Abstract
Chronic lymphocytic leukemia (CLL) is a lymphoproliferative disease with a highly variable outcome. The prognosis of patients with CLL may be predicted using a number of biomarkers, including the level of CD38 expression at the leukemic cell surface. This study investigates the hypothesis that CD38 expression by CLL cells reflects interactions with nonmalignant cells within pseudofollicles in secondary lymphoid tissue where tumor cell proliferation is thought to occur. CD38 expression is higher in tissues that contain pseudofollicles compared with those that do not. In addition, we show that CD38 expression in CLL is dynamic, changes in response to contact with activated CD4+ T cells, and identifies cells that are primed to proliferate. Finally, we demonstrate close contact between activated CD4+ T cells and proliferating tumor in primary patient tissue. Proliferating tumor cells in lymph nodes express CD38, which is in turn associated with an increased number of CD31+ vascular endothelial cells. Although the factors resulting in colocalization of tumor, T cells, and endothelium remain unclear, the existence of these cellular clusters may provide an explanation for the association between CD38 expression and adverse outcome in CLL and suggests novel therapeutic targets.
Introduction
Chronic lymphocytic leukemia (CLL) is a low-grade lymphoproliferative disorder with a highly variable course whose clinical features arise through the accumulation of CD5+CD23+ B cells in the bone marrow, blood, and lymphoid tissue. Although the prognosis of CLL can be predicted by simple clinical parameters that reflect disease burden, such as the extent of lymphadenopathy and presence of bone marrow failure,1,2 these do not predict outcome for those with early stage disease
A number of biologic markers have recently been described that address this problem and allow the identification of poor risk patients with low bulk disease who might benefit from early more aggressive therapy. One of the most powerful of these biomarkers is the mutational status of the variable portion of the leukemic immunoglobulin heavy chain (IgVH) genes.3,4 Within the germinal center, normal B cells undergo somatic hypermutation of their IgVH genes after exposure to T-dependent antigens. Approximately 50% of CLL patients show evidence of somatic hypermutation, arbitrarily defined as a deviation of more than 2% from the germ line IgVH sequence. For reasons that are unclear, these patients have a far better prognosis than those with unmutated IgVH genes.
In one of the initial studies of IgVH mutational status in CLL, a high level of membrane CD38 expression by peripheral blood leukemic cells was shown to correlate with unmutated IgVH genes and poor prognosis, and CD38 was thus proposed as a surrogate marker for mutational status.3 Subsequent studies confirmed that CD38 is an important prognostic factor in CLL; however, expression levels were shown to be largely independent of IgVH gene mutational status.5-9 During the course of the disease, the level of expression of CD38 may increase,6-8,10 although patients with completely absent CD38 expression at the outset generally never express CD38.5
The CD38 gene encodes a type II single chain transmembrane protein that is expressed by most cells of the hematopoietic lineage at some time during their differentiation.11 It has a widespread distribution in other body tissues12 and may function as both a receptor and an ectoenzyme.13 In normal B lymphocytes, CD38 acts as an adhesion molecule and a receptor, signaling through which results in increased apoptosis of bone marrow-derived immature B cells14 but decreased apoptosis of human tonsillar germinal center B cells.15 In vitro studies have shown that interaction between CD38 and CD31, its cognate receptor, promotes the proliferation and survival of CLL cells both directly and through up-regulation of CD100,16 which interacts with plexin-B1 on stromal cells and some subsets of T cells.17 In keeping with this observation, CD38-positive (CD38+) CLL cells express higher levels of other activation markers compared with CD38-negative (CD38−) cells.18
Proliferation centers or pseudofollicles are structures found in lymph node, splenic white pulp, and bone marrow samples that are infiltrated with CLL.19-24 These areas contain larger prolymphocytes and para-immunoblasts, a higher proportion of which express the proliferation marker Ki-67 in comparison to surrounding small lymphocytes. In addition to the tumor, a variety of nonmalignant cells, including T cells, are found in the bone marrow25 and lymph nodes of CLL patients with higher numbers of T cells in pseudofollicles than elsewhere.21 Because some of these CD4+ T cells also express activation and costimulatory markers, such as CD40L,26,27 it has been proposed that T cell–derived signals might promote tumor cell proliferation in these areas.
Although the level of CD38 expression has proven to be a simple and clinically useful parameter in CLL, the biologic basis of its association with prognosis is as yet unclear. In this study, we address this issue and investigate the hypothesis that the level of CD38 expression in CLL reflects interactions between leukemic and nonmalignant cells within pseudofollicles in secondary lymphoid tissue. Several predictions can be made from this model. First, the level of CD38 expression within tissues that contain pseudofollicles should be higher than in those that do not. If this is the case, then CD38 expression should be dynamic and change as cells transit between these tissues and the peripheral blood and in response to stimuli of the type found within pseudofollicles. Finally, within secondary lymphoid tissues, the level of CD38 expression should be highest in or near areas of interaction with stromal elements. To test these predictions, we obtained peripheral blood as well as cryopreserved and paraffin-embedded lymph node, spleen, and bone marrow biopsies from patients with proven CLL and investigated the factors determining the level of CD38 expression at these sites.
Methods
Patient samples
For comparison of CD38 expression in peripheral blood and bone marrow aspirates, clinical diagnostic data obtained from patients referred to King's College Hospital Trust over a 3-year period were anonymized and reviewed. Sequential patients with a confirmed diagnosis of CLL,28 in whom a measurement of peripheral blood and bone marrow CD38 expression had been made not more than 30 days apart, were identified. CD38 expression by both peripheral blood and bone marrow CLL cells was assessed by 3-color immunofluorescence with anti-CD5-fluorescein isothiocyanate (FITC), anti–CD19-phycoerythrin (PE), and anti–CD38-PE-Cy5 (all Beckman Coulter, High Wycombe, United Kingdom) using appropriate isotype-matched controls.
For the in vitro coculture studies, peripheral blood mononuclear cells (PBMCs) were collected from a separate cohort of 15 randomly selected patients with a confirmed diagnosis of CLL either at presentation or during follow-up at an interval of more than 3 months from previous therapy. In addition to the routine diagnostic panel and measurement of CD38 expression, IgVH genes were sequenced as previously described.4 Nucleotide sequences were aligned to EMBL/GenBank and V-BASE sequence directories to determine mutational status.29 Patient characteristics are outlined in Table 1. PBMCs were separated by density-gradient separation (Histopaque-1077; Sigma Chemical, Poole, United Kingdom). Cells were frozen and stored in aliquots in RPMI 1640, 40% fetal calf serum (FCS) and 10% dimethylsulfoxide (all Sigma Chemical).
Patient characteristics
| Patient no. . | VH mutational status . | %CD38+ (whole blood) . | Binet stage . |
|---|---|---|---|
| CLL 01 | Mutated | 85 | C |
| CLL 02 | Mutated | 0 | A |
| CLL 03 | Mutated | 75 | B |
| CLL 04 | Mutated | 0 | A |
| CLL 05 | Mutated | 0 | A |
| CLL 06 | Unmutated | 0 | C |
| CLL 07 | Unmutated | 1 | B |
| CLL 08 | NA | 0 | A |
| CLL 09 | Mutated | 25 | A |
| CLL 10 | Unmutated | 0 | B |
| CLL 11 | Unmutated | 65 | C |
| CLL 12 | Unmutated | 37 | B |
| CLL 13 | Unmutated | 26 | C |
| CLL 14 | Mutated | 88 | C |
| CLL 15 | NA | 65 | A |
| Patient no. . | VH mutational status . | %CD38+ (whole blood) . | Binet stage . |
|---|---|---|---|
| CLL 01 | Mutated | 85 | C |
| CLL 02 | Mutated | 0 | A |
| CLL 03 | Mutated | 75 | B |
| CLL 04 | Mutated | 0 | A |
| CLL 05 | Mutated | 0 | A |
| CLL 06 | Unmutated | 0 | C |
| CLL 07 | Unmutated | 1 | B |
| CLL 08 | NA | 0 | A |
| CLL 09 | Mutated | 25 | A |
| CLL 10 | Unmutated | 0 | B |
| CLL 11 | Unmutated | 65 | C |
| CLL 12 | Unmutated | 37 | B |
| CLL 13 | Unmutated | 26 | C |
| CLL 14 | Mutated | 88 | C |
| CLL 15 | NA | 65 | A |
NA indicates not available.
The studies on tissue sections were performed on an additional group of 13 cases of confirmed CLL. Of these, 8 were lymph nodes (4 formalin-fixed, paraffin-embedded, 4 cryopreserved) and 5 spleens (all cryopreserved). Lymph nodes were removed for diagnostic purposes and spleens for hypersplenism or autoimmune cytopenias. All patients were Binet stage B or C, and none had received chemotherapy for at least 3 months. Ethical approval for all of this work was obtained from the local institutional review board of King's College Hospital, and informed written consent was obtained in accordance with the Declaration of Helsinki.
In vitro studies
Coculture experiments.
T cells were separated from CLL cells using a human CD3+ selection kit (StemCell Technologies, Grenoble, France) or CD3 beads (Invitrogen, Paisley, United Kingdom) and CD4 depletions performed using CD4 beads (Invitrogen) according to the manufacturer's instructions. Both the positive fraction containing T cells and the negative fraction (residual CLL PBMCs) were retained for subsequent analysis. Purified CD3+ cells were then activated by incubation with 10 μL CD3/28 beads (Invitrogen) per 106 cells at a density of 106/mL in RPMI 1640 supplemented with 10% FCS and penicillin/streptomycin solution (all Sigma Chemical) for 12 hours. Residual CLL PBMCs were labeled with carboxyfluorescein diacetate succinimidyl ester (CFSE; Invitrogen) and incubated in RPMI 1640 supplemented with 1% bovine serum albumin (Sigma Chemical), 10% FCS, and penicillin/streptomycin solution before the addition of autologous activated T cells at a ratio of 1:4 and a density of 0.5 × 106 cells/mL. In some experiments, cell-cell contact was prevented by use of a cell culture insert of 0.4-μm pore size (BD Biosciences, Oxford, United Kingdom) between the CLL cells and activated T cells. Control samples comprising unselected and residual CLL PBMCs were incubated at the same initial density in parallel with the cocultures.
Samples were analyzed from day 0 to day 6 by 3-color flow cytometry to determine the level of CD38 expression and number of cell divisions by the CLL cells. Samples were stained with a PE-Cy5 conjugated anti-CD19 (AbD Serotec, Oxford, United Kingdom) and a PE-conjugated anti-CD38 (BD Biosciences), and CD38 expression by CD19+ cells was determined in comparison with the appropriate isotype-matched controls. The number of cell divisions was assessed by measurement of the intensity of CFSE staining on CD19+ cells in both cocultures and controls in the whole cohort of 15 patients and CD38 up-regulation in a randomly selected subset of 11 cases. Light chain restriction of CD19+ cells in the culture system was demonstrated using PE-conjugated antilambda or antikappa (BD Biosciences). Cells counts were performed using calibrated counting beads (Invitrogen) according to the manufacturer's instructions. T-cell activation was assessed at 0 and 24 hours using both PE-Cy5–conjugated anti-CD4 and anti-CD8 (AbD Serotec) with either PE-conjugated anti-CD40L (BD Biosciences) or FITC-conjugated anti-CD25 (AbD Serotec).
Immunofluorescence microscopy
Paraffin sections were cut at 3-μm thickness, and heat antigen retrieval was performed in 10 mM sodium citrate buffer. Frozen sections were cut at 7-μm thickness and subsequently fixed in ice-cold acetone for 10 minutes. For staining of both paraffin and frozen material, sections were prehydrated for 30 minutes in phosphate-buffered saline (PBS) before addition of 5% donkey serum (Jackson ImmunoResearch Laboratories, West Grove, PA) in PBS. Primary antibodies were diluted in PBS and sections incubated overnight at 4°C. Primary antibodies used were anti-CD3 (clone CD3-12), anti-CD25 (clone MEM-181), anti-CD31 (clone WM59), anti-CD38 (clone AT13/5; all AbD Serotec), anti-CD20 (goat polyclonal, Santa Cruz Biotechnology, Heidelberg, Germany), anti-CD23 (goat polyclonal; R&D Systems Europe, Abingdon, United Kingdom), anti-CD31 (rabbit polyclonal; Novus Biologicals, Littleton, CO), anti-CD34 (clone 8G12; BD Biosciences), anti-CD38 (clone SPC32), anti–Ki-67 (clone MM1; both VisionBiosystems, Newcastle, United Kingdom), anti-FOXP3 (clone 225; Abcam, Cambridge, United Kingdom) and anti–Ki-67 (rabbit polyclonal; both Abcam, Cambridge, United Kingdom). Primary antibodies were visualized with appropriate donkey whole IgG affinity purified antibodies multiply absorbed against alternate species conjugated to 7-amino-4-methylcoumarin-3-acetic acid, FITC, CY2, Cy3, and Cy5 (Jackson ImmunoResearch Laboratories). 7-Amino-4-methylcoumarin-3-acetic acid and FITC-conjugated antibodies were used at a dilution of 1:50; Cy2, Cy3, and Cy5 antibodies were used at a dilution of 1:100. Samples were mounted in SlowFade Gold antifade reagent (Invitrogen).
Immunofluorescent confocal microscopy was performed using a Zeiss Axiovert LSM 510 microscope (Carl Zeiss, Jena, Germany) fitted with Plan-Neofluar 10×/0.3, Plan-Neofluar 20×/0.5, Plan-Neofluar 40×/075, and C-Apochromat 63×/1.2 W Corr objectives, one 30 mW argon laser exciting at 488 nm, one 1 mW helium-neon laser exciting at 543 nm, and one 5 mW helium-neon laser exciting at 633 nm. Confocal microscopy data were collected using proprietary image acquisition software (LSM, release 3.5; Carl Zeiss). Images were edited for optimal color contrast using Adobe Creative Suite 2 premium (Adobe Systems, San Jose, CA).
Data analysis
All flow cytometric data were acquired using CellQuest Software (BD Biosciences) and analyzed using this program or FlowJo (TreeStar, Ashland, OR).
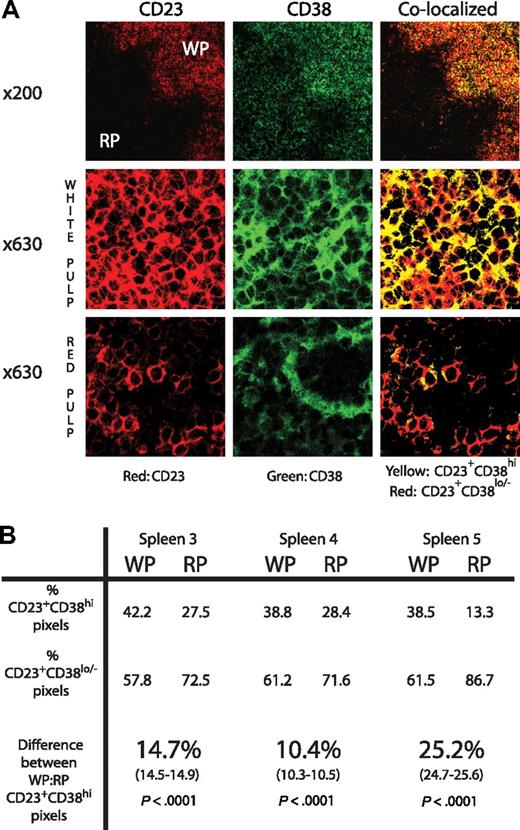
For analysis of CD38 staining on splenic tissue, quantitative colocalization of CD23 and CD38 was performed as follows: the voltage of the photomultiplier was set so that the most intense CD38 signal had a channel intensity of 255; this was determined either on CD23+CD38+ cells located in the white pulp or non-CD23+ cells in the red pulp, depending on which was more intense. Images were subsequently analyzed using Adobe Photoshop (Adobe Systems) by thresholding both CD23 (red) and CD38 (green) at channel 100. Pixels were considered CD23+CD38hi (yellow) if signals from both the CD23 and CD38 channel were greater or equal to 100 and CD23+CD38lo/− (red) if the CD23 channel signal was greater than 100 but that from the CD38 channel was less than 100. Given the lower number of CLL cells in red pulp areas compared with white pulp, at least 10 views of red pulp were compared with one of white pulp. The percentage of CD23+CD38hi and CD23+CD38lo/− pixels were determined from the total number of CD23+ pixels obtained from these views. A difference in proportion test was used to calculate the 95% confidence intervals and χ2 analysis to determine the significance of differences in the number of CD23+CD38hi pixels between white and red pulp (GraphPad Prism, version 4.00 for Windows; GraphPad Software, San Diego, CA).
CD38 expression by lymph nodes was determined using residual germinal center B cells as positive controls and appropriate isotype-matched negative controls. For analysis of CD31 staining of lymph node sections, images were thresholded to only give the intense CD31 staining apparent on vessels. The CD31 pixel index was calculated as the total number of CD31 pixels as a proportion of the section area. Rainbow palette images of lymph node sections were generated using the LSM510 software, which assigns different colors to different pixel intensities.
For all tissue studies, multiple sections and fields were analyzed from each sample and counting of cells performed by 2 independent observers, Pixel data were analyzed using Adobe Photoshop and statistical analysis performed using GraphPad Prism software. Replicate sample analyses are shown as mean plus or minus SEM, and statistical tests used are indicated in the text.
Results
CD38 expression is higher in CLL tissue compartments that contain pseudofollicles
We first investigated whether CD38 expression by CLL cells varies between tissue compartments. Paired blood and bone marrow samples from 35 patients with confirmed CLL were analyzed by flow cytometry and the percentage of CD19+CD5+ cells that express CD38 determined. Significantly higher numbers of bone marrow CLL cells expressed CD38 compared with those in the peripheral blood (26.9% ± 5.3% vs 18.8% ± 4.3%, P = .009, paired t test).
Multicolor immunofluorescent confocal microscopy was next used to assess differences in the level of CD38 expression in splenic tissue from 5 different cases showing infiltration with CLL. Immunostaining with CD23 showed that, although both the white and red pulp was infiltrated by tumor in all cases, white pulp was clearly delineated by the denser expression of this antigen. Furthermore, as previously noted,19,23 only white pulp contained large proliferating CD23+Ki-67+ cells, which were absent from red pulp areas. Both white and red pulp areas contained CD3+ cells and tumor cells that expressed CD38 (data not shown).
Using the same settings to examine both white and red pulp areas, CD38 expression by CD23+CLL cells was assessed. CD23+ cells with high (CD23+CD38hi) and low or absent CD38 expression (CD23+CD38lo/−) were identified by quantitative colocalization in both white and red pulp areas (Figure 1A). In 2 cases, CD38 expression, assessed by the frequency of CD23+CD38hi pixels, was low in white pulp (< 35% of total CD23+ pixels), and in these there was no difference between the percentage of CD23+CD38hi pixels in the red and white pulp. In the 3 cases with higher levels of CD38 (> 35% of the CD23 pixels also CD38hi), there were 10.4% to 25.2% more CD23+CD38hi pixels in the white compared with the red pulp (P < .001 by χ2 test, Figure 1B).
CD38 expression is higher on CLL cells derived from splenic white pulp compared with splenic red pulp. (A) Sections of spleen stained for CD23 (shown in red) and CD38 (shown in green) at ×200 and ×630 original magnifications. In the colocalized images, yellow represents CD23+CD38hi and red represents CD23+CD38lo/− pixels. Although the ×200 views show that CD38 staining is apparent in both red and white pulp, the high-power views show that CD38 colocalizes with CD23+ tumor in the white pulp areas, whereas in the red pulp it does not and must be expressed by cells other than the tumor. (B) The table shows the percentage of CD23+CD38hi and CD23+CD38lo/− pixels in both white pulp (WP) and red pulp (RP) in sections of the 3 available spleens with high CD38 staining detectable in the white pulp. CD38 expression by the tumor cells is at least 10% greater in the WP compared with the RP (95% confidence interval shown in brackets). Significance was demonstrated using the χ2 test.
CD38 expression is higher on CLL cells derived from splenic white pulp compared with splenic red pulp. (A) Sections of spleen stained for CD23 (shown in red) and CD38 (shown in green) at ×200 and ×630 original magnifications. In the colocalized images, yellow represents CD23+CD38hi and red represents CD23+CD38lo/− pixels. Although the ×200 views show that CD38 staining is apparent in both red and white pulp, the high-power views show that CD38 colocalizes with CD23+ tumor in the white pulp areas, whereas in the red pulp it does not and must be expressed by cells other than the tumor. (B) The table shows the percentage of CD23+CD38hi and CD23+CD38lo/− pixels in both white pulp (WP) and red pulp (RP) in sections of the 3 available spleens with high CD38 staining detectable in the white pulp. CD38 expression by the tumor cells is at least 10% greater in the WP compared with the RP (95% confidence interval shown in brackets). Significance was demonstrated using the χ2 test.
Effect of activated autologous T cells on CLL cells
Because CLL cells from locations that contain pseudofollicles had higher levels of CD38 expression, we next determined whether stimuli of the type observed at these sites might alter the level of expression of this molecule. Activated CD40L-expressing T cells are known to contact proliferating tumor cells in pseudofollicles,27 and we therefore investigated the effect of coculture with activated autologous T cells on the expression of CD38 and proliferation status of CLL cells. These studies were performed on cryopreserved CLL PBMCs, which, after thawing, had a viability of more than 80% and a CD38 level similar to the initial value. Activation of purified autologous T cells was confirmed by an increase in the percentage expressing CD25 (to a mean 90.6% ± 1.7%) and CD40L (to a mean 62% ± 2.4%).
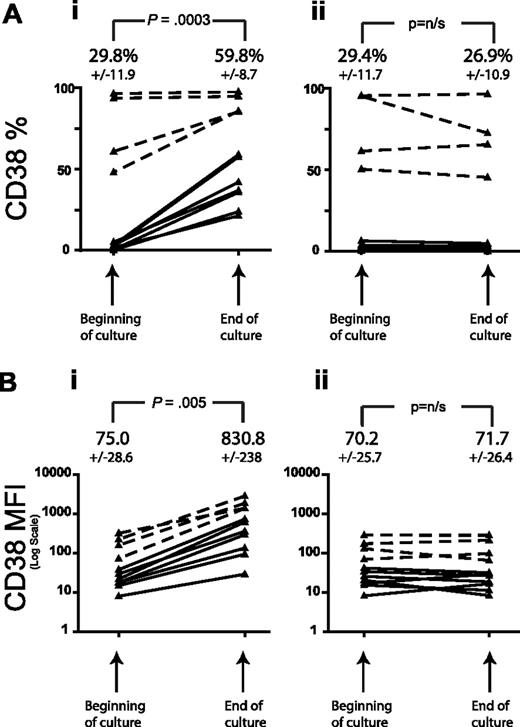
In all cases studied, in the presence of activated T cells, both the percentage of CLL cells expressing CD38 (%CD38) and the mean fluorescent intensity increased over the period of culture (P = .003 and .005, respectively, n = 11, paired t test) regardless of the initial level of expression, whereas incubation of CLL cells with unstimulated autologous T cells had no effect on either %CD38 or CD38 MFI over the culture period (Figure 2). We observed CD38 up-regulation from as early as 16 hours in the coculture period, an effect that was significantly reduced by partial depletion of CD4+ T cells (mean CD4 depletion 64%, mean reduction in CD38 up-regulation 35%, P = .04, n = 8, paired t test). Separation of the activated T cells from leukemic cells using a 0.4-μm cell culture insert demonstrated that the up-regulation of CD38 was largely contact dependent with a much smaller contribution from soluble mediators. We have previously reported that coculture with activated T cells does not alter tumor cell apoptosis over the time period of these experiments, indicating that the change in CD38 expression was not the result of changes in cell viability.30
CD38 expression by CLL cells increases in coculture with autologous activated T cells. T cells from 11 patients with CLL were positively selected from peripheral blood mononuclear cells (PBMCs) using CD3 magnetic beads. These were activated overnight with CD3/CD28 and then cocultured with the residual CLL PBMCs. Control experiments with unstimulated PBMCs were performed in parallel. CD38 expression by the CD19+ cells is shown on day 0 and day 4. (A) A significant increase in the percentage of CD19+CD38+ cells is demonstrated for CLL PBMCs cocultured with activated T cells (i) but not for CLL PBMCs cultured alone (ii). Note that even cases initially negative for CD38 in the coculture showed an increase in expression. (B) A significant increase in the mean fluorescent intensity (MFI) of CD38 expression by CD19+ cells is demonstrated in the same manner for cocultures (i) but not for PBMCs cultured alone (ii). The dashed lines show the results for the 4 cases with the highest initial CD38 percentage. Figures shown represent the mean plus or minus SEM values from the 11 patients in 4 independent experiments. Significance was assessed by the paired t test.
CD38 expression by CLL cells increases in coculture with autologous activated T cells. T cells from 11 patients with CLL were positively selected from peripheral blood mononuclear cells (PBMCs) using CD3 magnetic beads. These were activated overnight with CD3/CD28 and then cocultured with the residual CLL PBMCs. Control experiments with unstimulated PBMCs were performed in parallel. CD38 expression by the CD19+ cells is shown on day 0 and day 4. (A) A significant increase in the percentage of CD19+CD38+ cells is demonstrated for CLL PBMCs cocultured with activated T cells (i) but not for CLL PBMCs cultured alone (ii). Note that even cases initially negative for CD38 in the coculture showed an increase in expression. (B) A significant increase in the mean fluorescent intensity (MFI) of CD38 expression by CD19+ cells is demonstrated in the same manner for cocultures (i) but not for PBMCs cultured alone (ii). The dashed lines show the results for the 4 cases with the highest initial CD38 percentage. Figures shown represent the mean plus or minus SEM values from the 11 patients in 4 independent experiments. Significance was assessed by the paired t test.
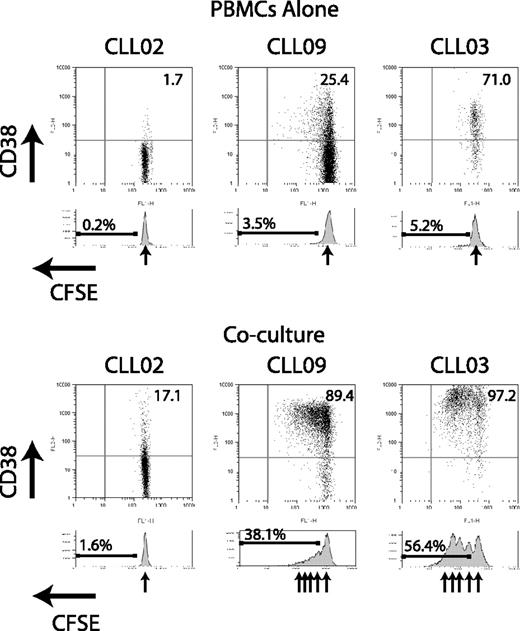
We next examined whether coculture of CLL cells with activated autologous T cells can result in CLL cell division. Using the cytoplasmic dye CFSE, CLL cells were observed to divide in some cocultures but never in the absence of activated T cells (Figure 3). Light chain restriction was maintained at the end of the coculture period, confirming expansion of the CLL clone and not residual polyclonal normal B cells (data not shown). In a randomly selected subset of 9 cases, cell counts were performed using flow cytometry counting beads as an internal control. These experiments confirmed that, compared with unstimulated CLL PBMCs, coculture with activated T cells caused an absolute increase in the number of CD19+ cells (P = .008, paired t test). Although CD38 up-regulation occurred by 16 hours, cell division was never seen before 96 hours of coculture. Experiments in which activated T cells were separated from CLL cells by a 0.4-μm insert demonstrated that cell division was completely contact dependent (data not shown).
Coculture of CLL cells with activated autologous T cells can result in CLL cell division in addition to CD38 up-regulation. CLL cells were labeled with CFSE and incubated with activated autologous T cells for 4 to 6 days. At the end of the culture period, the samples were analyzed by flow cytometry using CD19 expression to identify CLL cells. Flow cytometer plots gated on CD19+ cells are shown from 3 representative patient samples. Residual CLL PBMCs cultured alone show only a single CFSE peak, indicating no cell division. In the case with initial low CD38 expression (CLL02), coculture shows CD38 up-regulation but no division. In 2 cases with initial positive CD38 expression (CLL09 and CLL03), both CD38 up-regulation and division are seen in coculture.
Coculture of CLL cells with activated autologous T cells can result in CLL cell division in addition to CD38 up-regulation. CLL cells were labeled with CFSE and incubated with activated autologous T cells for 4 to 6 days. At the end of the culture period, the samples were analyzed by flow cytometry using CD19 expression to identify CLL cells. Flow cytometer plots gated on CD19+ cells are shown from 3 representative patient samples. Residual CLL PBMCs cultured alone show only a single CFSE peak, indicating no cell division. In the case with initial low CD38 expression (CLL02), coculture shows CD38 up-regulation but no division. In 2 cases with initial positive CD38 expression (CLL09 and CLL03), both CD38 up-regulation and division are seen in coculture.
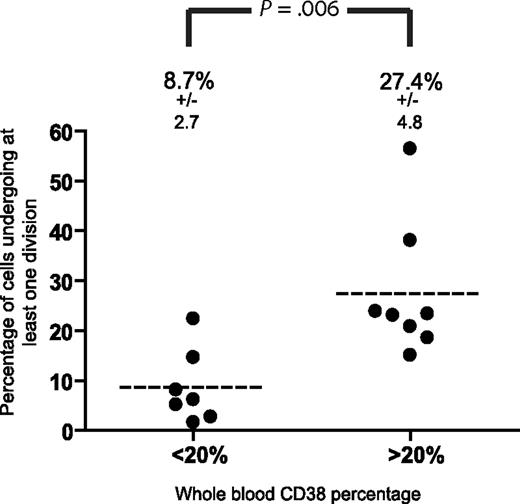
Because the extent of CLL cell division in coculture was highly variable between patients, we examined factors that might identify cases with a greater proliferative potential. Neither clinical stage nor the mutational status of the IgVH genes predicted for cell division in the coculture system. In contrast, the initial level of CD38 expression by peripheral blood leukemic cells was strongly associated with an increased chance of cell division in the in vitro system (Figure 4). In cases of CLL with a peripheral blood CD38 level of 20% or more, an arbitrary threshold that has also been used to define CD38 positivity for clinical purposes,7 27.4% plus or minus 4.8% of CD19+ cells underwent at least one division in the cocultures compared with 8.7% plus or minus 2.7% in those with an initial level of less than 20% (P = .006, n = 7, unpaired t test).
Whole blood %CD38 by CLL cells predicts cell division in coculture. Comparison of the capacity of CLL cells with a high (≥ 20%) and low (< 20%) %CD38 to undergo division after coculture with activated autologous T cells. Significantly more CD38hi CLL cells underwent at least one division compared with the CD38lo group. Statistical significance was determined by the unpaired t test.
Whole blood %CD38 by CLL cells predicts cell division in coculture. Comparison of the capacity of CLL cells with a high (≥ 20%) and low (< 20%) %CD38 to undergo division after coculture with activated autologous T cells. Significantly more CD38hi CLL cells underwent at least one division compared with the CD38lo group. Statistical significance was determined by the unpaired t test.
Proliferating tumor cells colocalize with activated CD4 T cells
Having demonstrated that CD38 expression is higher in tissues that contain proliferating tumor and that CD38 expression by CLL cells is dynamic and changes in response to contact with activated T cells, we next made a more detailed study of the relationship of these cells to each other in CLL lymph nodes.
Morphologic assessment of hematoxylin and eosin-stained lymph node sections (n = 8) showed effacement of the normal lymph node architecture with predominantly small monotonous lymphocytes. In addition, larger prolymphocytes and para-immunoblasts were present in all sections. Residual germinal centers were present in 4 of 8 cases. Morphologically distinct pseudofollicles comprising areas of paler staining with multiple para-immunoblasts were observed in 3 of 4 cases in which paraffin-embedded material was available. Pseudofollicles could not be identified in the thicker sections of cryopreserved tissue (data not shown).
Both the cryopreserved and paraffin-embedded CLL lymph node sections were assessed by multiparameter confocal immunofluorescence microscopy. CLL cells were identified using both CD20 and CD23, which gave an identical staining pattern. Dual staining using either CD20 or CD23 with Ki-67 showed that proliferating tumor cells were present in all cases in either a diffuse or patchy distribution. The areas containing proliferating tumor were also heavily infiltrated with CD3+ T cells (Figure 5A) of which in all cases greater than 80% also expressed CD4 (Figure 5B). These cells expressed CD25 but not FOXP-3 (data not shown) compatible with an activated T-helper phenotype. We were unable to demonstrate CD40L expression in tissue sections, presumably because of technical factors and because, as previously reported,31 expression levels may be relatively low because of receptor-mediated down-regulation induced by contact with CD40 on the tumor surface.
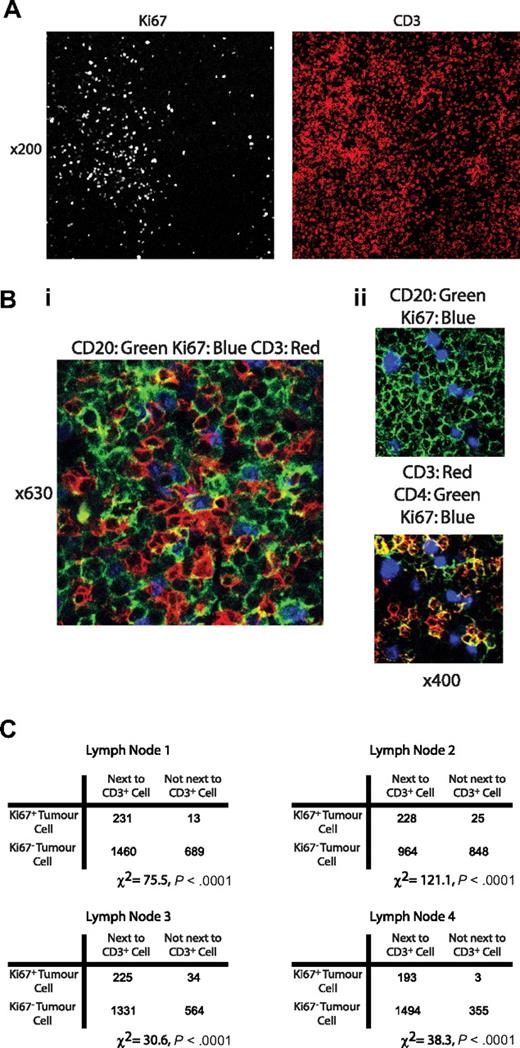
Large Ki-67+ tumor cells are present in lymph node sections and are found in close proximity to CD+ T cells. Confocal immunofluorescence microscopy of CLL lymph nodes. (A) View (original magnification ×200) of a CLL lymph node stained with Ki-67 and CD3. Ki-67+ cells were confirmed to be CLL cells using costaining with CD20 (data not shown). In this example, Ki-67+CD20+ cells had a patchy distribution. In areas of high Ki-67, staining with CD3 showed these areas also had a higher density of T cells. (Bi) View (original magnification ×630) of an area rich in proliferating CLL cells stained with CD20, Ki-67, and CD3. CD3+ cells are shown to be in intimate contact with Ki-67+ tumor cells. (Bii) View (original magnification ×400) of an area of lymph node stained with CD20 (green), CD3 (red), CD4 (green), and Ki-67 (blue). The CD3+ cells adjacent to CD20+Ki-67+ tumor cells show colocalization with CD4 (yellow). In all sections examined, more than 80% of these CD3+ cells colocalized with CD4. (C) Quantitative assessment of the spatial relationship between Ki-67+ tumor cells and CD3+ cells in serial views of 4 separate lymph node specimens confirms that proliferating tumor cells are significantly more probable to be touching an adjacent T cell compared with Ki-67− cells in all cases (χ2 test).
Large Ki-67+ tumor cells are present in lymph node sections and are found in close proximity to CD+ T cells. Confocal immunofluorescence microscopy of CLL lymph nodes. (A) View (original magnification ×200) of a CLL lymph node stained with Ki-67 and CD3. Ki-67+ cells were confirmed to be CLL cells using costaining with CD20 (data not shown). In this example, Ki-67+CD20+ cells had a patchy distribution. In areas of high Ki-67, staining with CD3 showed these areas also had a higher density of T cells. (Bi) View (original magnification ×630) of an area rich in proliferating CLL cells stained with CD20, Ki-67, and CD3. CD3+ cells are shown to be in intimate contact with Ki-67+ tumor cells. (Bii) View (original magnification ×400) of an area of lymph node stained with CD20 (green), CD3 (red), CD4 (green), and Ki-67 (blue). The CD3+ cells adjacent to CD20+Ki-67+ tumor cells show colocalization with CD4 (yellow). In all sections examined, more than 80% of these CD3+ cells colocalized with CD4. (C) Quantitative assessment of the spatial relationship between Ki-67+ tumor cells and CD3+ cells in serial views of 4 separate lymph node specimens confirms that proliferating tumor cells are significantly more probable to be touching an adjacent T cell compared with Ki-67− cells in all cases (χ2 test).
We next examined the spatial relationship of the activated T cells to proliferating tumor. The number of Ki-67+ and Ki-67− tumor cells in contact with CD3+ cells were counted in multiple sections of diffusely infiltrated cryopreserved lymph nodes from 4 patients with CLL. In each case, Ki-67+ CLL cells were significantly more probable to be touching an adjacent T cell than their Ki-67− counterparts (χ2 test, P = < .001 in each case tested, Figure 5C).
Distribution of CD38 expression in CLL lymph nodes
Using residual germinal center B cells as a positive control, CLL cells within the lymph nodes expressed CD38 at a relatively low level in the 8 cases studied. In 4 cases in which data were available, the percentage of CD38+ CLL cells in the peripheral blood paralleled the intensity of expression in the lymph node. CD38 expression was not detectable in a lymph node section from a patient with 2% CD38-expressing cells in the peripheral blood; however, progressively more intense CD38 staining was observed in cases with 13%, 15%, and 66% CD38+ cells in the peripheral blood. Of the 8 CLL lymph nodes examined, tissue CD38 expression was present in 6 and absent in 2 cases. In these 6 cases, all the large Ki-67+ proliferating tumor cells expressed CD38 (Figure 6A). However, as noted earlier, not all CD38+ cells expressed Ki-67, implying persistence after exit from the cell cycle. This may explain why there was no overall correlation between the number of cells expressing CD38 and Ki-67 in any of the lymph nodes examined. Similarly, although almost all Ki-67+ tumor cells were next to an activated T-cell, there was a considerable background T-cell infiltrate in all cases. As a result, no correlation between the number of proliferating cells and the overall number of T cells was observed.
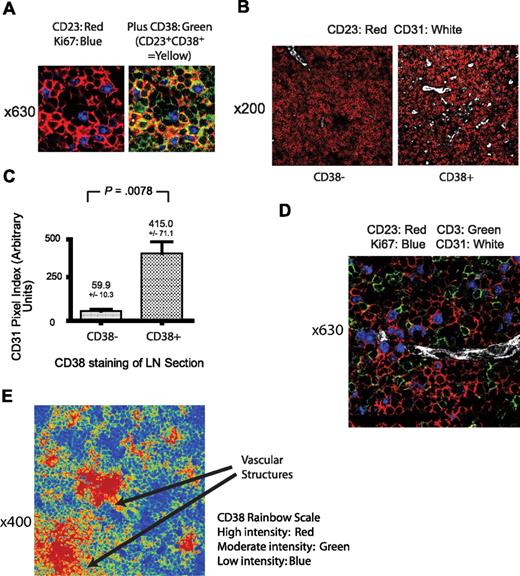
Relationship of lymph node CD38 expression with tumor proliferation and the microvasculature. (A) View (original magnification ×630) of a CLL lymph node from a patient with a peripheral blood CD38 level of 13% stained with CD38 (green), Ki-67 (blue), and CD23 (red). All proliferating CLL cells stain for both CD23 and CD38 (yellow). Identical appearances were observed in all CD38+ lymph nodes, defined by the presence of detectable CD38 staining as detailed in “Distribution of CD38 expression in CLL lymph nodes.” (B) View (original magnification ×200) of a CLL lymph node stained with CD31 (white) and CD23 (red), illustrating the larger number of vessels in a CD38+ compared with a CD38− case. (C) Quantification of the difference in lymph node vascular density assessed by the number of CD31+ pixels in CD38− and CD38+ cases of CLL. Significantly increased vessel density is apparent in the CD38+ cases. Results shown are the mean CD31 pixel index plus or minus SEM with the unpaired t test result. (D) View (original magnification ×630) of a CD38+ CLL lymph node, illustrating the relationship between proliferating CLL cells stained for Ki-67 (blue) and CD23 (red), T cells stained for CD3 (green), and a microvessel detected by CD31 (white). (E) View (original magnification ×400) of a CLL lymph node frozen section stained for CD38 and shown as a rainbow palette image in which different levels of expression are assigned a different color. The level of CD38 expression varies markedly within the lymph node and is most dense in areas around vascular structures. This image is representative of 12 frozen sections.
Relationship of lymph node CD38 expression with tumor proliferation and the microvasculature. (A) View (original magnification ×630) of a CLL lymph node from a patient with a peripheral blood CD38 level of 13% stained with CD38 (green), Ki-67 (blue), and CD23 (red). All proliferating CLL cells stain for both CD23 and CD38 (yellow). Identical appearances were observed in all CD38+ lymph nodes, defined by the presence of detectable CD38 staining as detailed in “Distribution of CD38 expression in CLL lymph nodes.” (B) View (original magnification ×200) of a CLL lymph node stained with CD31 (white) and CD23 (red), illustrating the larger number of vessels in a CD38+ compared with a CD38− case. (C) Quantification of the difference in lymph node vascular density assessed by the number of CD31+ pixels in CD38− and CD38+ cases of CLL. Significantly increased vessel density is apparent in the CD38+ cases. Results shown are the mean CD31 pixel index plus or minus SEM with the unpaired t test result. (D) View (original magnification ×630) of a CD38+ CLL lymph node, illustrating the relationship between proliferating CLL cells stained for Ki-67 (blue) and CD23 (red), T cells stained for CD3 (green), and a microvessel detected by CD31 (white). (E) View (original magnification ×400) of a CLL lymph node frozen section stained for CD38 and shown as a rainbow palette image in which different levels of expression are assigned a different color. The level of CD38 expression varies markedly within the lymph node and is most dense in areas around vascular structures. This image is representative of 12 frozen sections.
Relationship of CD38 expression and vascular endothelium
In the course of analyzing lymph node CD38 expression, vascular structures were noted in the areas of highest antigen density. Because CD31, the only known ligand for CD38, is expressed by vascular endothelial cells,32 we next determined the relationship between CD38-expressing tumor cells and CD31+ vasculature. Strong staining with CD31 was noted in vascular structures in all 8 lymph nodes examined. Colocalization with CD34 confirmed that the cells expressing CD31 were vascular endothelial cells (data not shown). In addition to the strong expression of CD31 by vascular endothelium, in some cases weaker staining was observed in tumor cells. No expression of CD31 was detected in T cells.
We next determined the significance of the apparent association between lymph node blood vessel density and CD38 expression by the tumor. In the 2 cases that lacked CD38 expression, the vascularity of the tumor areas within the lymph node was low compared with the 6 cases with positive CD38 staining (Figure 6B). A total of 32 ×200 views from within 8 lymph node sections were obtained from areas of known tumor infiltration and CD31 staining of vessel structures assessed by confocal microscopy. A CD31 pixel index was calculated for each ×200 view. Significantly increased CD31 staining was apparent on the 24 fields with detectable CD38 compared with the 8 without (415.0 ± 71.1 vs 59.9 ± 10.3, P = .008, unpaired t test, Figure 6C).
In addition to the overall correlation between vascular endothelial CD31 expression and CD38, a spatial relationship between proliferating tumor, blood vessels, and T cells was also apparent. Figure 6D shows a lymph node section from a patient with a peripheral blood CD38 level of 45%. Fine blood vessels are shown interweaving areas of proliferating Ki-67+ tumor and T cells. We also noted that CD38 expression varied considerably in frozen lymph node sections with the highest levels around vascular structures. This is illustrated in Figure 6E, which is representative of 12 frozen sections examined. More homogeneous CD38 staining was observed in paraffin-embedded material. Analysis of a case in which both fixed and frozen sections were available suggests that this may be a result of the fixation and/or antigen retrieval process.
Discussion
In this study, we investigated the hypothesis that CD38 expression in CLL is determined by interactions with non-neoplastic cells in the tumor microenvironment. Although our results are open to a number of interpretations, several points clearly emerge. First, unlike other prognostic biomarkers, CD38 expression in CLL is not a fixed parameter and varies within anatomic sites such as the spleen and lymph nodes, between tissue compartments such as the peripheral blood and bone marrow, and in response to physiologic stimuli such as contact with activated T cells. These findings are in keeping with other reports showing higher CD38 levels in the lymph node33 and bone marrow5 compared with the peripheral blood and studies showing that interferon-α18 or agonistic CD38 antibody and interleukin-216 can increase CD38 expression. Unlike the later reports, however, incubation with activated T cells increased CD38 expression regardless of the initial level and appeared to depend, at least in part, on cell contact.
In addition, we also documented a striking in vitro and in vivo relationship between CD38 expression and tumor proliferation. In an in vitro system designed to simulate some of the possible interactions in tissue pseudofollicles, CLL cells with high baseline CD38 expression were more probable to divide after contact with activated autologous T cells than those with low levels. Similarly, multiparameter confocal immunofluorescence microscopy of CLL lymph nodes showed that, in those cases with detectable CD38, all proliferating tumor cells express high levels of CD38. This finding is supported by recent studies showing that, within the same patient, the highest CD38-expressing CLL cells are more probable to be Ki-67+ than those with low levels.34,35 When sorted on the basis of CD38 level, these populations have short but similar length telomeres34 and identical cytogenetic abnormalities (Christopher Pepper, University of Cardiff, oral communication, October 30, 2007). Taken with our data, these findings strongly suggest that, rather than identifying distinct subclones with different biologic properties and replicative potential, CD38 may be acquired or lost by CLL cells according to their activation or proliferation status. The association between CD38 expression and proliferation does not, however, appear to be causal because each can be observed in the absence of the other.
Having shown that contact with activated T cells may change CD38 levels and cause proliferation in vitro, we next investigated the relationship between CD38 expression, tumor proliferation, and the microenvironment in vivo by analyzing CLL lymph nodes using multiparameter confocal microscopy. These studies revealed a highly significant association between tumor proliferation and contact with T cells. In all CLL lymph nodes examined, Ki-67+ CLL cells were far more probable to contact a T cell than Ki-67− cells. The T cells involved in these interactions had a CD4+CD25+FOXP3− activated helper phenotype similar to those involved in the induction of CD38 expression and proliferation in the in vitro system described. Although CD4+ T cells have previously been shown to be increased in CLL lymph node sections,21,22,26 and to preferentially localize to pseudofollicles,26 only one other study has shown contact between CD40L-expressing T cells and Ki-67+ CLL cells.27
In addition to the colocalization of T cells and proliferating CLL cells, we also demonstrated an association between the level of CD38 expression and tumor vascularity. Lymph node sections containing CD38+ CLL cells contained significantly more CD31+ vessels than CD38− sections. Although not formally quantified in the present study, CLL cells with the highest CD38 expression appeared to localize preferentially with vascular structures.
Taken as a whole, and when considered with the results of others described earlier, the data presented here provide persuasive evidence that CD38 expression and tumor proliferation in CLL are linked through interactions in the leukemic microenvironment involving T cells and the vascular endothelium. Whether this is because the microenvironment itself induces CD38 expression and proliferation or that these cells preferentially migrate and localize to these areas is however unclear and will be an important topic for future study. The adverse prognosis associated with expression of CD38 may thus relate to increased tumor proliferation and the consequent increase in tumor bulk and higher chance of progression through acquisition of additional genetic lesions.
Our results also raise a number of questions relevant to understanding the pathogenesis of CLL and the design of novel therapies that target the nonmalignant component of the tumor microenvironment. First, it will be important to determine the mechanism through which activated T cells accumulate at the sites of tumor proliferation and what effects these have on growth of the leukemic clone. There is now good evidence that ongoing antigenic stimulation of the B-cell receptor plays a role in the pathogenesis of CLL,36 and it will be of great interest therefore to determine whether the T-cell infiltrate also represents a response to antigen and, if so, its nature and route of presentation. It has previously been suggested that activated T cells may induce tumor proliferation in pseudofollicles through CD40/CD40L signaling.27 Although we were unable to stain tissue sections for CD40L and did not perform experiments with blocking CD40 antibody, the CD4+CD25+FOXP3− phenotype of the lymph node T cells and our in vitro results showing proliferation of CLL cells after contact with CD4+CD25+CD40L+ autologous T cells would be compatible with this theory. Alternatively, the lymph node T cells may represent a regulatory response similar to that observed in follicular and Hodgkin lymphoma,37,38 although the lack of FOXP3 expression is somewhat against this. It will thus be important to identify which of the many costimulatory and inhibitory molecules involved in the interaction between normal T and B cells play a role in CLL.
The relationship between CD38 expression by CLL cells and the microvasculature also warrants further exploration because ligand/receptor pairs expressed by the endothelium and CLL cells, such as CD31/CD38, PlexinB1/CD100,16 and integrins,39 have been shown to play a role in CLL. More recently, it has been shown ZAP70, another adverse prognostic marker in CLL, is phosphorylated after CD38 ligation and is a limiting factor in signaling through this pathway.40 CLL cells that express both CD38 and ZAP70 show enhanced migration in response to the chemokine SDF1α/CXCL12, and it is possible therefore that the colocalization of CLL cells that express the highest level of CD38 and the vascular endothelium results from enhanced migratory capacity. Several groups have shown that CLL cells secrete vascular endothelial growth factor41,42 with the highest levels in CD38+ disease,43 and this may provide a further explanation for the association between CD38 expression and increased tumor vascularity.
In conclusion, we have demonstrated that, far from being a stable biomarker, CD38 expression in CLL is dynamic, changes in response to contact with activated T cells, and identifies cells that are primed to proliferate. We showed that proliferating tumor cells express CD38 and colocalize with activated T cells, whereas CD38 expression was associated with increased vascularity. These observations provide a biologically plausible explanation for the association between CD38 expression and adverse outcome in CLL and suggest that targeting the nonmalignant component of the tumor microenvironment may yield novel therapies for this common disorder.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by a Leukemia Research Fund clinical research training fellowship (P.E.M.P.), a grant from Cancer Research United Kingdom (J.R.), and the Gretna Trust and an unrestricted educational grant from Chugai Pharma.
Authorship
Contribution: P.E.M.P. and A.G.S.B. designed and performed research, collected, analyzed, and interpreted data, and drafted the manuscript; J.R. performed research; A.W. contributed vital research material and interpreted data; J.S. analyzed and interpreted data; G.J.M. contributed analytical tools and drafted the manuscript; T.J.H. designed research and drafted the manuscript; S.D. designed research, collected, analyzed, and interpreted data, and drafted the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Piers E. M. Patten, Department of Haematological and Molecular Medicine, King's College London, Rayne Institute, 123 Coldharbour Lane, London, SE5 9NU, United Kingdom; e-mail: piers.patten@kcl.ac.uk.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal