Abstract
The etiologic agent of adult T-cell leukemia (ATL) is human T cell lymphotropic virus type I (HTLV-I). The HTLV-I protein Tax alters gene expression, including those of cytokines and their receptors, which plays an important role in early stages of ATL. Here we demonstrate that expression of interleukin-9 (IL-9) is activated by Tax via an NF-κB motif in its proximal promoter, whereas IL-9 receptor-α (IL-9Rα) expression is not induced by Tax. However, supporting a role for IL-9/IL-9Rα in ATL, a neutralizing monoclonal antibody directed toward IL-9Rα inhibited ex vivo spontaneous proliferation of primary ATL cells from several patients. Fluorescence-activated cell sorter analysis of freshly isolated peripheral blood mononuclear cells from these patients revealed high level expression of IL-9Rα on their CD14-expressing monocytes. Furthermore, purified T cells or monocytes alone from these patients did not proliferate ex vivo, whereas mixtures of these cell types manifested significant proliferation through a contact-dependent manner. Taken together, our data suggest that primary ATL cells, via IL-9, support the action of IL-9Rα/CD14-expressing monocytes, which subsequently support the ex vivo spontaneous proliferation of malignant T cells. In summary, these data support a role for IL-9 and its receptor in ATL by a paracrine mechanism.
Introduction
Adult T-cell leukemia (ATL) is a highly aggressive neoplasm characterized by a clonal expansion of CD4+ lymphocytes and by a monoclonal integration of human T cell lymphotropic virus type I (HTLV-I) provirus(es) in the tumor cells.1,2 HTLV-I is a type C retrovirus endemic in southern Japan, the Caribbean basin, Central and Southern Africa, and South America.3,4 Less than 5% of HTLV-I–infected persons develop either ATL or a chronic inflammatory disease of the central nervous system termed HTLV-I–associated myelopathy/tropical spastic paraparesis (HAM/TSP). Although the precise mechanisms of ATL leukemogenesis remain unclear, it has been suggested that the transformation of T cells in the early stages of the disease is mediated by the HTLV-I Tax protein (p40). Tax activates long terminal repeat-directed transcription by recruiting members of the cAMP response element-binding/and activating transcription factors family to the viral promoter. In addition, Tax activates other cellular transcription factors, such as NF-κB. Tax is associated with the expression of cellular genes,5 including the cytokine6-10 and cytokine receptor genes.7,11-13 It has been suggested that the dysregulation of cytokines and their receptors in HTLV-I–infected persons may play an important role in the early course of disease via autocrine stimulation.9,11
Interleukin-9 (IL-9) is a T cell–derived cytokine with pleiotropic activities on various cell types.14-16 IL-9 is mainly expressed by activated CD4+ T cells.17 The functions of IL-9 are mediated through the IL-9 receptor (IL-9R), which is a member of the hematopoietin receptor superfamily.18 The IL-9 receptor consists of the ligand-specific α-chain and the common γ-chain that is shared with IL-2, IL-4, IL-7, IL-15, and IL-21 receptors.19 More recently, a number of observations have suggested that IL-9 may play a role in asthma and allergic immune responses.16,17
However, the understanding of IL-9/IL-9R in the context of HTLV-I infection and ATL is incomplete. A number of studies have shown that IL-9 expression is increased by HTLV-I infection, and transcripts for IL-9 are readily detected in many HTLV-I–infected cell lines and primary ATL cells20,21 ; however, the mechanism of this activation has not been fully explored. In addition, IL-9Rα expression has not been detected on ATL cells and the weak response of ex vivo, primary ATL cells to exogenous IL-9 further obscures the potential role of IL-9 in ATL disease.21,22 Here we show that IL-9 expression is associated with the expression of HTLV-I Tax protein in primary ATL cells. Conversely, the expression of IL-9Rα does not appear to be induced by Tax. Yet, despite these observations, in 5 of the 9 cases with spontaneous proliferation of peripheral blood mononuclear cells (PBMCs) ex vivo, a monoclonal antibody directed to IL-9Rα inhibited the spontaneous proliferation of the primary ATL cells, strongly suggesting a role for IL-9/IL-9Rα in the expansion of HTLV-I–infected lymphocytes ex vivo. Fluorescence-activated cell sorter (FACS) analysis of freshly isolated PBMCs from those ATL patients examined revealed high expression of IL-9Rα on their monocytes, whereas monocytes from normal subjects were negative for IL-9Rα expression. Furthermore, the purified T cells or monocytes from these patients when cultured alone did not proliferate ex vivo, whereas the mixture of purified T cells and monocytes manifested significant proliferation through a contact-dependent manner. In addition, the proliferation was partially inhibited by antibody directed to IL-9Rα. These studies suggested that an action of IL-9Rα/CD14-expressing monocytes is mediated by IL-9, secreted by HTLV-I–infected ATL cells, which, in turn, are stimulated to proliferate by a paracrine mechanism. Taken together, our data suggest a role for IL-9/IL-9Rα in the spontaneous proliferation of primary ATL cells in ex vivo PBMC cultures.
Methods
Cell culture, plasmids, antibodies, and reagents
All cells were cultured in RPMI 1640 plus 10% fetal bovine serum (FBS). NK-92 was maintained with addition of 60 U of rhIL-2.23 Human PBMCs were isolated by Ficoll-Hypaque density centrifugation. The pMT2T-Tax, pMT2T-p50, and pMT2T-p65 plasmids were previously described.24-27 The IL-2–neutralizing monoclonal antibody was from R&D Systems (Minneapolis, MN), and the IL-9– and IL-9Rα–neutralizing antibodies were purchased from BioLegend (San Diego, CA). All the antibodies used for FACS were from BD Biosciences (San Diego, CA), except αIL-9Rα, which was purchased from BioLegend. The IL-9 enzyme-linked immunosorbent assay (ELISA) master kit was from BioLegend; the dual-luciferase assay kit was from Promega (Madison, WI). The study protocol was approved by the Institutional Review Board of the National Cancer Institute. Informed consent was obtained in accordance with the Declaration of Helsinki.
Taqman real-time PCR
The Taqman polymerase chain reaction (PCR) was done following the manufacturer's instruction. The Taqman Universal PCR Master Mix, the IL-9 primer/probe, HTLV-I Tax primer/probe, and the HPRT1 primer/probe sets were purchased from Applied Biosystems (Foster City, CA).
IL-9 and IL-9Rα gene promoter cloning
We used Genome Walker Library (Clontech, Mountain View, CA) to clone the IL-9 and IL-9Rα gene 5′ regulatory regions.17 Two antisense primers, 5′-CTGCATCTTGTTGATGAGGAAGTTGATG-3′ and 5′-ACGGAGCACAGGAGCAGGGCAGAGGT-3′, were used in 2 consecutive PCRs to amplify the upstream region of the IL-9 exon 1. The IL-9Rα 5′ region was amplified using antisense primers 5′-GATGGTGAAATTGTCAGAT-3′ and 5′-GGCAGCACGACGGTGCACTCACT-3′. The amplified fragments were cloned into PCR2.1 plasmid and later into the luciferase reporter plasmid pGL3.
Gel shift assay
The gel shift assay was done using the gel shift assay system from Promega.27,28 Briefly, 5 μg of nuclear extract was incubated at room temperature with 100 000 cpm of 32P-labeled probe in the binding buffer with or without unlabeled specific competitor or nonspecific competitor (SP1 consensus probe) for 20 minutes. The DNA-protein complex was separated from the free probe by electrophoresis on the Novex 6% DNA retardation gel (Invitrogen, Carlsbad, CA). Gels were then dried and analyzed using the phosphorimaging instrument.
CFSE staining
Cell division generation was measured in ATL PBMCs in accordance with previously described protocols for dye dilution.29 Briefly, PBMCs were suspended at 10 × 106 cells/mL, stained under low light with 5 μM (final concentration) of the cell division tracking dye carboxyfluorescein diacetate succinimidyl ester (CFSE) in phosphate-buffered saline (PBS) plus 5% FBS for 5 minutes at room temperature, and washed 3 times with PBS plus 5% FBS, then resuspended in RPMI 1640 plus 10% FBS. The stained cells were cultured for 6 days and the dividing cells were analyzed by FACS sorting after 6 days in culture.
T cells and monocyte purification and proliferation
T cells and monocyte separation was performed using the human pan T cell isolation kit II and human monocyte isolation kit II (Miltenyi Biotec, Auburn, CA). T cells and monocyte separation are both based on negative selection. The separated T cells were either cultured alone at 1 × 106 cells/mL or with 1 × 106 cells/mL separated monocytes for 6 days in a 96-well plate or transwell plate. The proliferation was measured by 3H thymidine uptake after 6 days in culture.
Microarray analysis
Microarray analysis was performed as described by Shaffer et al.30 Total RNA was prepared using RNAeasy kit (Qiagen, Valencia, CA). The NCI human gene expression operon array was used in this study. The raw data from each cDNA array were normalized as described30 and deposited in the online Gene Expression Omnibus (GEO) of the National Center for Biotechnology Information under GEO series accession number GSE10789.31
Results
IL-9 secretion by PBMCs from ATL patients and HTLV-I–infected T-cell lines
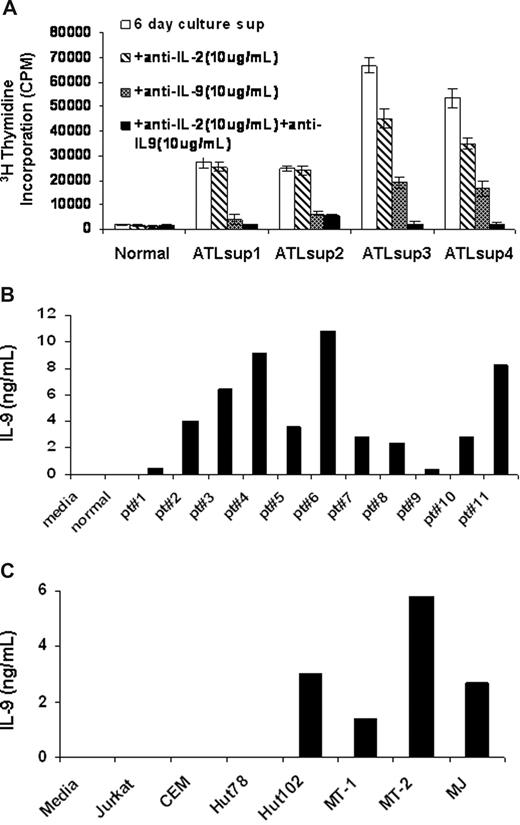
To determine what cytokines were produced in supernatants from ex vivo cultured PBMCs of ATL patients, we performed a proliferation assay using the cytokine-dependent human natural killer cell line NK-92 as an indicator.23 PBMCs from ATL patients and normal controls were cultured for 6 days in RPMI 1640 media plus 10% FBS, and the culture supernatants were harvested. Little or no stimulatory activity was observed in the supernatants from normal human PBMC cultures. In contrast, the culture supernatants obtained from PBMCs of ATL patients often showed high NK-92 stimulatory activity. In a number of cases, this stimulatory activity could be inhibited in part or completely by a neutralizing antibody to IL-2 alone or to a combination of antibodies to IL-2 and IL-9. In 5 of 11 cases studied, the proliferation could be inhibited by a neutralizing antibody to IL-9 but only modestly by an antibody to IL-2 (Figure 1A). To determine the IL-9 protein levels in these culture supernatants, we performed an ELISA using a pair of antibodies specifically raised against human IL-9. Consistent with the proliferation data, culture supernatants from ATL PBMCs from all 11 patients examined contained detectable levels of IL-9 (1-10 ng/mL) (Figure 1B). In contrast, no IL-9 was detectable in the culture supernatants of PBMCs obtained from normal donors (n = 10, patient vs normal, P < .001). We next examined, by ELISA, the IL-9 protein levels in culture supernatants from HTLV-I–infected T-cell lines, including Hut102, MT-1, MT-2, and MJ as well as the HTLV-I–negative T-cell lines Jurkat, CEM, and Hut78. As shown in Figure 1C, the IL-9 protein was not detected in culture supernatants from the HTLV-I–negative T-cell lines, whereas the HTLV-I–infected cell lines had high levels of IL-9 in their supernatants (1-6 ng/mL). These findings suggested that IL-9 expression was elevated in some HTLV-I–infected T-cell lines and ATL PBMCs.
IL-9 was secreted into the supernatants of ATL PBMCs and HTLV-I–infected cell lines. (A) NK-92 cell line assay of 6-day culture supernatants of PBMCs from smoldering and chronic ATL patients were performed. Monoclonal antibodies to IL-2 or IL-9 or to both were added to the assay to detect the presence of IL-2 or/and IL-9 in the 6-day culture supernatants. The normal control was typical of that observed from 10 normal donors. (B) IL-9 ELISA was performed to define the IL-9 levels in the 6-day culture supernatants of PBMCs from 11 patients with ATL. The growth medium and the supernatants from normal donor PBMCs culture were used as a control (n = 10, patient vs control, P < .001). (C) An IL-9 ELISA was performed to determine the IL-9 levels in the culture supernatants of HTLV-I–infected cell lines Hut102, MT-1, MT-2, and MJ as well as HTLV-I–negative cell lines Jurkat, CEM, and Hut78.
IL-9 was secreted into the supernatants of ATL PBMCs and HTLV-I–infected cell lines. (A) NK-92 cell line assay of 6-day culture supernatants of PBMCs from smoldering and chronic ATL patients were performed. Monoclonal antibodies to IL-2 or IL-9 or to both were added to the assay to detect the presence of IL-2 or/and IL-9 in the 6-day culture supernatants. The normal control was typical of that observed from 10 normal donors. (B) IL-9 ELISA was performed to define the IL-9 levels in the 6-day culture supernatants of PBMCs from 11 patients with ATL. The growth medium and the supernatants from normal donor PBMCs culture were used as a control (n = 10, patient vs control, P < .001). (C) An IL-9 ELISA was performed to determine the IL-9 levels in the culture supernatants of HTLV-I–infected cell lines Hut102, MT-1, MT-2, and MJ as well as HTLV-I–negative cell lines Jurkat, CEM, and Hut78.
Effect of HTLV-I Tax expression on IL-9 mRNA levels
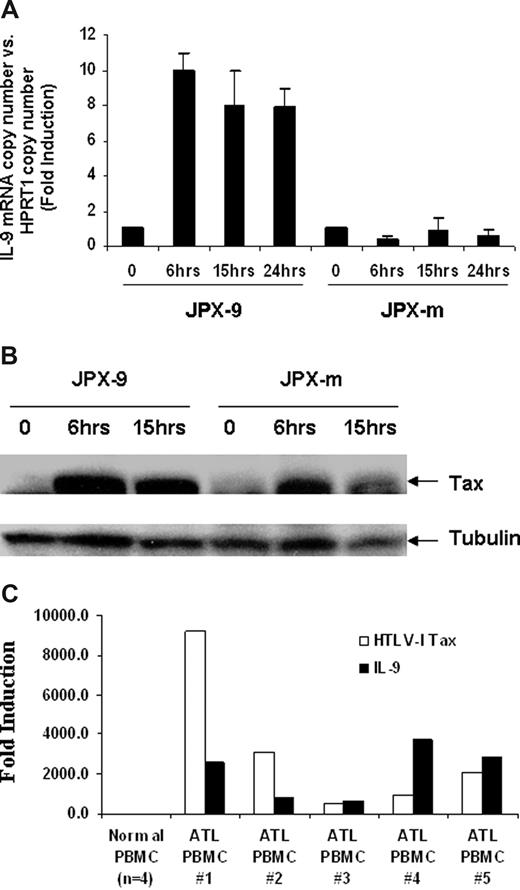
We hypothesized that IL-9 expression levels might be increased through the action of the HTLV-I–encoded protein Tax. To explore the influence of Tax on IL-9 expression, we used 2 cadmium chloride (CdCl2) inducible Jurkat Tax transfectants, JPX-9 and JPX-m, which expresses wild-type Tax or a nonfunctional Tax protein, respectively.32 As shown in Figure 2A, the IL-9 mRNA levels were increased approximately 10-fold on induction of wild-type Tax protein in JPX-9 cells but were not changed in JPX-m cells, which produced a mutant nonfunctional Tax protein after induction. The IL-9 mRNA level could be detected as early as 6 hours after addition of CdCl2 (Figure 2A), and this correlated with the expression of Tax protein induced by CdCl2. As shown in Figure 2B, Tax expression was induced 6 hours after addition of CdCl2 in both JPX-9 and JPX-m cells. These data suggest that the Tax protein is involved in the elevated expression of IL-9 observed in HTLV-I–infected cells. Tax is an inducer of NF-κB. However, in ATL cells from patients, NF-κB remains activated despite the eventual shutdown of Tax expression in some cases, suggesting that Tax may be needed to initiate but not to maintain NF-κB activation.33 To address this issue, in the present study, TaqMan real-time PCR was performed on the ex vivo cultured PBMCs using IL-9 and Tax primer/probes. Very modest amounts of message for both Tax and IL-9 were expressed in cells immediately ex vivo. However, as noted in Figure 2C, ex vivo cultured cells from all 5 patients examined coexpressed large quantities of both Tax and IL-9.
HTLV-I Tax-transactivated IL-9 expression in Jurkat T cells. (A) IL-9 mRNA levels after Tax induction were detected by Taqman real-time PCR. The copy number of IL-9 mRNA was normalized by the copy number of hypoxanthine guanine phosphoribosyl transferase 1 (HPRT1) mRNA. The data are representative of 3 independent experiments. (B) Western blot analysis using a monoclonal antibody to Tax for relative expression of wild-type HTLV-I Tax (JPX-9) and nonfunctional mutant Tax (JPX-m) in JPX-9 and JPX-m before and after addition of 20 μM CdCl2. (C) Taqman real-time analysis of HTLV-I Tax and IL-9 message in ATL PBMCs ex vivo cells.
HTLV-I Tax-transactivated IL-9 expression in Jurkat T cells. (A) IL-9 mRNA levels after Tax induction were detected by Taqman real-time PCR. The copy number of IL-9 mRNA was normalized by the copy number of hypoxanthine guanine phosphoribosyl transferase 1 (HPRT1) mRNA. The data are representative of 3 independent experiments. (B) Western blot analysis using a monoclonal antibody to Tax for relative expression of wild-type HTLV-I Tax (JPX-9) and nonfunctional mutant Tax (JPX-m) in JPX-9 and JPX-m before and after addition of 20 μM CdCl2. (C) Taqman real-time analysis of HTLV-I Tax and IL-9 message in ATL PBMCs ex vivo cells.
HTLV-I Tax transactivated the IL-9 promoter through a putative NF-κB binding site
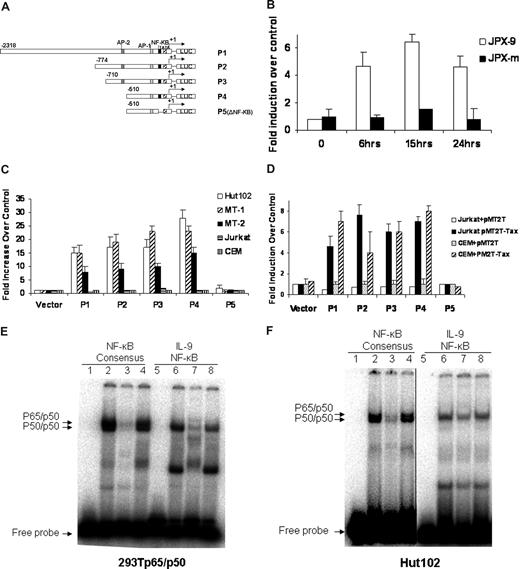
To dissect the transcriptional regulation of IL-9 in the context of HTLV-I infection, we cloned the IL-9 promoter. The transcriptional start site was determined by 5′ rapid amplification of cDNA ends (RACE) analysis on RNAs obtained from Hut102 cells. The IL-9 promoter was subsequently cloned into the pGL3 luciferase reporter construct for use in the promoter activity study. The structures of the IL-9 promoter reporter constructs P1, P2, P3, P4, and P5 are shown in Figure 3A. P1 to P4 have a series of tandem deletions from the proximal promoter, whereas with P5 the putative NF-κB binding site has been deleted. The promoter constructs were then transiently transfected into T-cell lines to determine their promoter activity. The reporter activity of the IL-9 promoter construct P1(-2318) increased approximately 5-fold when wild-type Tax protein was expressed in JPX-9 (Figure 3B). However, in JPX-m cells, which expressed a nonfunctional Tax after CdCl2 induction, the reporter activity of P1 was not changed after addition of CdCl2. These data suggest that wild-type Tax could transactivate the IL-9 promoter.
Activation of the IL-9 promoter was mediated by NF-κB and the IL-9 NF-κB motif ATGTCAGGGTTTTTCCGTGTTTG bond to NK-κB in the gel shift assay. (A) Schematic representation of the IL-9 luciferase reporter constructs P1, P2, P3, P4, and P5 (ΔNF-κB). (B) IL-9 luciferase reporter construct P1 (10 μg) and CMV-Renilla (1 μg) were transfected into JPX-9 and JPX-m cells. The Tax expression was induced by addition of CdCl2, and the promoter activities were assayed at different time points after CdCl2 addition. The promoter activities were normalized by the Renilla value. The results are representative of 3 independent experiments. (C) 10 μg of the IL-9 luciferase construct and 1 μg CMV-Renilla were transfected into Tax expressing Hut102, MT-1, MT-2, and Tax nonexpressing Jurkat, CEM cells by electroporation. Dual-luciferase assays were performed 48 hours later. Normalized results using Renilla values are representative of 3 independent experiments. Experimental variations are indicated by SE bars. (D) The IL-9 luciferase constructs were transfected into Jurkart and CEM T cells in the absence (pMT2T, empty vector) or in the presence of 10 μg of the Tax expression construct pMT2T-Tax by electroporation. Dual-luciferase assays were performed following the manufacturer's recommendations. The data are representative of 3 independent experiments. (E) Gel shift assay. Extracts obtained from 293T cells transfected with p65 and p50 expression constructs were used for the binding of cNF-κB AGTTTGAGGGGACTTTCCCAGGC and IL-9 NF-κB ATGTCAGGGTTTTTCCGTGTTTG (the underlined sequences are the NF-κB binding sites). The typical p50/p65 heterodimer and p50/p50 homodimer can be readily seen with cNF-κB, whereas IL-9 NF-κB forms a complex that comigrates with the p50/p50 homodimer of the cNF-κB, as shown by an arrow (lanes 2, 6). The binding of cNF-κB to p50/p65 and p50/p50 complexes can be competed off specifically by addition of a 50-fold molar excess of unlabeled IL-9 NF-κB probe (lane 3) but was not affected by addition of a 50-fold molar excess of unlabeled nonspecific SP1 probe (lane 4). Similarly, the binding of IL-9 NF-κB to the p50/p50 complex was specifically competed off by addition of a 50-fold molar excess of unlabeled cNF-κB probe (lane 7) and was not affected by addition of a 50-fold molar excess of unlabeled nonspecific SP1 probe (lane 8). Lanes 1 and 5 represent the negative controls. (F) Gel shift assay. Nuclear extracts from the HTLV-I–positive cell line Hut102 were used for the binding of cNF-κB and IL-9 NF-κB. The patterns of cNF-κB and IL-9 NF-κB binding are very similar to panel E. A vertical line has been inserted to indicate a reposition of gel lanes.
Activation of the IL-9 promoter was mediated by NF-κB and the IL-9 NF-κB motif ATGTCAGGGTTTTTCCGTGTTTG bond to NK-κB in the gel shift assay. (A) Schematic representation of the IL-9 luciferase reporter constructs P1, P2, P3, P4, and P5 (ΔNF-κB). (B) IL-9 luciferase reporter construct P1 (10 μg) and CMV-Renilla (1 μg) were transfected into JPX-9 and JPX-m cells. The Tax expression was induced by addition of CdCl2, and the promoter activities were assayed at different time points after CdCl2 addition. The promoter activities were normalized by the Renilla value. The results are representative of 3 independent experiments. (C) 10 μg of the IL-9 luciferase construct and 1 μg CMV-Renilla were transfected into Tax expressing Hut102, MT-1, MT-2, and Tax nonexpressing Jurkat, CEM cells by electroporation. Dual-luciferase assays were performed 48 hours later. Normalized results using Renilla values are representative of 3 independent experiments. Experimental variations are indicated by SE bars. (D) The IL-9 luciferase constructs were transfected into Jurkart and CEM T cells in the absence (pMT2T, empty vector) or in the presence of 10 μg of the Tax expression construct pMT2T-Tax by electroporation. Dual-luciferase assays were performed following the manufacturer's recommendations. The data are representative of 3 independent experiments. (E) Gel shift assay. Extracts obtained from 293T cells transfected with p65 and p50 expression constructs were used for the binding of cNF-κB AGTTTGAGGGGACTTTCCCAGGC and IL-9 NF-κB ATGTCAGGGTTTTTCCGTGTTTG (the underlined sequences are the NF-κB binding sites). The typical p50/p65 heterodimer and p50/p50 homodimer can be readily seen with cNF-κB, whereas IL-9 NF-κB forms a complex that comigrates with the p50/p50 homodimer of the cNF-κB, as shown by an arrow (lanes 2, 6). The binding of cNF-κB to p50/p65 and p50/p50 complexes can be competed off specifically by addition of a 50-fold molar excess of unlabeled IL-9 NF-κB probe (lane 3) but was not affected by addition of a 50-fold molar excess of unlabeled nonspecific SP1 probe (lane 4). Similarly, the binding of IL-9 NF-κB to the p50/p50 complex was specifically competed off by addition of a 50-fold molar excess of unlabeled cNF-κB probe (lane 7) and was not affected by addition of a 50-fold molar excess of unlabeled nonspecific SP1 probe (lane 8). Lanes 1 and 5 represent the negative controls. (F) Gel shift assay. Nuclear extracts from the HTLV-I–positive cell line Hut102 were used for the binding of cNF-κB and IL-9 NF-κB. The patterns of cNF-κB and IL-9 NF-κB binding are very similar to panel E. A vertical line has been inserted to indicate a reposition of gel lanes.
To determine which cis-element was responsible for the activation of the IL-9 gene in HTLV-I–infected T cells, we measured the reporter activity of the IL-9 promoter P1 to P5 in HTLV-I–infected T-cell lines Hut102, MT-1, and MT-2, which express Tax constitutively. As shown in Figure 3C, IL-9 reporter plasmids P1, P2, P3, and P4 were activated approximately 10- to 25-fold in HTLV-I–infected T-cell lines Hut102, MT-1, and MT-2, whereas they were not activated in the HTLV-I–negative T-cell lines, such as Jurkat and CEM. Deletion of the putative NF-κB site in the IL-9 promoter (P5) strongly diminished the IL-9 promoter activity in HTLV-I–infected cell lines. Moreover, cotransfecting Jurkat and CEM T cells with luciferase reporter plasmids P1, P2, P3, and P4 in combination with the Tax expression plasmid pMT2T-Tax increased the basal promoter activity by 4- to 8-fold (Figure 3D), whereas cotransfection of pMT2T-Tax with the putative NF-κB deletion construct (P5) showed no promoter activity. These data strongly suggest that the transactivation action of HTLV-I Tax on IL-9 expression is mediated via an NF-κB site in the proximal IL-9 promoter.
NF-κB bound specifically to a putative NF-κB motif within the IL-9 promoter
To determine whether the NF-κB proteins and the putative NF-κB motif within the IL-9 promoter are functionally related, we performed a gel shift assay comparing the putative IL-9 NF-κB sequence with the consensus NF-κB binding sequence.26,27 We first examined the binding with extracts from 293T cells transfected with plasmids expressing the p50 and p65 NF-κB subunits. As shown in Figure 3E, the IL-9 putative NF-κB site bound to the NF-κB protein complex that comigrated with p50/p50 as observed in the NF-κB consensus probe sample (Figure 3E, lane 6). Moreover, the binding of the NF-κB consensus probe was specifically competed off by the addition of a 50-fold molar excess of the unlabeled IL-9 putative NF-κB probe (Figure 3E, lane 3). Reciprocally, the binding of the IL-9 putative NF-κB probe was specifically competed off by the addition of a 50-fold molar excess of the unlabeled NF-κB consensus probe (Figure 3E, lane 7). As a nonspecific competitor control, the bindings of the NF-κB consensus probe or IL-9 putative NF-κB probe to the NF-κB protein complex were not affected by the addition of a 50-fold molar excess of an unlabeled SP1 consensus probe (Figure 3E, lanes 4 and 8). These data indicate that the putative NF-κB binding sequence within the IL-9 promoter was recognized by NF-κB proteins.
We next examined the promoter binding in the context of HTLV-I infection using nuclear extracts from the HTLV-I–negative Jurkat and HTLV-I-infected Hut102 T-cell lines. We saw a similar pattern of NF-κB protein binding to the IL-9 putative NF-κB sequence and NF-κB consensus sequence using Hut102 nuclear extract (Figure 3F), and the binding was markedly lower when nuclear extracts from Jurkat cells were used (data not shown). The increased binding in Hut102 cells was the result of the constitutively active NF-κB protein as a consequence of HTLV-I infection, whereas unactivated Jurkat cells did not have significant levels of activated NF-κB protein.
HTLV-I Tax did not transactivate IL-9Rα expression
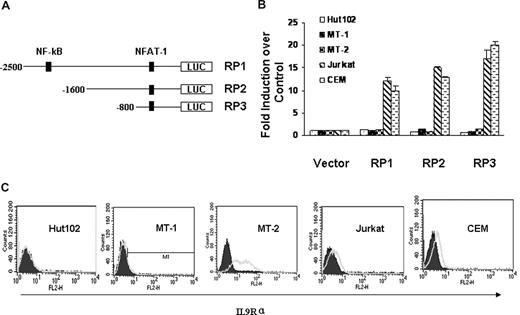
To dissect the transcriptional regulation of IL-9Rα in the context of HTLV-I infection, we cloned the IL-9Rα promoter and subsequently introduced it into a luciferase reporter construct. Figure 4A is the schematic representation of the IL-9Rα promoter construct RP1, RP2, and RP3. We first tested the IL-9Rα promoter activity in HTLV-I–positive Hut102, MT-1, and MT-2 cells and HTLV-I–negative Jurkat and CEM cells. Surprisingly, the IL-9Rα promoter constructs were constitutively active in HTLV-I–negative Jurkat and CEM cells but were quite silent in HTLV-I–positive Hut102, MT-1, and MT-2 cells (Figure 4B). These data suggest that HTLV-I Tax may not be involved in the regulation of the expression of IL-9Rα. Moreover, when IL-9Rα promoter constructs were cotransfected with an HTLV-I Tax expression construct, pMT2T-Tax, in Jurkat and CEM cells, the IL-9Rα promoter activity was not affected by the expression of Tax (data not shown). This suggests that Tax is not involved in the regulation of IL-9Rα expression. FACS analysis of the surface expression of IL-9Rα confirmed that IL-9Rα was expressed on Jurkat and CEM cells but not on HTLV-I–positive Hut102 and MT-1 cells (Figure 4C). These data show a correlation between IL-9Rα promoter activity and endogenous IL-9Rα expression. The only exception was MT-2 cells; with these cells, the IL-9Rα promoter was not activated, but IL-9Rα was highly expressed on the cell surface. This observation can be explained by an earlier study by others demonstrating that a long terminal repeat of HTLV-I had integrated into the 5′ coding sequence of the IL-9Rα gene, resulting in its dysregulated expression.34
HTLV-I Tax did not transactivate IL-9Rα. (A) Schematic representation of IL-9Rα promoter constructs RP1, RP2, and RP3. (B) IL-9Rα promoter assay in HTLV-I–positive Hut102, MT-1, MT-2 cells and HTLV-I–negative Jurkat, CEM cells. The promoter activities were normalized by comparison to the Renilla value. The results are representative of 3 independent experiments. (C) FACS analysis of surface expression of IL-9Rα on HTLV-I-positive Hut102, MT-1, MT-2 cells and HTLV-I–negative Jurkat and CEM cells. PE-conjugated mouse IgG2b was used as the control.
HTLV-I Tax did not transactivate IL-9Rα. (A) Schematic representation of IL-9Rα promoter constructs RP1, RP2, and RP3. (B) IL-9Rα promoter assay in HTLV-I–positive Hut102, MT-1, MT-2 cells and HTLV-I–negative Jurkat, CEM cells. The promoter activities were normalized by comparison to the Renilla value. The results are representative of 3 independent experiments. (C) FACS analysis of surface expression of IL-9Rα on HTLV-I-positive Hut102, MT-1, MT-2 cells and HTLV-I–negative Jurkat and CEM cells. PE-conjugated mouse IgG2b was used as the control.
Antibody directed to IL-9Rα inhibited the ex vivo spontaneous proliferation of primary ATL PBMCs
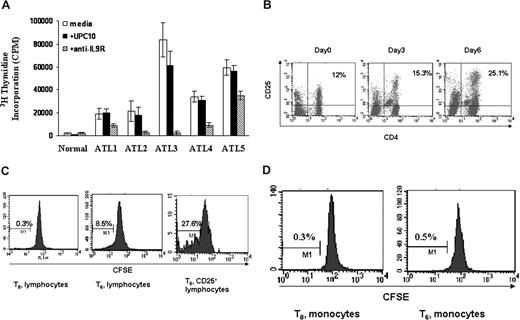
It has been suggested that the spontaneous T-cell proliferation seen in HAM/TSP is probably a lymphocyte response to Tax epitopes.35 Furthermore, the ex vivo proliferation of HAM/TSP PBMCs was partially inhibited by the addition of antibodies to IL-2 and IL-2 receptors with an augmentation of this inhibition of proliferation by addition of antibodies to IL-15 or its receptor.36,37 Similar to HAM/TSP, the HTLV-I–associated PBMCs of some patients with ATL also proliferate spontaneously in the absence of any exogenous mitogen or antigen. Spontaneous proliferation of primary ATL PBMCs with and without daclizumab (humanized anti-IL2Rα) was performed routinely in 6-day ex vivo cultures to predict the clinical response of ATL patients to daclizumab treatment. The PBMCs from most ATL patients (19 of 25) proliferated ex vivo (geometric mean 27 630 cpm, SD 2.198) with 3H thymidine uptake more than 5000 cpm/105 cells compared with less than 3000 cpm/105 cells for 20 normal controls (geometric mean 389 cpm, SD 2.812). In some cases, the proliferation of the PBMCs from ATL patients could be inhibited partly or completely by the addition of either an antibody to IL-2 or daclizumab. To explore the biologic function of IL-9 in the context of ATL spontaneous proliferation, we added a monoclonal antibody directed to IL-9Rα to the proliferation assay at T0. In our subsequent studies, we focused on 5 ATL patients wherein the antibody directed to IL-9Rα inhibited the proliferation, whereas the nonspecific control antibody UPC10 had no effect (Figure 5A). This suggested that signaling through IL-9Rα was important for the spontaneous proliferation of these ATL T cells. To define which cell population within the ATL PBMCs was proliferating ex vivo, we first assessed the activated T cells (CD4+/CD25+). We found that CD4+/CD25+-expressing cells in the PBMCs proliferated so that there developed an increasing percentage of these cells in the ex vivo cultures over time (Figure 5B). More specifically, CFSE staining of the lymphocytes from these ATL patient PBMCs confirmed that the lymphocytes were dividing and that approximately 28% of the activated lymphocytes (CD25+) were dividing after 6 days in culture (Figure 5C). The monocytes from the same patients were not dividing as monitored by CFSE staining (Figure 5D).
Antibody directed to IL9Rα inhibited the spontaneous proliferation of ATL PBMCs ex vivo. (A) Six-day spontaneous proliferation of ATL PBMCs with and without antibody to IL9Rα in the ex vivo culture. A nonspecific antibody, UPC10, was used as a control. The monoclonal antibody to IL9Rα or UPC10 was added to the 96-well plates at day 0. 3H thymidine was added to the culture during the last 6 hours of culture. Cells were then harvested and analyzed for the 3H thymidine incorporation. (B) CD4+CD25+ T cells from ATL patients proliferated in ex vivo culture. FACS analysis of CD4/CD25 expression was performed at different time points (day 0, day 3, and day 6). (C) CFSE staining of CD3+ lymphocytes to monitor their cell division. CFSE was labeled at day 0, and the cells were then put in culture without any stimulation. FACS analysis of CFSE-positive or CD25 CFSE double-positive cells was done at day 6. Unlabeled cells were used as controls. (D) CFSE staining of monocytes (CD14-expressing cells) to monitor cell division.
Antibody directed to IL9Rα inhibited the spontaneous proliferation of ATL PBMCs ex vivo. (A) Six-day spontaneous proliferation of ATL PBMCs with and without antibody to IL9Rα in the ex vivo culture. A nonspecific antibody, UPC10, was used as a control. The monoclonal antibody to IL9Rα or UPC10 was added to the 96-well plates at day 0. 3H thymidine was added to the culture during the last 6 hours of culture. Cells were then harvested and analyzed for the 3H thymidine incorporation. (B) CD4+CD25+ T cells from ATL patients proliferated in ex vivo culture. FACS analysis of CD4/CD25 expression was performed at different time points (day 0, day 3, and day 6). (C) CFSE staining of CD3+ lymphocytes to monitor their cell division. CFSE was labeled at day 0, and the cells were then put in culture without any stimulation. FACS analysis of CFSE-positive or CD25 CFSE double-positive cells was done at day 6. Unlabeled cells were used as controls. (D) CFSE staining of monocytes (CD14-expressing cells) to monitor cell division.
The IL-9Rα subunit was expressed on monocytes of the primary ATL PBMCs from select patients
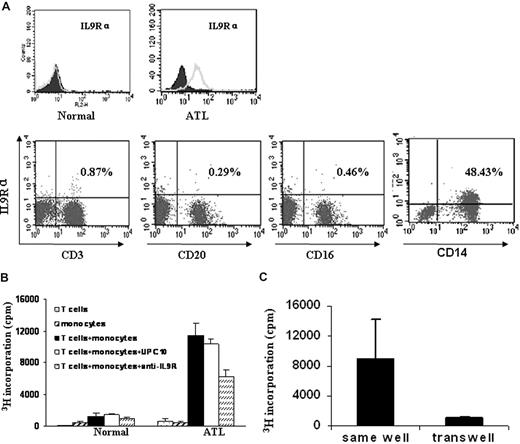
To better understand the mechanism of anti-IL9Rα inhibition in the context of ATL, it was important to know what cells were expressing the IL-9 receptor. We used FACS analysis to monitor IL-9Rα expression on the freshly isolated PBMCs from the ATL patients. In our study, we found that IL-9Rα was not expressed on the T cells (CD3+), B cells (CD20+), or NK cells (CD16+) in the freshly isolated PBMCs. However, IL-9Rα was expressed by the monocytes of the 5 patients who showed inhibition of spontaneous proliferation by addition of an anti–IL-9Rα antibody (Figure 6A). These IL-9Rα–expressing cells coexpressed CD14. As a control, monocytes of PBMCs obtained from normal donors did not express IL-9Rα (Figure 6A). These studies suggest that a paracrine loop may exist in ATL, in which the IL-9 made by the T cells stimulates IL-9Rα–producing monocytes.
IL-9Rα–expressing monocytes were required for the spontaneous proliferation of ATL PBMCs through a contact-dependent manner. (A) IL-9Rα was expressed on the monocyte population of ATL PBMCs, which also expressed CD14. IL-9Rα was not expressed on CD3 (T), CD20 (B), or CD16 (NK) positive cells. PBMCs from normal donors were used as a control. (B) Six-day spontaneous proliferation of purified T cells, monocytes, and mixture of purified T cells and monocytes (T cells: monocytes = 1:1). 3H thymidine was added to the culture during the last 6 hours of culture. Anti–IL-9Rα or control antibody UPC10 was added to the culture at T0. Cells were then harvested and analyzed for their 3H thymidine incorporation. (C) Separated T cells and monocytes were cultured in the same chamber or different chambers of the transwell (0.4 μm) for 6 days. 3H thymidine was added to the culture during the last 6 hours of culture.
IL-9Rα–expressing monocytes were required for the spontaneous proliferation of ATL PBMCs through a contact-dependent manner. (A) IL-9Rα was expressed on the monocyte population of ATL PBMCs, which also expressed CD14. IL-9Rα was not expressed on CD3 (T), CD20 (B), or CD16 (NK) positive cells. PBMCs from normal donors were used as a control. (B) Six-day spontaneous proliferation of purified T cells, monocytes, and mixture of purified T cells and monocytes (T cells: monocytes = 1:1). 3H thymidine was added to the culture during the last 6 hours of culture. Anti–IL-9Rα or control antibody UPC10 was added to the culture at T0. Cells were then harvested and analyzed for their 3H thymidine incorporation. (C) Separated T cells and monocytes were cultured in the same chamber or different chambers of the transwell (0.4 μm) for 6 days. 3H thymidine was added to the culture during the last 6 hours of culture.
Mixture of purified T cells and monocytes from ATL PBMCs induced spontaneous proliferation ex vivo through a contact-dependent manner
To further define whether a paracrine IL-9/IL-9R loop was involved in the spontaneous proliferation of the primary ATL PBMCs, we performed a proliferation assay using purified T cells and monocytes from 2 of the 5 ATL PBMCs that manifested the inhibition of spontaneous proliferation by the monoclonal anti–IL-9Rα antibody. We demonstrated that the purified T cells or monocytes did not proliferate when cultured alone (Figure 6B), whereas the addition of the separated monocytes to the purified T cells induced significant proliferation. This proliferation of cocultured monocytes and purified T cells was inhibited by the addition of an antibody to IL-9Rα (Figure 6B). Furthermore, the T cells and monocytes did not proliferate when cultured in different compartments of the transwell (Figure 6C). This phenomenon suggests that the spontaneous proliferation observed with ex vivo PBMCs from select ATL patients represents a cooperative action requiring both IL-9–producing T cells and IL-9Rα–expressing monocytes through a contact-dependent manner.
Discussion
The increased expression of IL-9 in HTLV-I–infected T cells suggested a potential role for IL-9 in the development of HTLV-I– associated ATL. ATL is characterized by the clonal expansion of activated CD4+ lymphocytes.1,2 It has been suggested that Tax-induced trans-activation of IL-2, IL-2Rα, IL-15, and IL-15Rα resulted in 2 autocrine loops that result in cytokine-dependent T-cell proliferation in the early stages of HTLV-I–associated diseases.9,13,36,37 Supporting this hypothesis, we have observed that PBMCs from 19 of 25 patients in the early phases of ATL spontaneously proliferated in culture (> 5000 cpm/105 cells, geometric mean 27 630 cpm) in the absence of any mitogen or antigen, whereas 20 normal PBMCs, studied in the same fashion, manifested less than 3000 cpm/105 cells (geometric mean 389 cpm). In several but not all of the patients, the proliferation was inhibited in part by addition of antibodies to IL-2, IL-15, or by a combination of these antibodies, thereby emphasizing the role of these 2 autocrine loops in the proliferation. However, with other ATL patients, this proliferation was only partially inhibited or not inhibited at all by such antibodies to IL-2, IL-15, and their private receptors, suggesting that another cytokine (or cytokines) might be involved. Analyzing the culture supernatants from these ex vivo cells using the cytokine-dependent indicator cell line, NK-92, revealed that the majority of 6-day culture supernatants of PBMCs from ATL patients contained high amounts of IL-9 (Figure 1). The presence of IL-9 in the culture supernatants was also confirmed by ELISA analysis. Furthermore, in some of the ATL patients within this group, the spontaneous proliferation was blocked by a monoclonal antibody to IL-9Rα. This suggested that the IL-9/IL-9R system might be involved in the expansion of the HTLV-I–infected CD4+ T cells of some patients in the early stages of ATL.
Elevated IL-9 mRNA expression had been shown previously in HTLV-I–infected cell lines and ATL PBMCs.21 In addition, after the cloning and characterization of human, genomic IL-9, it was speculated that HTLV-I Tax might transactivate IL-9 expression in light of the presence of a putative NF-κB binding site in the proximal IL-9 promoter.20 However, the observation that IL-9 mRNA expression did not correlate with Tax expression in HTLV-I–infected cell lines did not support this speculation.21 Moreover, the dramatic induction of IL-9 mRNA expression after IL-2 addition in the HTLV-I–infected, IL-2–dependent cell line, T1, raised the possibility that HTLV-I Tax protein alone was not sufficient for the induction of IL-9 expression.38 Here we present data that demonstrate that in the ex vivo cultured ATL cell Tax expression is associated with the transactivation of IL-9. A luciferase reporter construct with the IL-9 promoter was activated when transfected into the HTLV-I–positive cell lines (Figure 3C), and it was also activated in the HTLV-I–negative T cell lines, Jurkat and CEM, when they were cotransfected with a Tax cDNA expression vector (Figure 3D). These findings directly indicate the ability of Tax to transactivate the human IL-9 gene. Noting the strong correlation between Tax trans-activation of many cellular genes and the NF-kB pathway, it could be predicted that this would also be true for IL-9 induction. This notion was, indeed, supported by our data. Here we demonstrated that the deletion of the NF-κB motif from the IL-9 promoter reporter constructs dramatically reduced reporter activity to the basal level in HTLV-I–infected T-cell lines Hut102, MT-1, and MT-2 (Figure 3C). Moreover, the NF-κB deletion reporter construct was not activated when cotransfected with the Tax expression plasmid into Jurkat and CEM T cell lines (Figure 3D). The involvement of NF-κB in IL-9 expression was further supported by the observation that the putative IL-9 NF-κB motif could bind specifically to the NF-κB protein in electrophoresis mobility shift assays (Figure 3E,F). HTLV-I Tax is an inducer of NF-κB. However, in ATL cells from patients, NF-κB remains activated despite the eventual shutdown of Tax expression, in some cases suggesting that Tax may be needed to initiate but not to maintain NF-κB activation.5,33 Sustained NF-κB may lead to IL-9 activation without the presence of Tax. However, our data showed a nice correlation of Tax expression and IL-9 expression in select ATL PBMCs (Figure 2C), suggesting this is not the case in the ATL patients studied.
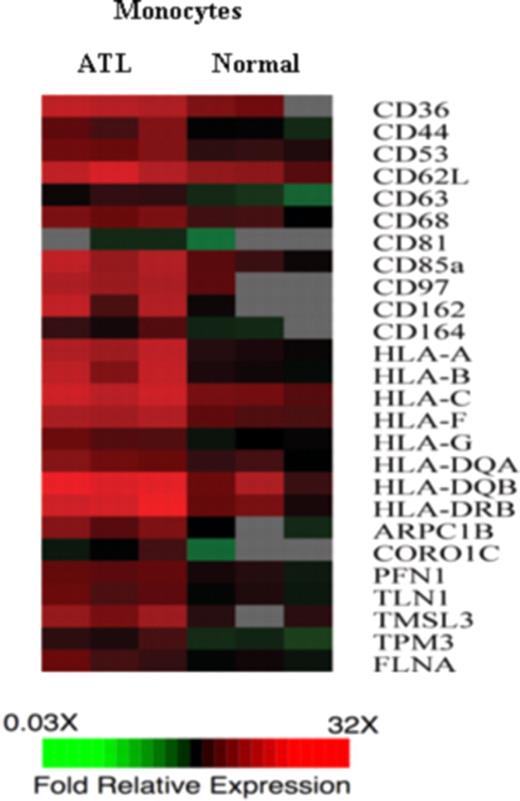
IL-9 expression has been frequently reported in HTLV-I-infected T cells; however, the direct involvement of IL-9 in the proliferation of primary ATL cells is rare.21 This may be the result of the lack of expression of IL-9Rα on ATL T cells. In that context, we demonstrate here that IL-9Rα promoter expression constructs were silent in HTLV-I–infected T-cell lines (Figure 4), suggesting that the Tax protein was not acting on the IL-9Rα promoter. Furthermore, by FACS analysis, we were unable to detect IL-9Rα expression on CD3 or CD4, CD8-positive lymphocytes from the 5 ATL patients who demonstrated inhibition of ex vivo proliferation by an antibody to IL-9Rα; this strongly suggested that IL-9Rα expression was present on a cell with a “non-T cell” phenotype. This paradox was potentially resolved when it was demonstrated that CD14-expressing monocytes from these same patients were expressing high levels of IL-9Rα (Figure 6A). Therefore, this led us to hypothesize that, unlike IL-2, IL-2Rα, and IL-15, IL-15Rα, which act through autocrine loops on the HTLV-I–infected CD4 T cells in ATL, IL-9 and IL-9Rα exert their biologic function through a paracrine loop. That is, IL-9 may not stimulate T lymphocytes directly because there is no expression of IL-9Rα on these cells. However, IL-9 may stimulate monocytes, which in trans support the proliferation of the T cells. The mechanism whereby IL-9–stimulated monocytes promote proliferation of cocultured ATL cells remains undefined. To obtain preliminary clues concerning this contribution, transcription profiles were obtained between monocytes derived from ATL patients and those derived from healthy donors as assessed by cDNA microarray (Figure 7). There was a 2-fold or greater increase in the expression of 700 genes in the monocytes derived from 3 ATL patients compared with those of 3 healthy donors. Three sets of functionally related genes were observed in the monocytes derived from ATL patients, including those encoding a series of adhesion molecules, those encoding class 1 and 2 major histocompatibility complex (MHC) genes, and those encoding cytoskeleton binding proteins. To support a role of some of those genes in the spontaneous proliferation, in studies of Makino et al,39,40 there was a 40% to 90% suppression of spontaneous proliferation of PBMCs in ex vivo cultures from patients with HAM/TSP on the addition of the antibodies to HLA-ABC, HLA-DR, CD58, and CD86. Thus, it is possible that IL-9–stimulated monocytes promote proliferation of ATL cells through a direct monocyte–T cell interaction that involves the surface MHC and adhesion molecules.
Genes encoding adhesion molecules, MHC class I and class II, and cytoskeleton binding proteins were up-regulated in monocytes derived from ATL patients. The gene expression of monocytes from 3 ATL patients were compared with the gene expression of monocytes derived from 3 normal healthy donors. The monocytes were purified using negative selection. cDNA from ATL monocytes or normal monocytes was labeled with Cy5 and hybridized with human universal reference cDNA labeled with Cy3. Red indicates Cy5/Cy3 ratio more than 1, green indicates Cy5/Cy3 ratios less than 1, black indicates no significant change in gene expression, and gray indicates the spot did not meet data selection criteria. These ratios were depicted according to the color scale shown at the bottom. Three sets of functional related genes, including genes encoding adhesion molecules, MHC class I and MHC class II gene, and genes encoding cytoskeleton binding proteins, which had at least 2-fold increases in the ATL monocytes.
Genes encoding adhesion molecules, MHC class I and class II, and cytoskeleton binding proteins were up-regulated in monocytes derived from ATL patients. The gene expression of monocytes from 3 ATL patients were compared with the gene expression of monocytes derived from 3 normal healthy donors. The monocytes were purified using negative selection. cDNA from ATL monocytes or normal monocytes was labeled with Cy5 and hybridized with human universal reference cDNA labeled with Cy3. Red indicates Cy5/Cy3 ratio more than 1, green indicates Cy5/Cy3 ratios less than 1, black indicates no significant change in gene expression, and gray indicates the spot did not meet data selection criteria. These ratios were depicted according to the color scale shown at the bottom. Three sets of functional related genes, including genes encoding adhesion molecules, MHC class I and MHC class II gene, and genes encoding cytoskeleton binding proteins, which had at least 2-fold increases in the ATL monocytes.
It has already been documented that adherent cells are important elements in the spontaneous proliferation of HTLV-I–infected T cells.41,42 In HAM/TSP patients, removal of adherent cells from ex vivo cultures led to a significant loss of proliferation manifested by the T cells.38,42 Our in vitro studies with spontaneously proliferating ATL cells draw a strong parallel to those observed in HAM/TSP. More specifically, the monocytes in ATL PBMCs did not divide in the ex vivo cultures but were required for the spontaneous proliferation of T cells through a contact-dependent manner (Figure 6B,C), clearly emphasizing the need for a paracrine interaction that in some circumstances involves IL-9 and its receptor. Interestingly, it was reported that the spontaneous proliferation of HAM/TSP T cells was not completely inhibited by addition of an antibody to IL-2 or to IL-2Rα.37 Inhibition of the IL-15/IL-15Rα system increased the effectiveness of IL-2 blockade but did not result in inhibition to baseline.36 Antibodies to IL-9 or its receptor also inhibited the proliferation to some extent (J.C. and T.A.W., unpublished data, January 2008). It would be interesting to look at the role of the IL-9/IL-9R system in this disease setting.
In conclusion, here we present data that suggest that IL-9 is also involved in the spontaneous ex vivo proliferation of ATL cells via a paracrine loop. It would be of interest in the future to explore the relationship between the cytokine expression (eg, IL-2, IL-15, IL-9) and the course and severity of HTLV-I–associated diseases (such as ATL) to better understand the roles of those cytokines in initiation of leukemogenesis and in the persistence of the leukemic state. Furthermore, it would be of value to evaluate inhibitors of Jak3, which is required for the signaling by IL-2, IL-9, and IL-15 for their therapeutic action in HTLV-I–associated diseases.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Steven Jacobson (National Institute of Neurological Disorders and Stroke, National Institutes of Health) for the kind gift of monoclonal anti-Tax antibody, Musafumi Yamada for technical help with the CFSE staining, and Lloyd Lam for help with microarray analysis.
This work was supported by the Intramural Research Program of the National Cancer Institute, National Institutes of Health.
National Institutes of Health
Authorship
Contribution: J.C. designed and performed research, collected, analyzed, and interpreted data, and wrote the manuscript; M.P., B.R.B., V.P.N., M.S., and C.K.G. performed research and collected and analyzed data; J.C.M. and J.E.J. provided patient care and collected patient samples; R.B. provided critical insights in research design and revised the manuscript; T.A.W. designed research and revised the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Thomas A. Waldmann, National Institutes of Health, 10 Center Drive, Building 10, Room 4N115, Bethesda, MD 20892-1374; e-mail: tawald@helix.nih.gov.