Abstract
Vascular endothelial cell growth factor (VEGF) is a multifunctional cytokine involved in tumor formation. In chronic lymphocytic leukemia (CLL), it is known that the malignant cells secrete VEGF and possess VEGF receptors. This suggests that an autocrine loop might be important in the pathogenesis of CLL. Here we show that, in patients with lymphadenopathy, autocrine VEGF and α4β1 integrin are involved in the chemokine-dependent motility of CLL cells on and through endothelium—processes important for the invasion of lymphoreticular tissues, a major determinant of disease outcome. In contrast, normal lymphocytes were not dependent on autocrine VEGF or α4β1 for either type of cell movement. Moreover, in contrast to normal B lymphocytes, CLL cells failed to cluster and activate αLβ2 in response to chemokines, unless VEGF receptor(s) and α4β1 were also engaged by their respective ligands. This is the first demonstration that autocrine VEGF is involved in CLL-cell motility, and that the αLβ2 on the malignant cells is functionally altered compared with that of normal B cells in not undergoing activation in response to chemokine alone. Given the importance of cell motility for tissue invasion, the present results provide a rationale for a trial of VEGF and α4 blockade in patients with CLL who have tissue disease. (Blood. 2005;105:4813-4819)
Introduction
There is currently much interest in the role of vascular endothelial cell growth factor (VEGF) in tumor formation and development.1-3 Indeed, inhibitors of VEGF or of the function of its receptors are being tested for their clinical antitumor effects.4,5
VEGF is known to have multiple roles in tumor formation. For example, it is a major stimulating factor for the endothelial-cell migration and proliferation required for tumor vascularization.6,7 Moreover, the cytokine also directly affects the migration, proliferation, and survival of certain tumor cells themselves.8,9
Work from the Department of Haematology at University of Liverpool, United Kingdom, has shown that the malignant lymphocytes of chronic lymphocytic leukemia (CLL) produce VEGF, both in vitro and in tissues, and that secreted cytokine is capable of stimulating endothelial-cell proliferation and new-vessel formation. On the basis of these studies, we proposed that VEGF made by CLL cells might be involved in the neovasculogenesis necessary to provide nutrients for the enlarged lymphoreticular tissues in the disease.10
Since our original study, we and others have shown that CLL cells possess at least 2 VEGF receptors (VEGFR1 and VEGFR2)11-13 and that both these receptors are able to signal.12 There is therefore the possibility that CLL-cell-derived VEGF might mediate important autocrine effects on CLL cells. Indeed, it has recently been reported that autocrine VEGF can enhance CLL-cell survival.13
Since VEGF is known to stimulate the motility of a range of cell types,14,15 we hypothesized that autocrine VEGF might also be involved in the cell motility and transendothelial migration (TEM) known to be important in the pathogenesis of CLL.16-21
Our previous work has demonstrated that in patients with CLL who have clinical lymphadenopathy the malignant cells express α4β1 integrin and undergo chemokine-induced TEM that is dependent on both α4β1 and αLβ2. In patients with CLL who had no lymph node enlargement, the malignant cells did not express α4β1 and were unable to undergo TEM.17
In the present study, we used α4-expressing malignant cells to examine the role of VEGF in CLL-cell motility. We show that autocrine VEGF is indeed involved in the motility of the malignant cells on and through endothelium—processes important in CLL cell homing to tissues. Strikingly, the motility of normal B cells did not depend on either VEGF or α4β1, but was fully dependent on αLβ2. Furthermore, by examining the factors involved in this dependence of CLL-cell motility on both VEGF and α4β1, we demonstrate that the αLβ2 of the malignant cells differs from that of normal B cells in not being activated by chemokines alone. However, autocrine VEGF and α4β1 engagement together overcome this defect in the activation of αLβ2 on CLL cells. We suggest that these results may be therapeutically relevant and encourage a trial of the effects of VEGF blockade in patients with CLL who have organomegaly.
Patients, materials, and methods
Patients and donors
All patients had typical CLL with respect to morphology and surface-marker expression (low-density, light-chain-restricted, surface immunoglobulin, together with CD5 and CD23 positivity). All the patients had lymphocyte counts greater than 50 × 109/L and clinical lymphadenopathy (greater than 1 cm at 2 or more sites as detected by clinical examination). Cases were numbered sequentially, and these numbers were used for all experiments. For example, cells 1 were always from patient no. 1.
Normal lymphocytes were obtained either from buffy coats prepared by the Liverpool Blood Transfusion Service (n = 5) or from the peripheral blood (PB) of volunteers (n = 3). Donors were assigned letters of the alphabet and these letters always referred to the same donor.
For later experiments, some of the patient and normal cells were no longer available and it was therefore necessary to study additional material (CLL cases 7 and 8; healthy donors g and h).
Human umbilical vein endothelial cells (HUVECs) were prepared from umbilical cords obtained from the Liverpool Women's Hospital.
All samples were obtained with informed consent and with the approval of the Liverpool Research and Ethics Committee, Royal Liverpool and Broadgreen University Hospitals Trust, and of the Research and Development Committee, Liverpool Women's Hospital.
Cell preparation and culture
CLL cells were isolated from peripheral blood by Ficoll-Hypaque density gradient centrifugation and stored in liquid nitrogen prior to use. Because all of the patients had a high white blood cell (WBC) count, the mononuclear preparations contained a high proportion of CLL cells (CD19 > 95%; CD3 < 5%; and CD14 < 1%). After thawing, CLL cells were cultured for 1 hour in RPMI containing 1% bovine serum albumin (BSA; Sigma, Poole, United Kingdom), 2 mM L-glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin (Invitrogen, Paisley, Scotland). TEM assays were also performed in this medium. For time-lapse video microscopy (TLVM), CLL cells were cultured in CO2-independent medium (Invitrogen) containing 1% BSA, 2 mM L-glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin.
Normal PB lymphocytes were isolated by Ficoll-Hypaque density gradient centrifugation, and B cells positively selected using CD19-conjugated magnetic beads and a magnetic-activated cell-sorting (MACS) column (Miltenyi Biotech, Bisley, United Kingdom). B cells purified in this way were more than 98% CD20 positive. In some experiments, the cells were purified by depletion of CD3+ and CD14+ cells using magnetic beads (Miltenyi Biotech); B cells purified in this way were more than 95% CD20+.
Endothelial cells were stripped from the umbilical vein with trypsin and cultured to confluence in Iscove modified minimal essential medium (Invitrogen) containing 20% newborn calf serum (Sigma), 2 mM L-glutamine, 100 μg/mL penicillin, 100 μg/mL streptomycin, and 15 μg/mL epidermal growth factor (EGF; Invitrogen). HUVECs were used for up to the third passage.
Chemokines, antibodies, and inhibitors
The chemokines CC chemokine ligand 21 (CCL21) and CXC chemokine ligand 12 (CXCL12; R&D Systems Europe, Oxford, United Kingdom) were used.
For inhibition experiments, blocking mAbs were used against VEGF (clone 26503; immunoglobulin G2b [IgG2b]), α4 (IgG2a) and αL (IgG1) integrins, and vascular-cell adhesion molecule-1 (VCAM-1; IgG1). Class-specific nonimmune immunoglobulins were used as a control for nonspecific effects (all antibodies and controls were from R&D Systems). All antibodies were titrated, and used at concentrations that produced maximum inhibition; control immunoglobulins were also used at these concentrations.
In addition, an inhibitor of the VEGF receptor kinase (SU5416; Merck Biosciences, Nottingham, United Kingdom) was used. A range of concentrations of SU5416 were tested (1-10 μM); marked inhibition of migration (mean 73% of control; n = 5) was observed at 1 μM, but slightly greater inhibition occurred in the presence of 10 μM SU5416 (mean 80%; n = 5). For this reason, the inhibitor was used at 10 μM for subsequent experiments. At this concentration, SU5416 is reported to specifically inhibit both VEGFR1 and R2.22 Finally, pertussis toxin (1 μg/mL; Sigma) was used to inhibit G-protein signaling via chemokine receptors.
To examine the effect of αLβ2 engagement by intracellular adhesion molecule-1 (ICAM-1), cells were exposed to complexed ICAM-1. The complexes were prepared by incubating ICAM-1-Fc (0.1 μg/mL; R&D Systems) overnight at 4°C with goat F(ab′)2 anti-human Fc (2 μg/mL; Caltag, Burlington, CA).
For analysis of the purity of B cells after both MACS purification and transmigration through HUVECs, a fluoroscein isothiocyanate (FITC)- conjugated anti-CD20 monoclonal antibody (mAb) (Becton Dickinson, Oxford, United Kingdom) was used. Fluorescent-conjugated anti-integrin mAbs (α4-phycoerythrin [PE] and αL-FITC; Becton Dickinson) were also used to determine the levels of these integrins on CLL and normal cells and also to stain these cells prior to live cell imaging.
TEM assay
HUVECs were grown to confluence on the inserts of Transwell plates (5-μm pore size; Corning, Koolhovenlaan, the Netherlands). The HUVECs were washed, and 5 × 105 cells (CLL or normal B) were added to the inserts. CCL21 (1 μg/mL) or CXCL12 (100 ng/mL) was then added to the bottom wells; these concentrations had been previously shown to induce maximum TEM.17 The plates were then incubated for 6 hours (CLL cells) or 4 hours (normal B cells) at 37°C, 5% CO2 in air; these times were chosen after time-course experiments. After incubation, the undersides of the inserts were then scraped to remove any cells that had recently transmigrated, and the cells then harvested from the bottom wells. As some of the transmigrating lymphocytes had adhered to the bottom wells, EDTA (ethylenediaminetetraacetic acid; 0.2%) was added for 5 minutes at 37°C and the cells harvested and pooled. These transmigrated cells were then counted and the migration index (MI) calculated (MI = no. of CD20+ cells transmigrating in the presence of chemokine divided by the number of CD20+ cells transmigrating in the absence of chemokine).
Live-cell imaging
HUVECs were grown to confluence on 3.5 mm2 Petri dishes (Corning). HUVECs were washed and 5 × 105 CLL or normal B cells were added. The cells were then filmed for 1 hour by TLVM (CK2 microscope; Olympus, Southall, United Kingdom) and a 5720A time-lapse video recorder (Panasonic, Bracknell, United Kingdom) with the video slowed down by a factor of 16. The movement of cells was then determined by sequential tracing of cell outlines on the screen. A cell was considered to have moved when its position had changed by more than 2 cell diameters. This methodology allows the percentage of motile cells to be calculated. For the velocity calculations, the cells were assumed to be 8 μm in diameter.
Motility studies were also performed on lymphocytes incubated on the recombinant cellular ligands for α4 and αL viz. VCAM-1 and ICAM-1 (R&D Systems). Briefly, Petri dishes were coated overnight with either VCAM-1 or ICAM-1. Both proteins were titrated between 0.1 to 50 μg/mL, and maximum motility was observed at 10 μg/mL of both ligands; therefore this concentration was used for subsequent experiments. After overnight incubation, the Petri dishes were washed and preincubated with CCL21 (1 μg/mL) or CXCL12 (100 ng/mL) for 30 minutes at 37°C, 5% CO2 in air, prior to TLVM. In addition, CLL and normal B cells were incubated with the complexed ICAM-1 or anti-αL mAb for 30 minutes at 4°C, prior to TLVM on VCAM-1 in the presence of CCL21.
Live cell imaging was also performed using cells stained with nonblocking mAbs to αL and α4 conjugated to FITC and PE, respectively. In order to acquire images at high magnification, 3.5 mm2 Petri dishes with a glass insert were used (Iwaki, Chiba, Japan) along with a 63×/1.4 oil-immersion objective lens. Images were taken every 30 seconds for 1 hour (Zeiss LSM 510 microscope with metadetector controlled by Zeiss AIM software version 3.2; Zeiss, Welwyn Garden City, United Kingdom).
Inhibition studies
The same methodologies were used in order to study inhibition of both TEM and motility. Cells were incubated at 4°C for 30 minutes with either anti-VEGF (2.5 μg/mL), anti-α4 (5 μg/mL), anti-αL (10 μg/mL), anti-VCAM-1 (60 μg/mL), or the relevant isotypic control mAb. In addition, cells were incubated with SU5416 (10 μM) or 0.25% dimethyl sulfoxide (DMSO; the diluent) for 2 hours at 37°C. CLL cells were also incubated for 2 hours at 37°C with 1 μg/mL of pertussis toxin.
Statistics
The Student t test was used to determine the statistical significance of the results.
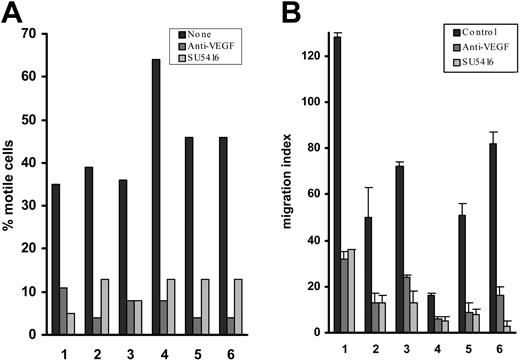
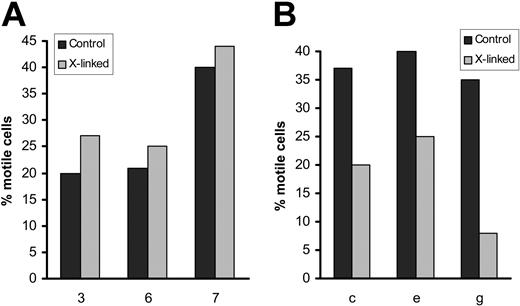
Effect of VEGF blockade on CLL-cell migration on and through endothelium. (A) Percentage of CLL cells motile on HUVECs and the reduction in the number of motile cells induced by a blocking anti-VEGF mAb and by an inhibitor of VEGF receptor kinase activity (SU5416). In the presence of VEGF blockade, the residual motile CLL cells closely resembled their untreated counterparts with regard to their velocity and extent of movement. (B) Effects of these inhibitors on CCL21-dependent CLL-cell TEM, expressed as a migration index (see “Patients, materials, and methods”). When the patients were considered together as a group, the inhibition of motility/TEM with or without blocking anti-VEGF mAb or SU5416 was always significant (P always < .003). Although there was case-to-case variation in both the percentage of motile cells and migration indices, when a given case was tested on more than one occasion similar results were obtained.
Effect of VEGF blockade on CLL-cell migration on and through endothelium. (A) Percentage of CLL cells motile on HUVECs and the reduction in the number of motile cells induced by a blocking anti-VEGF mAb and by an inhibitor of VEGF receptor kinase activity (SU5416). In the presence of VEGF blockade, the residual motile CLL cells closely resembled their untreated counterparts with regard to their velocity and extent of movement. (B) Effects of these inhibitors on CCL21-dependent CLL-cell TEM, expressed as a migration index (see “Patients, materials, and methods”). When the patients were considered together as a group, the inhibition of motility/TEM with or without blocking anti-VEGF mAb or SU5416 was always significant (P always < .003). Although there was case-to-case variation in both the percentage of motile cells and migration indices, when a given case was tested on more than one occasion similar results were obtained.
Results
To test the hypothesis that autocrine VEGF is involved in CLL-cell motility, we first examined the effects of blockade of this cytokine on the motility of CLL cells on and through endothelium.
Blocking VEGF inhibits CLL-cell motility on and through endothelium
We have previously shown that the malignant cells from patients with CLL who have lymphadenopathy migrate through endothelium in response to the CCR7-binding chemokines CCL21 and CCL19 (secondary lymphoid-tissue chemokine (SLC) and Epstein-Barr virus-induced receptor ligand chemokine (ELC), respectively), by a process involving both α4-VCAM-1 and αL-ICAM-1 interactions.17
Using CLL cells from these patients, we tested the effect of a blocking anti-VEGF mAb and of a VEGF receptor kinase inhibitor (SU5416) on migration on and through HUVEC monolayers. The presence of either reagent induced a marked reduction of both the percentage of motile cells and of the number of CLL lymphocytes undergoing TEM (Figure 1A-B), while neither a class-specific IgG2b control nor 0.25% DMSO (diluent for SU5416) had any effect (data not shown). At the concentration used (10 μm), SU5416 inhibits both VEGFR1 and VEGFR2.22 Therefore, although these experiments clearly show that receptor inhibition largely abrogates motility, they do not identify which receptor (or both) is (are) involved.
In these experiments, CLL-cell TEM was dependent on the chemotactic gradient produced by the addition of CCL21 below the membrane-supported HUVEC monolayer. “Spontaneous” movement on HUVECs was not affected by the addition of CCL21, but was inhibited by pertussis toxin, an irreversible inhibitor of the trimeric G protein necessary for signaling via chemokine receptors (data not shown). These results indicate that CLL-cell movement on HUVEC monolayers is also likely to be dependent on a chemokine.
Regarding the origin of the VEGF, the growth factor could theoretically have originated not only from CLL cells, but also from the HUVECs. However, using both enzyme-linked immunosorbent assay (ELISA) and immunocytochemistry, we could not demonstrate production or secretion of VEGF protein by HUVECs (data not shown). The present results therefore indicate that, in this group of patients, autocrine VEGF is important for CLL-cell motility on and through endothelium. This conclusion was confirmed in experiments presented later examining VEGF-dependent motility on VCAM-1. We next asked whether the inhibitory effect of VEGF blockade is a CLL-related phenomenon by performing similar experiments with normal B cells.
Blocking VEGF does not inhibit normal B-cell motility on and through endothelium
In marked contrast to their effect on CLL cells, neither anti-VEGF mAb nor SU5416 affected the motility or TEM of normal PB B cells in any consistent or significant manner (Figure 2A-B), regardless of whether the B cells had been purified by positive (Figure 2) or negative enrichment (not shown). Also, VEGF blockade had no effect on the TEM of normal T cells (data not shown).
Taken together with the results in the previous section, this indicates that CLL-cell movement on and through endothelium is dependent on a mechanism involving VEGF, while that of normal B cells is not. Also, the results show that a VEGF-independent motility mechanism present in normal B cells is not activated in CLL cells.
We next examined the mechanisms underlying these differences in the motile behavior of CLL versus normal B cells. Because binding of both α4β1 and αLβ2 to their respective ligands (VCAM-1 and ICAM-1) is known to be important for the interaction of normal leukocytes with endothelium,23,24 and because we have demonstrated the critical role of both integrin heterodimers in CLL-cell TEM,17 we focused on the role of these integrins/ligands. This seemed all the more important given that it is well established that VEGF cooperates with integrins in the induction of a number of cellular functions, including motility.25-27 We therefore next examined the effect of blocking antibodies to α4 and αL on the motility of normal and CLL B cells on and through endothelium.
Effect of VEGF blockade on normal B-cell migration on and through endothelium. The experiments were performed exactly as in Figure 1. (A) Percentage of motile normal B cells on HUVECs. (B) TEM. When the donors were considered together as a group, none of the differences in motility/TEM with or without blocking anti-VEGF mAb or SU5416 were significant (P always > .4).
Effect of VEGF blockade on normal B-cell migration on and through endothelium. The experiments were performed exactly as in Figure 1. (A) Percentage of motile normal B cells on HUVECs. (B) TEM. When the donors were considered together as a group, none of the differences in motility/TEM with or without blocking anti-VEGF mAb or SU5416 were significant (P always > .4).
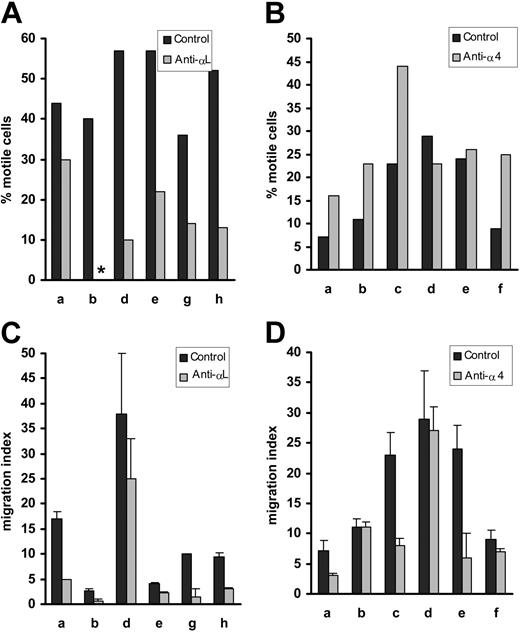
Blocking αL, but not α4, inhibits the motility of normal B cells
The motility of normal B cells on HUVECs was reduced by anti-αL mAb, but not by a blocking mAb against α4 (Figure 3A-B). Indeed, in 4 of the 6 donors studied, motility may have been enhanced in the presence of α4 blockade. Like motility, TEM was consistently reduced in the presence of a blocking anti-αL mAb (Figure 3C). The effect of anti-α4 on TEM was variable. In 3 of the 6 donors studied the migration index was unchanged, while in the other 3 it was reduced (Figure 3D).
Effect of αL and α4 blockade on normal B-cell motility on, and migration through, HUVECs. Panels A and B show the effect of blocking mAbs on motility on HUVECs, while C and D illustrate the effect of these mAbs on TEM. αL blockade induced significant inhibition of both motility (P = .0006) and TEM (P = .006), whereas α4 had no significant effect on normal B-cell motility on (P = .07) or through (P = .08) HUVECs. Asterisk indicates no motile cells. Error bars indicate standard error of the mean.
Effect of αL and α4 blockade on normal B-cell motility on, and migration through, HUVECs. Panels A and B show the effect of blocking mAbs on motility on HUVECs, while C and D illustrate the effect of these mAbs on TEM. αL blockade induced significant inhibition of both motility (P = .0006) and TEM (P = .006), whereas α4 had no significant effect on normal B-cell motility on (P = .07) or through (P = .08) HUVECs. Asterisk indicates no motile cells. Error bars indicate standard error of the mean.
Taken together with the results in the previous section dealing with VEGF blockade, these experiments show that normal B-cell motility on and through HUVECs is dependent on αL, but independent of VEGF. The effects of blocking α4 were less consistent and differed for motility versus TEM.
This importance of αL in normal B-cell motility was confirmed by showing that normal B cells were motile (47% ± 30%, n = 3) on a surface coated with chemokine (CCL21) and the αL ligand, ICAM-1 (10 μg/mL). No cell movement was observed in the absence of chemokine (data not shown). Furthermore, and as expected, blocking autocrine VEGF with either anti-VEGF mAb or SU5416 had no effect on the motility of normal B cells on ICAM-1 in the presence of CCL21 (n = 3, data not shown).
The motility of CLL cells differs from that of normal B cells with regard to involvement of both α4 and αL
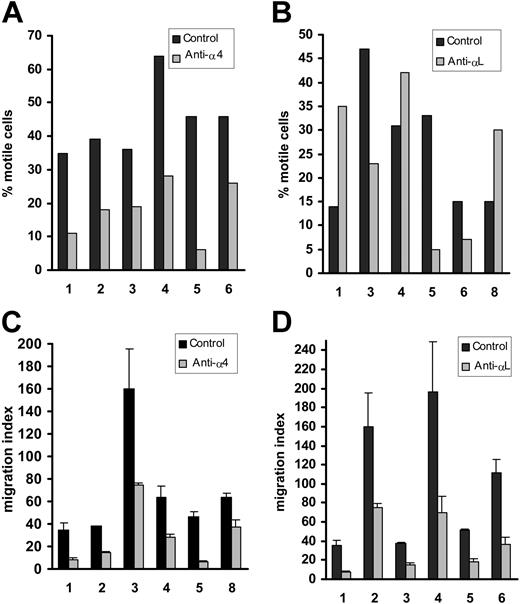
In contrast to normal B-cell motility, that of CLL cells on and through HUVECs was consistently inhibited by a blocking mAb to α4 (Figure 4A,C). As expected, blocking the α4 ligand, VCAM-1, had the same effect (data not shown, n = 2). Taken together with the earlier VEGF blocking experiments, these results indicate that CLL-cell motility on and through HUVECs, unlike that of normal B cells, is consistently dependent on α4-VCAM-1 interactions and VEGF. This conclusion was confirmed by showing that CLL cells were motile on a VCAM-1-coated surface in the presence of chemokine (28% ± 11%, n = 3). Furthermore, this motility was inhibited by the blocking anti-VEGF mAb and SU5416 (0% and 9% ± 8% respectively, n = 3), confirming that autocrine VEGF is indeed required for CLL-cell motility involving α4-VCAM-1 interactions.
CLL cells also differed from normal B cells with regard to their use of αL in that a blocking mAb had no consistent effect on their motility on HUVECs (Figure 4B). Thus, among the 6 cases studied, blocking αL reduced motility in 3 cases (nos. 3, 5, and 6; Figure 4B), but enhanced motility in the remaining 3 (nos. 1, 4, and 8; Figure 4B). These differences were not the result of variable αL expression since both the number of cells expressing this integrin and the intensity of staining were comparable in all 6 patients studied (79% ± 8%, MFI = 84 ± 8). With regard to TEM, αL consistently and markedly reduced the number of transmigrating CLL cells (Figure 4D).
At this point, we concluded that α4-expressing CLL cells require both autocrine VEGF and α4-VCAM-1 interactions for their TEM, whereas this requirement varies with regard to their motility on HUVECs. In contrast, normal B cells do not require VEGF and are less clearly dependent on α4. With regard to αL, this integrin is known to be important in the TEM of both CLL17 and normal B cells.28,29 We therefore suggest that a dual signal generated by α4 engagement and VEGF results in the activation of αL necessary for the chemokine-induced TEM of CLL cells. In contrast, chemokine alone is able to activate the αL of normal B cells for TEM. This points to a functional defect in CLL cells of αL activation by chemokine.
We next sought to confirm this hypothesis by examining αL clustering in CLL and normal B cells on HUVECs in the presence or absence of VEGF blockade. We used this approach because receptor clustering is known to play an important role in the activation and function of integrins.30-32
Effect of α4 and αL blockade on CLL-cell motility and TEM. (A-B) Effect of blocking antibodies on motility on HUVECs as in Figure 3. (C-D) Effect of these antibodies on TEM. Both anti-integrin mAbs significantly inhibited TEM (P < .005), whereas the effect on motility was only significant in the case of anti-α4 (P = .004; for αL, P = .4).
Effect of α4 and αL blockade on CLL-cell motility and TEM. (A-B) Effect of blocking antibodies on motility on HUVECs as in Figure 3. (C-D) Effect of these antibodies on TEM. Both anti-integrin mAbs significantly inhibited TEM (P < .005), whereas the effect on motility was only significant in the case of anti-α4 (P = .004; for αL, P = .4).
αL clustering on CLL cells differs from that of normal B cells, in being dependent on VEGF and α4
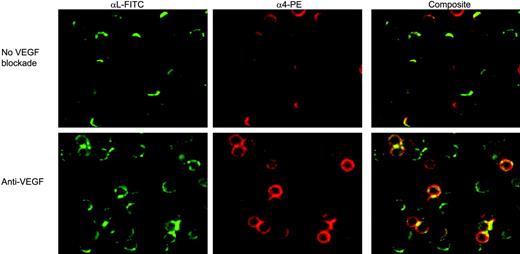
Using immunofluorescence live-cell imaging, we found that αL on both CLL (Figures 5 and 6A) and normal B cells (Figure 6B) was clearly clustered to the leading edge of cells that were motile on HUVECs. The number of CLL cells exhibiting this clustering was markedly reduced in the presence of either VEGF blockade (Figures 5 and 6A) or of a blocking anti-α4 mAb (Figure 6A). In contrast, such inhibition had no effect on the clustering of αL on normal B cells motile on HUVECs (Figure 6B). These experiments therefore confirm our earlier conclusion that the αL of CLL cells differs from that of normal B cells in requiring α4 and VEGF signals for activation in the presence of chemokine. This conclusion was further confirmed by culturing CLL and normal B cells on ICAM-1 in the presence of CCL21. While the αL of normal B cells became clearly clustered, that of CLL cells did not. (For normal B cells, the percentage of polarized cells = 36% ± 9%; for CLL cells, 5% ± 5%; n = 3.)
Effect of VEGF blockade on CLL-cell integrin clustering. CLL cells were prestained with nonblocking anti-αL-FITC or anti-α4-PE mAbs. After staining, cells were preincubated with the anti-VEGF blocking mAb before being placed on HUVEC monolayers, and filmed for 60 minutes using live cell imaging. The images shown were obtained after 5 minutes. In the absence of VEGF blockade, both α4 and αL were clearly polarized on most cells (top row), and this polarization was markedly reduced in the presence of the blocking anti-VEGF mAb (bottom row). Similar results were obtained when the SU5416 inhibitor was used to inhibit VEGF (data not shown). Comparable staining was observed throughout the 60 minutes of culture. These images are representative of 3 experiments in 3 separate patients, and the results for coclustered α4 and αL (composite staining) are shown quantitatively in Figure 6.
Effect of VEGF blockade on CLL-cell integrin clustering. CLL cells were prestained with nonblocking anti-αL-FITC or anti-α4-PE mAbs. After staining, cells were preincubated with the anti-VEGF blocking mAb before being placed on HUVEC monolayers, and filmed for 60 minutes using live cell imaging. The images shown were obtained after 5 minutes. In the absence of VEGF blockade, both α4 and αL were clearly polarized on most cells (top row), and this polarization was markedly reduced in the presence of the blocking anti-VEGF mAb (bottom row). Similar results were obtained when the SU5416 inhibitor was used to inhibit VEGF (data not shown). Comparable staining was observed throughout the 60 minutes of culture. These images are representative of 3 experiments in 3 separate patients, and the results for coclustered α4 and αL (composite staining) are shown quantitatively in Figure 6.
Since it is known that VEGF can activate β1 integrins,25,27 we next used the same approach to examine the effect of the growth factor on α4β1 activation as measured by the clustering of this integrin on motile cells in the presence or absence of VEGF blockade. α4 was clearly clustered to 1 pole of CLL cells motile on either HUVECs (Figure 5) or VCAM-1 (+CCL21; Figure 6C). This α4 clustering was reduced by either the blocking anti-VEGF mAb or SU5416 (Figures 5 and 6). Furthermore, when the CLL cells were incubated on either VCAM-1 or HUVECs, both α4 and αL were colocalized to 1 pole of the cells and the clustering of αL on both substrata was inhibited by VEGF blockade (Figures 5 and 6). Thus, it seems that VEGF induces the activation of CLL-cell α4 for motility in a process that leads to the clustering of αL and the colocalization of the 2 integrins. This conclusion was confirmed by showing that a blocking mAb to α4 also inhibited the αL clustering observed on HUVECs (Figure 6A).
Effect of VEGF and α4 blockade on integrin clustering on CLL and normal B cells. (A) Quantitative data from the experiments illustrated in Figure 5 involving CLL cells incubated on HUVECs. (B) Results for similar experiments performed with normal B cells motile on HUVECs. (C) Results of experiments where CLL cells were cultured on VCAM-1 (+ CCL21). In all 3 panels, for the experiments involving VEGF inhibition, more than 98% of cells showed copolarization of both α4 and αL and the results therefore show the percentage of copolarized cells. However, when the effect of α4 inhibition was examined the cells were stained with αL only, and the percentages given represent the number of cells in which this integrin was clustered. All data were obtained after 5 minutes of incubation.
Effect of VEGF and α4 blockade on integrin clustering on CLL and normal B cells. (A) Quantitative data from the experiments illustrated in Figure 5 involving CLL cells incubated on HUVECs. (B) Results for similar experiments performed with normal B cells motile on HUVECs. (C) Results of experiments where CLL cells were cultured on VCAM-1 (+ CCL21). In all 3 panels, for the experiments involving VEGF inhibition, more than 98% of cells showed copolarization of both α4 and αL and the results therefore show the percentage of copolarized cells. However, when the effect of α4 inhibition was examined the cells were stained with αL only, and the percentages given represent the number of cells in which this integrin was clustered. All data were obtained after 5 minutes of incubation.
The normal inhibitory effect of αL engagement on α4 function is absent in CLL cells
The work presented earlier showed that normal and CLL B cells behave very differently with regard to their use of α4 for motility on HUVECs. Thus, a blocking anti-α4 antibody inhibited the motility of CLL cells on HUVECs, but had no consistent effect on the movement of normal B cells under the same conditions. Given that it is known that, in normal cells, αL engagement can have an inhibitory effect on the function of α4,24 and given our demonstration that αL is functionally defective on CLL cells, we postulated that their α4 was not being inhibited as a result of defective αL-ICAM-1 interaction. To test this hypothesis, we examined the chemokine-induced motility of normal and CLL lymphocytes on VCAM-1 in the presence or absence of αL engagement by soluble ICAM-1 complexes (“Patients, materials, and methods”) or by anti-αL mAb.
We found that both cell types were motile on VCAM-1 in the presence of CCL21 (Figure 7). However, the effect of αL engagement differed in the 2 cell types (Figure 7). Thus, αL engagement inhibited the movement of normal B cells on this substratum (Figure 7A), but had no effect on the motility of CLL cells (Figure 7B).
These data therefore lend further support to our observations indicating that αL is functionally defective in CLL. Furthermore, they indicate that the reason that normal B cells do not use their α4 for movement on HUVECs is that engagement of their αL inhibits α4-mediated motility on VCAM-1. In contrast, we conclude that CLL cells use α4 for motility because the functional state of αL is altered so that engagement of this integrin does not generate the inhibitory signal to α4.
VEGF and α4 are also required for CLL-cell motility induced by CXCL12
Several studies have indicated that CXCL12 (stromal cell-derived factor-1α [SDF-1α]) stimulates CLL-cell motility and is probably important for the homing of the malignant lymphocytes to bone marrow.18,33 Employing the same approaches used for CCL21, we therefore examined the effect of blocking VEGF (with SU5416 and a blocking anti-VEGF mAb) and α4 on CXCL12-induced TEM and cell motility on VCAM-1. All 3 forms of blockade inhibited both types of CLL-cell motility (n = 3; data not shown). This indicates that VEGF and α4 are required not only for CLL-cell motility induced by CCL21 (via CCR7), but also for that produced by CXCL12 (via CXCR4).
Effect of αL cross-linking on the movement of CLL and normal B cells on VCAM-1. CLL (A) or normal B cells (B) were preincubated with ICAM-1 complexes (see “Patients, materials, and methods”) to cross-link αL. Cells were then filmed by TLVM during culture for 60 minutes on VCAM-1- and CCL21-coated plates.
Effect of αL cross-linking on the movement of CLL and normal B cells on VCAM-1. CLL (A) or normal B cells (B) were preincubated with ICAM-1 complexes (see “Patients, materials, and methods”) to cross-link αL. Cells were then filmed by TLVM during culture for 60 minutes on VCAM-1- and CCL21-coated plates.
Discussion
The aim of the present study was to examine the role of autocrine VEGF in (patho)physiologically relevant CLL-cell motility. We used lymphocytes from patients with lymphadenopathy in whom we had previously shown that the malignant cells express α4β1 integrin and are able to undergo TEM dependent on α4, αL, and CCR7 ligands such as CCL21.17 We examined cell movement on and through endothelial monolayers since both processes are important for homing to lymphoreticular tissues.34-36 The TEM was completely dependent on chemokine, and motility on endothelium was probably similarly dependent since it was abrogated by pertussis toxin. Importantly, this chemokine-induced movement of CLL cells on and through endothelium was dependent on VEGF since blocking the cytokine and its receptors inhibited both types of cell movement. Also, VEGF was shown to be important for the CLL-cell motility induced by 2 different physiologically relevant chemokines (CCL2117,19 and CXCL1217,19 ). This is the first demonstration that VEGF is important in chemokine-induced CLL-cell motility. Furthermore, since we could find no evidence of VEGF synthesis or secretion by HUVECs, we conclude that autocrine VEGF derived from the CLL cells themselves is involved in this motility. This importance of autocrine VEGF was further confirmed by showing that blocking VEGF inhibited the chemokine-induced movement of CLL cells on purified VCAM-1, the endothelial cell ligand for α4. CLL cells lacking α4β1 are unable to undergo TEM,17,19 and yet produce VEGF.10,17 Therefore, it is the combined stimulation by VEGF and α4 engagement that is critical for chemokine-induced TEM. All CLL clones produce variable amount of VEGF,10 and we have found that there is no correlation between the levels of secretion and CLL-cell TEM and/or lymphadenopathy (K.J.T., unpublished observation, June 2001). Therefore it seems that it is the expression of α4β1, rather than the precise level of VEGF production, that is critical for the extent of integrin-dependent motility and transmigration. Importantly, the dependence on VEGF for cell motility was a CLL-related phenomenon since the movement of normal PB B and T cells was not affected by VEGF inhibition.
Since lymphoreticular tissues are important sites of malignant-cell survival and proliferation37,38 and since the extent of tissue invasion in CLL is an important determinant of prognosis,39,40 we suggest that the present results are not only important for the pathogenesis of tissue-phase CLL, but may also be therapeutically relevant. Thus, anti-VEGF therapy might be expected to block the entry of CLL cells into lymphoreticular tissues, thereby depriving the malignant cells of microenvironmental stimuli favoring their survival and proliferation. Furthermore, since we show here that VEGF is not involved in the movement of normal lymphocytes on and through endothelium, this treatment would have the additional benefit of having no effect on the tissue entry of normal B and T cells in these already immunocompromised patients.
The fact that VEGF blockade inhibited CLL-cell motility without influencing the movement of normal lymphocytes implies that CLL cells are unable to activate a motility mechanism present in normal lymphocytes and that VEGF can compensate for this deficiency. We next analyzed the mechanism(s) involved in this specific dependence of chemokine-induced CLL-cell motility on VEGF. To do this, we examined the involvement of α4β1 and αLβ2 integrins since both are known to be important in normal24,41,42 and CLL17 cell interactions with endothelium. In particular, it has been established that both α4β1-VCAM-136,43 and αLβ2-ICAM-144,45 interactions can mediated cell motility, and that αLβ2 is a central component of TEM into peripheral lymph nodes.29,46
We first showed that the movement of normal B cells, which also produce VEGF,10 was mediated by αL and could be directly stimulated by chemokine without the involvement of either autocrine VEGF or α4. Thus, movement on and through endothelium and chemokine-induced motility on ICAM-1 were all unaffected by blockade of either VEGF or α4; motility on and through endothelium was, however, inhibited by anti-αL mAb. In contrast, when similar experiments were performed with CLL cells, TEM was found to be dependent on both VEGF and α4, as well as on αL. Given that all the CLL-cell clones consistently expressed αL, these results taken together suggest that the αLβ2 of CLL cells differs functionally from that of normal B cells in requiring combined stimulation by VEGF and α4β1 engagement, as well as chemokine, to mediate TEM.
The role of αLβ2 in CLL-cell motility on endothelium differed somewhat from its involvement in TEM. Thus, although the activation of αLβ2 for both movement on, and through, endothelium were dependent on VEGF and α4β1, a blocking anti-αL antibody did not consistently inhibit motility on HUVECs. It is well established that the mechanisms involved in movement on, as compared with through, endothelium differ.29,32 We therefore conclude that the function of CLL-cell αLβ2 is consistently altered in its ability to mediate TEM, but less consistently so with respect to motility on endothelium.
Since CLL cells were dependent on VEGF, α4, αL, and chemokine for TEM, this suggested that VEGF and α4 engagement were together able to overcome the functional defect of αL activation. Because integrin clustering is an important part of integrin activation and function in cell motility,30,31 we used live-cell imaging to examine the effect of blockade of VEGF or α4 on the distribution of α4 and αL on CLL and normal B cells cultured on HUVECs. αL clustering on the 2 cell types was different in that, in CLL cells, the clustering of αL at the leading edge of motile cells was dependent on both α4 and VEGF, whereas that of normal B cells was not. Furthermore, in CLL cells cultured on VCAM-1 not only α4, but also αL, was clustered, and this clustering was inhibited by VEGF blockade. We therefore conclude that in CLL cells there is a defect in chemokine-induced inside-out activation of αLβ2, and that VEGF and α4β1 signaling together overcome the defect. The presence of this defect was confirmed directly by showing that chemokine did not induce clustering of the αL of CLL cells on ICAM-1, while the αL of normal B cells became clearly polarized under identical conditions. Clearly, to understand the nature of the functional defect of αL, its origin, and the mechanism of its correction by VEGF and α4 engagement requires complex signaling studies, which are the subject of our ongoing investigations.
The normal αL signaling in PB B cells explains why in our experiments these cells, unlike CLL lymphocytes, were not able to use α4 for motility in the presence of αL engagement. Thus, it has been previously reported that αL engagement has a negative feedback effect on the α4 activity of normal lymphocytes,24 and we confirmed this here. In contrast, such negative feedback did not occur in CLL cells, presumably as a result of the altered functional state of αL demonstrated in the present study.
Since anti-α4 antibodies,47 as well as anti-VEGF therapies, are becoming available for clinical use, the dependence of CLL-cell motility on both VEGF and α4β1 engagement raises the possibility that such agents, when used either alone or in combination, might have therapeutic potential in patients with CLL who have tissue invasion.
Prepublished online as Blood First Edition Paper, February 24, 2005; DOI 10.1182/blood-2004-10-4054.
Supported by a grant from the Leukaemia Research Fund (United Kingdom).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal