑ | negative | — |
| 28 | 0 | ↑ | + | ↑ | ↑ | ↓ | ↑ | — | ↑ | ↑ | ↑ | negative | — |
| 29 | 0 | ↑ | neg | ∼ | ∼ | ↓ | ∼ | — | ↑ | ∼ | < 20% | positive | — |
| 30 | 0 | — | — | — | — | — | — | — | — | positive | — | ||
| 31 | 0 | ↑ | — | ↓ | ↑ | ↑ | ∼ | ↑ | ↑ | — | ↑ | ||
| 32 | 0 | ↑ | + | ↑ | ↑ | ↓ | ↑ | ↑ | ∼ | ↑ | ↑ | positive | ↓ |
| 33 | — | ↑ | + | ↑ | ↑ | ↓ | ↑ | ↑ | ↑ | ↑ | ↑ | — | — |
| 34 | 18 | — | — | — | — | ↑ | — | — | — | positive | — | ||
| 35 | 0 | ↑ | + | ∼ | ↑ | ↓ | ↑ | — | ↑ | ↑ | ↑ | positive | — |
| 36 | 0 | ↑ | neg | ∼ | ∼ | ↓ | ↑ | — | ↑ | ↑ | ↑ | positive | — |
| 37¶ | 0 | ↑ | — | — | ∼ | ∼ | ∼ | — | — | partial | < 20% | positive | — |
| 38¶ | 0 | ↑ | + | ↑ | ↑ | ↓ | ↑ | — | — | ↑ | ↑ | positive | — |
| 39** | 0 | ↑ | + | ↑ | ↑ | ↓ | ↑ | — | — | ↑ | ↑ | positive | — |
| 40 | 0 | ↑ | + | ↑ | ↑ | ↓ | ↑ | — | — | ↑ | ↑ | negative | — |
| 41 | 0 | ↑ | + | ↑ | ↑ | ↓ | ↑ | — | — | ↑ | ↑ | positive | — |
| 42 | 0 | ↑ | + | ↑ | ↑ | ↓ | ↑ | — | — | ↑ | ↑ | — | — |
| 43 | 67 | ↑ | + | ↑ | ↑ | positive | |||||||
| 44 | — | neg | ∼ | ∼ | — | ||||||||
| 46 | — | ↑ | + | ∼ | ∼ | — | |||||||
| 47 | — | ↑ | + | ↑ | ↑ | — |
neg indicates undetectable protein levels.
CLL samples were classified according their p53 FISH status.
No sample.
No change from control.
A total of 20% was used as a cutoff point for determining annexin positivity.
Negative in p21 functional assay.
CLL sample exposed to 3 μM SAHA.
CLL sample exposed to 3 μM MS275.
Determination of p53 functional status
The p53 status functional status was determined based on the induction of p21 protein in response to 10 Gy ionizing radiation for 2 hours in CLL cells. A 25% up-regulation in p21 levels was used as a cutoff point, as described.28
Cell lines and cell culture
H1299, HeLa, and K562 cells were purchased from ATCC (Manassas, VA). Cell lines and primary CLL cells were maintained in RPMI 1640 medium. Panabinostat (LBH589) was provided by Novartis (Basel, Switzerland). MS-275 and suberoylanilide hydroxamic acid (SAHA) were purchased from Axxora Life Sciences (San Diego, CA) and Cayman Chemical (Ann Arbor, MI). All reagents were dissolved in 100% dimethyl sulfoxide (Honeywell Burdick & Jackson, Muskegon, MI) to a stock concentration of 10−2 M and stored at −80°C. Cell lines and primary CLL cells were treated with 10 nM LBH589, 3 μM SAHA, or 3 μM MS275 for the indicated times.
Western blotting
Immunoblotting was performed with antibodies against H3AcK9/14, H3pSer10 (Millipore, Billerica, MA), myc (Santa Cruz Biotechnology, Santa Cruz, CA), E2F1 (Millipore, Billerica, MA), Itch (BD Biosciences, San Jose, CA), TAp73 (IMG-246, Imgenex, San Diego, CA), PUMA (ProSci, Poway, CA), caspase-9 (MBL, Nagoya, Japan), glyceraldehyde-3-phosphate dehydrogenase (GAPDH; Millipore), and actin antibody (Santa Cruz Biotechnology). Proteins were quantitated using the densitometry function on the Odyssey-LiCor, normalized to GAPDH or actin within the same sample and expressed as percent of control.
Real-time PCR
Total RNA was extracted from cells using the mir-Vana RNA extraction kit (Applied Biosystems, Foster City, CA). The relative quantities of miR106b and miR130a were measured on 5 ng of total RNA by quantitative reverse-transcribed polymerase chain reaction (qRT-PCR) using the mir-Vana reverse transcription kit followed by qPCR (Applied Biosystems) in the ABI PRISM 7700 Sequence Detection System (Applied Biosystems), and determined by the comparative CT method using snRNA RNU6B levels for normalization. The primer and probes for miR106b, miR130a, and RNU6B were obtained from Applied Biosystems. Relative expression of Mcm7, Itch, p73, GAPDH, and PUMA mRNA was performed using 20 to 500 ng total RNA with the ABI PRISM 7700 Sequence Detection System; all reagents except PUMA were purchased from Applied Biosystems. The PUMA primer probes were a kind gift from Dr Bingliang Fang (University of Texas M. D. Anderson Cancer Center).
ChIP assays and amplicon preparation
Chromatin immunoprecipitation (ChIP) assays (107 cells/assay) were performed using the EZ-ChIP kit from Millipore. The primary antibodies used in this study, H3AcK9, Pol II, E2F1, and nonspecific IgG control, were purchased from Millipore or provided with the EZ-ChIP kit. Myc was purchased from Santa Cruz Biotechnology. For PCR analysis of the ChIP samples before amplicon generation, QIAquick-purified immunoprecipitates were dissolved in 50 μL of water. Standard PCR reactions using 3 μL of the immunoprecipitated DNA were performed; primer sequences are indicated in Document S1 (available on the Blood website; see the Supplemental Materials link at the top of the online article). Calculation of transcription factor and Pol II occupancy on the miR106b promoter was performed according to the ChIP-qPCR primer assay data analysis template from Superarray Bioscience (Frederick, MD).
Luciferase assays
HeLa cells were cotransfected in 6-well plates with 1 μg of pGL3 firefly luciferase reporter vector containing the wild-type or mutated ITCH 3′ untranslated region (UTR), 0.5 μg of the β-galactosidase gene (Promega, Madison, WI), and 300 nM miRNA or scrambled precursors (Ambion, Austin, TX) using Fugene 6 (Roche Diagnostics, Indianapolis, IN). Forty-eight hours after transfection, the firefly luciferase and β-galactosidase activities were measured consecutively. Each reporter plasmid was transfected at least twice (on different days) in triplicate.
Microarray experiments, data analysis, and submission
RNA blot analysis was performed as described previously.29 All data were submitted using MIAMEXPRESS to the Array Express database (www.ebi.ac.uk) and can be retrieved using the accession number E-TABM-628. Each sample from the time course of treatment with LBH589 received ID numbers from P1_control-1 to P2_9h-2.
miRNA overexpression
Pre-miRNA precursor molecules miR106b and miR130a, antimiR-miRNA inhibitors miR106b AM and miR130aAM, and premiR-miRNA precursor molecules negative control #1 were obtained from Ambion. All miRNA were transfected at 300 nM using the Amaxa Biosystems nucleofector (Gaithersburg, MD) and the cell line nucleofector kit V, program U1330 into K562 and primary leukemia cells for 48 to 72 hours, after which cells were harvested for RNA and protein analysis. In standardization experiments, the transfection efficiency ranged between 41% and 65% based on the uptake of the fluorescent transfection indicator, siGLO (Dharmacon RNA Technologies, Lafayette, CO; data not shown).
Mitochondrial integrity and apoptosis assays
A total of 106 primary CLL cells was incubated with 25 nM TMRM (Invitrogen, Carlsbad, CA) and annexin V–fluorescein isothiocyanate (BD Biosciences) for 15 minutes. Fluorescence of at least 10 000 cells was determined on a BD Biosciences FACSCalibur flow cytometer to determine the mitochondrial membrane potential and percentage of apoptotic cells within the same population.
Results
Induction of p73 is associated with a down-regulation in the levels of the E3 ligase Itch in primary leukemia cells
To separate the miRNA-mediated regulation of p73 from the influence exerted by the p53 protein on p73 and its downstream targets, primary CLL samples were classified on the basis of the genomic integrity of p53 using interphase FISH techniques (Table 1). Homozygous or hemizygous deletions of the p53 locus (del17p), when present in at least 50% of the cells, are associated with a nonfunctional p53 protein that is characterized by an inability to up-regulate p21 in response to DNA damage.28 In our cohort, 18% (8 of 44) of the samples exhibited del17p in more than 50% of cells; further analysis in selected samples confirmed their inability to induce p21 protein in response to ionizing radiation (data not shown). Two additional samples were classified as p53-deficient solely on the basis of the p53 functional assay (Figure S1A). Overall, our results indicated that 23% (10 of 44) of the samples expressed a nonfunctional p53 protein. In contrast, samples with normal FISH cytogenetics that were assayed induced p21 protein in response to ionizing radiation (Figure S1A).
CLL cells exposed to LBH589, a pan deacetylase inhibitor, demonstrated a significant increase in the levels of p73 (35 ± 6 to 64 ± 7, n = 33), a proapoptotic member of the p53 family (Figure 1A). In contrast, the levels of p53 were either decreased or remained unchanged in the same leukemia samples (Figure S1B), suggesting that the cellular response of CLL cells to deacetylase inhibitors was probably independent of p53 function. Previous reports have indicated that transcriptional and posttranslational mechanisms contribute to the induction of p73.20 Therefore, the expression of p73 mRNA was quantified in 20 CLL samples exposed to LBH589. Our data demonstrate that p73 mRNA was induced in only 6 of the 20 samples exposed to LBH589, whereas p73 protein was increased in 16 of the samples evaluated in the aforementioned cohort (Figure S2), indicating that transcriptional induction of p73 mRNA was not the primary mechanism for the observed induction of p73 protein.
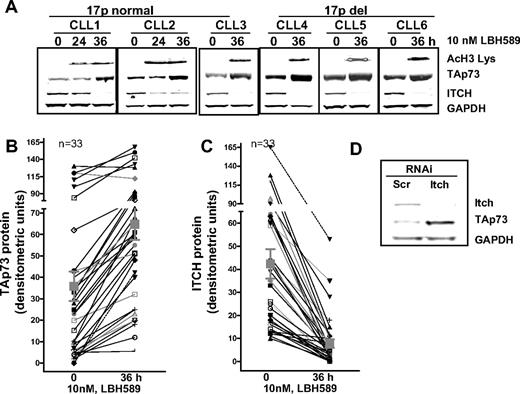
Inverse relation between p73 and Itch in primary CLL consequent to deacetylase inhibition. (A) Primary leukemia cells that were normal or del17p were treated with 10 nM LBH589 for 36 hours. Changes in acetylation levels of histone H3, expression levels of p73, Itch, and GAPDH were measured. (B,C) Quantification and inverse relation between p73 and Itch expression in response to LBH589 in 33 primary leukemia samples. The Pearson correlation between paired samples was conducted (P < .05, 2-tailed P value, Pearson correlation r = −0.4). (D) K562 cells were transfected with short interfering RNA that were scrambled or against Itch for 48 hours, followed by immunoblotting for Itch, p73, and GAPDH.
Inverse relation between p73 and Itch in primary CLL consequent to deacetylase inhibition. (A) Primary leukemia cells that were normal or del17p were treated with 10 nM LBH589 for 36 hours. Changes in acetylation levels of histone H3, expression levels of p73, Itch, and GAPDH were measured. (B,C) Quantification and inverse relation between p73 and Itch expression in response to LBH589 in 33 primary leukemia samples. The Pearson correlation between paired samples was conducted (P < .05, 2-tailed P value, Pearson correlation r = −0.4). (D) K562 cells were transfected with short interfering RNA that were scrambled or against Itch for 48 hours, followed by immunoblotting for Itch, p73, and GAPDH.
Significantly, the induction of p73 in leukemia samples was associated with a marked decline in the levels of the E3 ubiquitin ligase, Itch (42 ± 6 to 8 ± 1, n = 33; Figure 1A,C). Quantitative analysis of both p73 and Itch protein levels in paired CLL samples indicated that levels of p73 expression demonstrated an inverse correlation with the levels of the Itch protein after exposure to LBH589 (P < .05, 2-tailed P value, Pearson correlation r = −0.4, n = 33; Figure 1B compared with Figure 1C). In addition, 9 of 10 samples with nonfunctional p53 demonstrated the inverse relation between Itch and p73 (Table 1). Thus, CLL cells exposed to deacetylase inhibitors respond by down-regulating the levels of the E3 ligase Itch, which was associated with a reciprocal induction of the p73 protein, independent of p53 function.
To confirm the inverse relationship between Itch and p73,24 K562 leukemia cells were transfected with short interfering RNA against Itch. The levels of Itch were reduced (to ∼ 7% normalized expression) in comparison with K562 cells transfected with a nontargeting scrambled RNA (Figure 1D). Targeted down-regulation of Itch was associated with an increase in p73 levels in the same cells (Figure 1D).
Up-regulation of microRNA-106b in response to chromatin modifying agents in CLL
To determine the mechanisms underlying the decline in Itch protein, we hypothesized that exposure of primary leukemia cells to deacetylase inhibitors may specifically induce miRNA that target Itch, thereby initiating a signaling cascade that enables p73 apoptotic signaling. A bioinformatic search indicated that approximately 118 to 1331,32 miRNAs putatively target the 3′ UTR of Itch. To determine whether these miRNA were up-regulated in response deacetylase inhibitors, a miRNA array was used to compare the miRNA expression profile of 2 independent CLL samples that were exposed to 10 nM LBH589 for 3 or 5 hours with those of untreated cells. The preliminary data indicated that a large complement of mature and precursor miRNA were up-regulated after exposure to LBH589, including some that were predicted to target Itch (Table S1). However, only one mature miRNA computationally predicted to target Itch, miR106b, was up-regulated 2.6- and 5.8-fold, respectively, in both of the CLL samples exposed to the deacetylase inhibitor and hence was chosen for further evaluation.
miR106b is located as a cluster with miRs 93-25 on intron 13 of the Mcm7 on chromosome 7q22 and actively cotranscribed in the context of the Mcm7 primary RNA transcript.33 Therefore, the responses of Mcm7 and miR106b to LBH589 treatment for 3 or 6 hours were quantitated in 20 CLL samples (5 with del17p). Twelve samples demonstrated increases in Mcm7 and miR106b expression (using a 1.4-fold induction as a cutoff point) on exposure to LBH589 (Figures 2A and S3A). One additional sample showed an induction of miR106b without corresponding increases in Mcm7. Conversely, 7 samples that did not up-regulate miR106b in response to LBH589 also failed to induce Mcm7 expression (Figure 2A). Thus, our data indicate the exposure of CLL cells to deacetylase inhibitors results in the transcriptional induction of miR106b, largely in conjunction with its host gene.
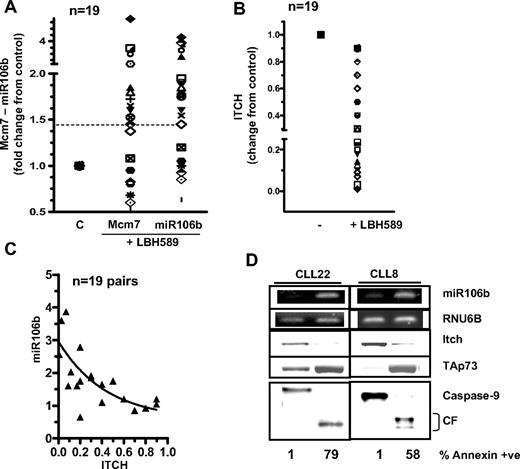
Up-regulation of miR106b levels in response to deacetylase inhibitors in primary leukemia cells. (A) Quantitation of Mcm7 and miR106b and in 20 primary leukemia cells exposed to LBH589. (B) Quantitation of Itch in the primary leukemia samples evaluated in panel A. (C) Pearson correlation demonstrating the inverse relation between miR106b and ITCH in paired samples (P < .005, 2-tailed P value, Pearson correlation r = −0.7). (D) Levels of miR106b, RNU6B (loading control for small RNA) as analyzed by qRT-PCR, are compared with the protein levels of Itch, p73, caspase-9 (CF indicates cleaved fragment), and annexin positivity in representative CLL samples.
Up-regulation of miR106b levels in response to deacetylase inhibitors in primary leukemia cells. (A) Quantitation of Mcm7 and miR106b and in 20 primary leukemia cells exposed to LBH589. (B) Quantitation of Itch in the primary leukemia samples evaluated in panel A. (C) Pearson correlation demonstrating the inverse relation between miR106b and ITCH in paired samples (P < .005, 2-tailed P value, Pearson correlation r = −0.7). (D) Levels of miR106b, RNU6B (loading control for small RNA) as analyzed by qRT-PCR, are compared with the protein levels of Itch, p73, caspase-9 (CF indicates cleaved fragment), and annexin positivity in representative CLL samples.
Importantly, up-regulation of miR106b (3 of 4 with del17p) was associated with a concomitant decrease in Itch protein (Figure 2B) in the same samples (Table 1), indicating an inverse correlation between LBH589-induced up-regulation in the levels of miR106b and the observed declines in the levels of ITCH (Figure 2C, P < .005, 2-tailed P value, Pearson correlation r = −0.7). The interrelationship between miR106b, Itch, its ubiquitin target, p73, caspase-9 and apoptosis was further evaluated in response to deacetylase inhibitors in primary leukemia cells (Figure 2D). The results indicate that exposure of CLL cells to LBH589 resulted in an induction in the levels of miR106b that were associated with declines in the levels of Itch. This in turn was associated with increases in the levels of p73 and apoptotic cell death. This reciprocal signaling cascade was not influenced by p53 function.
Modifications of specific lysines on histone H3 and increased recruitment of RNA polymerase II, E2F1, and myc to the miR106b promoter in response to LBH589
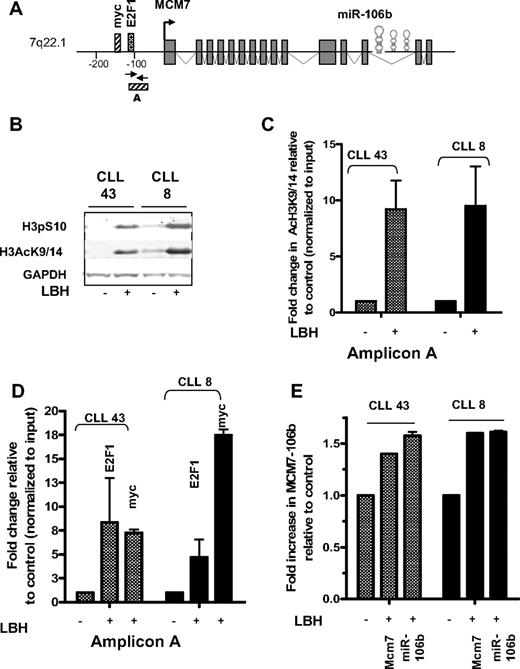
To evaluate the mechanisms by which miR106b is transcriptionally induced in response to deacetylase inhibitors, we investigated the modifications that occurred on specific lysines of histone H3 around the Mcm7-miR106b promoter before and after exposure to LBH589 for 5 hours. Covalent modifications, such as phosphorylation of Ser10 (H3pS10) or acetylation of lysines 9 or 14 (H3AcK9/14) around transcription start sites, are associated with the establishment of an open chromatin structure and transcriptional activation of genes.34 The structure and regulatory elements of the Mcm7-miR106b gene35 are shown (Figure 3A).
Pol II, E2F1, and myc are recruited to the miR106b promoter in response to deacetylase inhibition. (A) Structure and regulation of the Mcm7-miR106b gene. (B) Phosphorylation of serine 10 and acetylation of lysine 9/14 on histone H3 in response to LBH589. (C) Increase in H3AcK9/14 at Amplicon A within the promoter of miR106b from 2 individual CLL samples. (D) Increased recruitment of E2F1 and myc to the promoter of miR106b in the same CLL samples. (E) Coordinate expression of Mcm7 and miR106b by qRT-PCR in the aforementioned CLL samples.
Pol II, E2F1, and myc are recruited to the miR106b promoter in response to deacetylase inhibition. (A) Structure and regulation of the Mcm7-miR106b gene. (B) Phosphorylation of serine 10 and acetylation of lysine 9/14 on histone H3 in response to LBH589. (C) Increase in H3AcK9/14 at Amplicon A within the promoter of miR106b from 2 individual CLL samples. (D) Increased recruitment of E2F1 and myc to the promoter of miR106b in the same CLL samples. (E) Coordinate expression of Mcm7 and miR106b by qRT-PCR in the aforementioned CLL samples.
Exposure to LBH589 caused a significant increase in the levels of phosphorylated H3 on serine 10 and acetylated H3 on lysine 9 and 14 in leukemia cells (Figure 3B). To gain a further understanding of the covalent modifications to the chromatin around the promoter region of this miRNA, ChIP assays were used in conjunction with real-time PCR. Exposure to LBH589 caused a 10- to 12-fold increase in H3AcK9/14 around the E2F1/myc site (Amplicon A) within the Mcm7-miR106b promoter as shown in 2 representative samples of 4 that were evaluated (Figure 3C). This modification was associated with a recruitment of RNA Pol II to the promoter near the transcription start site (data not shown).
Activation marks on chromatin promote an open chromatin conformation that allows access to, and the recruitment of, additional transcription factors to the promoters of target genes. Ectopic expression of E2F1 and myc has been shown to activate the Mcm7 gene.35,36 Therefore, recruitment of E2F1 and myc to the Mcm7-miR106b promoter was evaluated in response to LBH589. There was 5- to 12-fold increase in E2F1 recruitment and an 8- to 15-fold increase in myc recruitment to the Mcm7-miR106b promoter within 5 hours of exposure to LBH589 in the region defined by Amplicon A (Figure 3D). Similar results were obtained for the other 2 CLL samples (data not shown). The concurrent presence of active chromatin marks on histone H3, along with the recruitment of Pol II, E2F1, and myc to the Mcm7-miR106b promoter of primary leukemia samples in response to LBH589, was associated with a transcriptional induction of both Mcm7 and miR106b mRNA (Figure 3E). Together, these findings provide evidence that deacetylase inhibition led to the acetylation of histones around the Mcm7-miR106b promoter and facilitated the recruitment of E2F1 and myc to the Mcm7-miR106b promoter. These events probably promote the transcriptional induction of miR106b in conjunction with its host gene Mcm7.
E2F1 and myc regulate the miR106b promoter response to LBH589
To further evaluate the requirement for E2F1 and myc in the induction of miR106b, 2 related constructs, which either contained the proximal E2F1 and myc sites (A8) or harbored mutations at these sites, were used (Figure S4A).35 These constructs have previously been shown to confer responsiveness to exogenously expressed E2F1 or myc.35,36 On transfection into H1299 cells, the constructs with the functional E2F1 and myc sites (A8) induced miR106b-reporter luciferase activity 2- to 4-fold in response to LBH589 treatment (Figure S4B). In contrast, mutation of the E2F1 and myc binding sites abrogated the induction of miR106b promoter in response to LBH589 (Figure S4B). These results indicated the requirement for E2F1 and myc for the deacetylase-induced transcriptional induction of miR106b.
To validate this finding in vivo, we analyzed E2F1 and myc expression in 24 primary CLL samples by Western blot. In one representative sample (Figure S4C), the levels of E2F1 were low in pretreatment samples but were induced within 3 to 6 hours of exposure to LBH589. Of the 4 samples that lacked myc expression, 3 also lacked E2F1 expression. With one exception, samples that lacked myc did not up-regulate Mcm7-miR106b transcripts (Figure S4D). More importantly, there was a strict positive correlation between expression of E2F1 (17 of 23) and up-regulation of Mcm7-miR106b, indicating that E2F1 probably positively regulates miR106b expression in response to LBH589 in primary CLL cells.
Identification of Itch as a target of miR106b
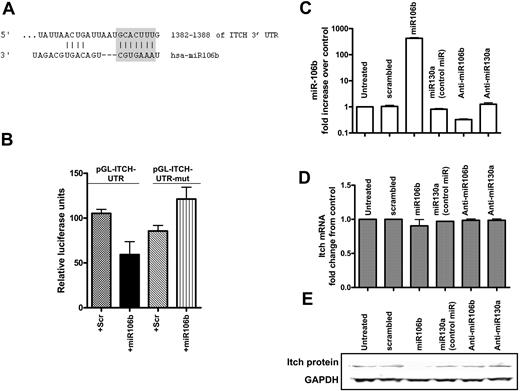
By analyzing the homology between miR106b and the Itch mRNA sequence, we found that 7 nucleotides from the 5′ end of this miRNA were complementary to the Itch cDNA (clone NM_031483; Figure 4A). To evaluate this putative interaction, precursor molecules for miR106b were cotransfected with reporter constructs containing the ITCH 3′UTR with the miR106b predicted binding site and either the 3′UTR in the antisense orientation or a mutated construct lacking the first 7 bases of the binding site, into HeLa cells. Our results indicate that overexpression of miR106b specifically inhibited the expression of the reporter construct containing the ITCH 3′UTR (Figure 4B), suggesting that miR106b directly interacts with the 3′UTR of the Itch mRNA.
Identification of Itch as a target of miR106b in K562 cells. (A) Complementary binding between miR106b and the 3′UTR of Itch. (B) Relative luciferase activity of luciferase reporter constructs containing full-length and either the antisense or mutated construct (values shown together as pGL-Itch-UTR mut) on addition of 300 nM of the precursor molecules for miR106b or scrambled miRNA. (C) Quantitation of miR106b expression in K562cells transfected with 300 nM precursor molecules for miR106b, miR130a (an unrelated miR not predicted to target Itch), anti-miR106bAM, anti-miR130aAM, and a nontargeting pre-miRNA control (scrambled) or left untreated for 48 hours. (D) The levels of Itch mRNA were quantitated after normalizing to GAPDH in the same samples. (E) Immunoblotting was performed for Itch and GAPDH in the same samples.
Identification of Itch as a target of miR106b in K562 cells. (A) Complementary binding between miR106b and the 3′UTR of Itch. (B) Relative luciferase activity of luciferase reporter constructs containing full-length and either the antisense or mutated construct (values shown together as pGL-Itch-UTR mut) on addition of 300 nM of the precursor molecules for miR106b or scrambled miRNA. (C) Quantitation of miR106b expression in K562cells transfected with 300 nM precursor molecules for miR106b, miR130a (an unrelated miR not predicted to target Itch), anti-miR106bAM, anti-miR130aAM, and a nontargeting pre-miRNA control (scrambled) or left untreated for 48 hours. (D) The levels of Itch mRNA were quantitated after normalizing to GAPDH in the same samples. (E) Immunoblotting was performed for Itch and GAPDH in the same samples.
To evaluate this interaction in cells, a nontargeting miRNA control (scrambled), precursor molecules encoding miRs 106b and 130a, and anti-miR inhibitors against 106b (AM106b) and 130a (AM130a) were transfected into K562 leukemia cells. miR130a was used in our study as an irrelevant control miRNA that is up-regulated in response to LBH589 (Figure S5) but is not predicted to target Itch.8 Figure 4C demonstrates that miR106b RNA is highly expressed in cells transfected with the precursor for miR106b, but not in cells expressing scrambled, miR130a, AM106b, or AM130a. Overexpression of miR106b was associated with a decrease in Itch protein levels (Figure 4E) but had no effect on Itch mRNA levels (Figure 4D). In contrast, expression of miR130a did not affect levels of Itch, confirming the specificity of the miR106b-Itch interaction.
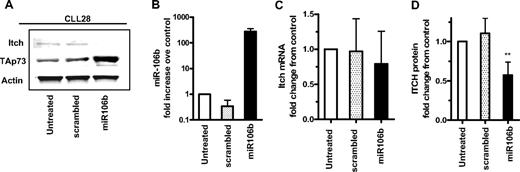
Parallel experiments were conducted in primary leukemia cells. Ectopic expression of miR106b, but not scrambled miRNA, was associated with a decrease in Itch protein and corresponding increases in p73 levels (Figure 5A). Figure 5B demonstrates the high levels of expression of miR106b in CLL cells transfected with the precursor miR106b or scrambled molecules. Again, enforced expression of miR106b caused a decline in Itch protein (P < .05; Figure 5D) but not mRNA (Figure 5C) as quantitated in 4 primary leukemia samples. Taken together, these studies identify Itch as a specific target for miR106b in cell lines and primary leukemia cells and demonstrate that up-regulation of miR106b is the apical step of a signaling cascade that leads to the activation of p73 via the down-regulation of Itch.
Identification of Itch as a target of miR106b in primary leukemia cells. (A) Primary leukemia cells were transfected with 300 nM precursor molecules for miR106b, nontargeting pre-miRNA control (scrambled) or left untreated for 72 hours. The expression levels of Itch, p73, and actin were evaluated. (B) Levels of miR106b quantitated by RT-PCR in leukemia cell samples transfected with miR106b or scrambled sequences as described in panel A. (C) Expression of Itch mRNA as normalized to GAPDH (C) and Itch protein as normalized to actin (D). Error bars represent SD from transfections of 2 independent leukemia samples analyzed in duplicate. One-way analysis of variance was performed for the quantified values of Itch protein under different transfection conditions with the Prism 5 software. ** Statistical significance (P < .05).
Identification of Itch as a target of miR106b in primary leukemia cells. (A) Primary leukemia cells were transfected with 300 nM precursor molecules for miR106b, nontargeting pre-miRNA control (scrambled) or left untreated for 72 hours. The expression levels of Itch, p73, and actin were evaluated. (B) Levels of miR106b quantitated by RT-PCR in leukemia cell samples transfected with miR106b or scrambled sequences as described in panel A. (C) Expression of Itch mRNA as normalized to GAPDH (C) and Itch protein as normalized to actin (D). Error bars represent SD from transfections of 2 independent leukemia samples analyzed in duplicate. One-way analysis of variance was performed for the quantified values of Itch protein under different transfection conditions with the Prism 5 software. ** Statistical significance (P < .05).
Induction of p73 is associated with an up-regulation of PUMA, mitochondrial dysfunction, and 53-independent cell death in primary leukemia cells
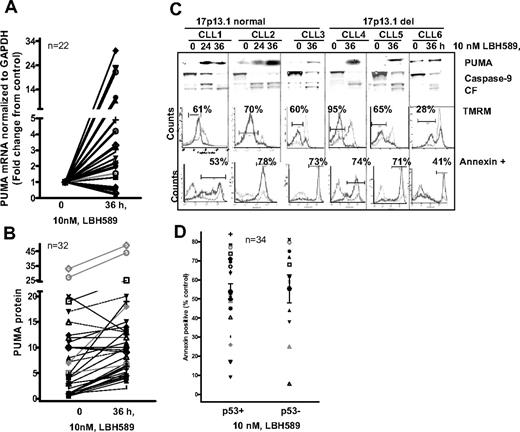
Overexpression paradigms have indicated a molecular link between p73, transactivation of PUMA, and mitochondrial-directed apoptosis.23,37 To determine whether this molecular pathway mediates apoptosis under therapeutically relevant conditions, the induction of PUMA mRNA and protein was evaluated in primary leukemia cells that were exposed to LBH589. Overall, PUMA mRNA was induced by 5- plus or minus 1-fold in 15 of 22 (4 of 6 with del17p) samples (Figure 6A), whereas PUMA protein was induced from 7-fold (± 1-fold) in control to 12-fold (± 1-fold) in 26 of 32 (7 of 10 with del17p) samples exposed to LBH589 (Figure 6B,C). These events were associated with processing of caspase-9 (Figure 6C), mitochondrial dysfunction as assessed by the loss of TMRM from within the mitochondria (Figure 6C), and p53-independent apoptosis (9 of 10 samples with nonfunctional p53, and 31 of 34 samples overall) as assessed by the increase in annexin positivity (Figure 6C,D) in primary CLL samples. Thus, LBH589-induced increases in p73 were significantly associated with the induction of apoptosis (Figure 1B compared with Figure 6D; P = .016, Fisher exact test, 2-tailed P value) in primary CLL cells by a process not influenced by p53.
Induction of p73 positively regulates PUMA to induce mitochondrial dysfunction and apoptosis in primary leukemia cells. (A) Induction of PUMA transcript in cells treated with 10 nM LBH589 for 36 hours. (B) Immunoblotting was performed on the samples in panel A to determine the levels of PUMA protein. (C top panels) Induction of PUMA was accompanied by the processing of caspase-9 (CF indicates cleaved fragment) in representative samples with or without deletions in 17p. (C middle panel) The same samples were evaluated for losses in TMRM at 48 hours, reflecting decreases in mitochondrial membrane integrity. (C bottom panel) The percentage of cell death at 48 hours was evaluated by annexin V staining in the same samples. (D) Quantitation of the apoptotic response of 34 primary leukemia samples that were normal (p53+) or with del17p (p53−) 10 nM LBH589 at 48 hours.
Induction of p73 positively regulates PUMA to induce mitochondrial dysfunction and apoptosis in primary leukemia cells. (A) Induction of PUMA transcript in cells treated with 10 nM LBH589 for 36 hours. (B) Immunoblotting was performed on the samples in panel A to determine the levels of PUMA protein. (C top panels) Induction of PUMA was accompanied by the processing of caspase-9 (CF indicates cleaved fragment) in representative samples with or without deletions in 17p. (C middle panel) The same samples were evaluated for losses in TMRM at 48 hours, reflecting decreases in mitochondrial membrane integrity. (C bottom panel) The percentage of cell death at 48 hours was evaluated by annexin V staining in the same samples. (D) Quantitation of the apoptotic response of 34 primary leukemia samples that were normal (p53+) or with del17p (p53−) 10 nM LBH589 at 48 hours.
To evaluate whether this signaling cascade was activated in response to histone deacetylation in general, CLL cells were exposed to the hydroxamate- and benzamide-based deacetylase inhibitors, MS275 and SAHA. Both miR106b (Figure S6A) and its host Mcm7 (Figure S6B) were up-regulated within 6 hours of exposure to MS275 and SAHA, respectively. Up-regulation of miR106b was associated with a decline in Itch levels, corresponding to induction of p73, caspase-9, and cell death (Figure S6C) in primary leukemia cells, demonstrating that these responses were associated with histone deacetylase inhibition, regardless of the specific inhibitory agent.
Discussion
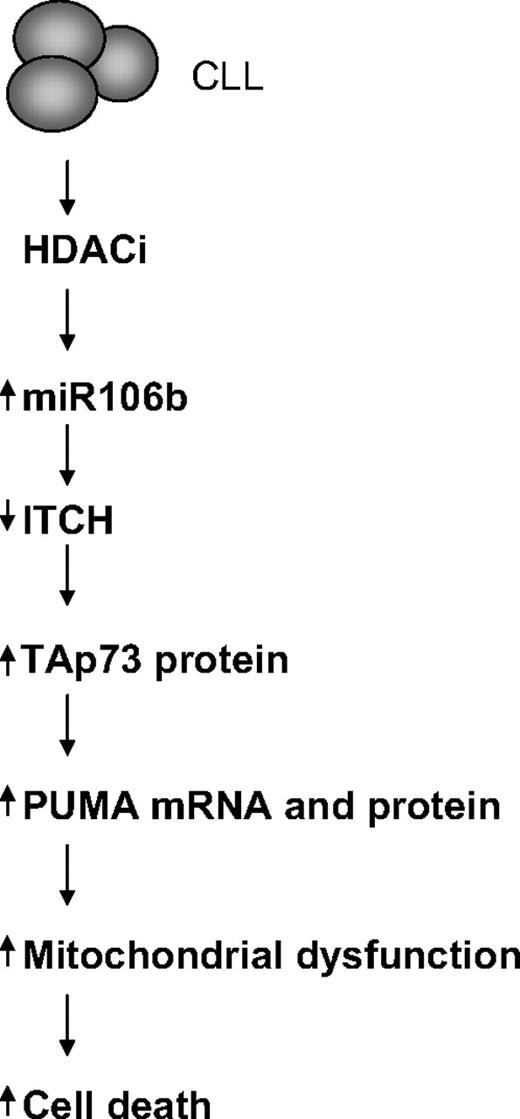
We hypothesized that deacetylase inhibition in CLL cells may transcriptionally up-regulate specific miRNA that in turn initiates a signaling cascade that activates p73 apoptotic signaling. In this study, we demonstrated that deacetylase inhibition facilitated an E2F1 and myc-regulated increase in miR106b that targeted the ubiquitin ligase Itch in primary CLL cells. Decreases in Itch were related to a posttranscriptional stabilization in the levels of p73 and associated with the transcriptional induction of PUMA, mitochondrial dysfunction, and CLL death (Figure 7).
The miR106b signaling cascade in CLL cells exposed to deacetylase inhibitors.
Deacetylase inhibition stabilized the acetylation of specific lysine residues on histone H3 around the Mcm7-miR106b promoter. This action was accompanied by the recruitment of E2F1 and myc to the promoter followed by transcriptional induction of Mcm-7-miR106b. E2F1 and the miRNAs regulated by it appear to have unique functions depending on the cellular context in which they are expressed. For instance, both E2F1 and miR106b are overexpressed in actively replicating gastric tumors where it mediated resistance to transforming growth factor-β by down-regulating p21.38 However, CLL being a quiescent tumor, both Mcm7 RNA and miR106b were undetectable or expressed at very low levels.39 Further, miR106b probably does not target p21 in this disease given that 80% of CLLs do not express this protein.40 Thus, it appears that, in quiescent cells, miR106b probably has targets other than p21 that may be physiologically relevant to the disease.
Experiments using ectopic expression of miR106b indicated that, in CLL, the E3 ligase Itch is a specific target of this miRNA and that miR106b-induced decreases in Itch were causally related to increases in p73 levels. Similarly, pharmacologic strategies that reversed the epigenetic silencing of miR106b expression in CLL cells were associated with a decrease in the levels of the E3 ligase Itch. However, it remains to be determined whether exogenous manipulations that either increase cellular miR106b levels or deplete Itch levels would serve to sensitize CLL cells to the action of deacetylase inhibitors. At a cellular level, Itch promotes the ubiquitylation and degradation of transcription factors, such as c-Jun,41 Notch,42 and p73.24 We focused on p73 because of its central role in mediating apoptosis.20,23 Using antibodies that specifically recognize the proapoptotic TAp73 isoform,43 we demonstrated that levels of TAp73 accumulate in response to the LBH589. Consequently, miR106b probably has a critical role in regulating cell survival in CLL through its effect on Itch, which in turn, acts as a negative regulator of the proapoptotic transcription factor, p73. However, a small subset of samples (5 of 19) demonstrated down-regulation of Itch and resultant induction of p73 despite lack of an induction of mir106b, suggesting the existence of alternate pathways that regulate Itch levels in response to deacetylase inhibition in this subgroup.
Induction of p73 was associated with increases in PUMA. The PUMA promoter has a p53/p73 response element and is an important mediator of mitochondrial dysfunction in response to cellular stress.23 However, because the p53 levels did not increase in CLL cells after exposure to LBH589, it seems evident that miR106b Itch-mediated increases in p73 were the event causally associated with the observed transcriptional induction of PUMA mRNA and protein in our samples.23 Deacetylase inhibition can activate other cellular signaling processes that converge at the level of caspase-9 processing and mitochondrial dysfunction.11 Although such mechanisms may also contribute to the appearance of processed caspase-9 and mitochondrial dysfunction in CLL cells exposed to deacetylase inhibitors, it is highly probable that the observed induction of PUMA in our samples was functionally associated with mitochondrial dysfunction and p53-independent death of primary CLL cells in our samples given the well-documented role of PUMA in mediating such processes.
In conclusion, quiescent tumors such as CLL may be characterized by the silencing of specific miRNA-regulated pathways. For instance, low levels of expression of miR106b may offer CLL cells a mechanism whereby the apoptotic potential of p73 is repressed. Deacetylase inhibitors facilitate an E2F1 and myc-regulated increase in miR106b, which in turn mediates the induction of the proapoptotic transcription factor, p73, by targeting the ubiquitin ligase Itch. These findings illustrate the existence of regulatory mechanisms wherein critical signaling pathways are modulated by miRNA in cancer cells. Chemotherapeutic drugs that activate miR106b could potentially circumvent the resistance associated with p53 dysfunction in CLL.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Peter Ataja for LBH589, Drs Lynne Abruzzo and Laura Rassenti for the p53 FISH analysis, Dr William Wierda for the providing clinical samples, and Yuling Chen and Brenita Tyler for technical assistance. The Mcm7 promoter constructs were a kind gift from Dr Tohru Kiyono (Nagoya University, Nagoya, Japan).
This work was supported by CLL Global Research Foundation (Houston, TX) and the National Institutes of Health (Bethesda, MD; grant CA81534).
National Institutes of Health
Authorship
Contribution: D.S. primarily designed and performed experiments and wrote the paper; G.A.C. and V.K.P. contributed to scientific design and performed experiments; C.T. analyzed, interpreted, and submitted microarray data; C.-G.L. performed microarray experiments; G.G., B.E., and C.L. performed experiments; M.J.K. helped with scientific design and contributed primary leukemia samples; and W.P. contributed to scientific design and interpretation of data and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Deepa Sampath, Department of Experimental Therapeutics, Box 71, University of Texas M. D. Anderson Cancer Center, 1515 Holcombe Blvd, Houston, TX 77030; e-mail: dsampath@mdanderson.org.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal