Abstract
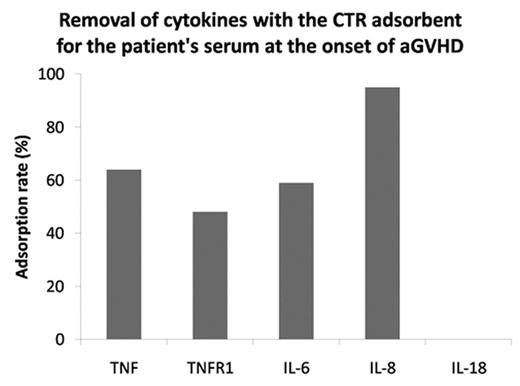
Acute graft-versus-host disease (aGVHD) remains the major cause of morbidity and mortality after allogeneic stem cell transplantation (SCT). Previous studies show that inflammatory cytokines such as tumor necrosis factor alfa (TNFα), interleukin-6 (IL-6), interleukin-8 (IL-8), and interleukin-18 (IL-18) are involved in the pathogenesis of aGVHD, and that the excess of these cytokines is associated with severity and mortality of aGVHD. We hypothesized that removal of these excessive cytokines from patients’ blood at the onset of aGVHD might improve the treatment outcome. A novel absorbent CTR can effectively adsorb small- to middle-sized proteins like cytokines and enterotoxins in vitro. In view of future exploitation of extracorporeal treatment using CTR column, we tested whether CTR could remove these inflammatory cytokines from blood. When the serum containing a mix of recombinant cytokines was incubated with a CTR adsorbent for 2 hrs, 55% of TNFα, 81% of IL-6, 83% of IL-8, and 22% of IL-18 were successfully removed. Next, we measured TNFα, soluble TNFα receptor 1 (TNFR1), IL-6, IL-8, and IL-18 levels in serum samples obtained from 5 patients (median age 38y, range 26–63y) who underwent myeloablative SCT in 4 and non-myeloablative SCT in 1. AGVHD developed in 2 with grade 3 and in 3 with grade 2. When cytokine levels in patients were expressed as a ratio to the mean cytokine level in control serum samples obtained from three healthy individuals, the mean ratios of TNFα, TNFR1, IL-6, IL-8, and IL-18 at the onset of aGVHD were 6.0 (range, 1.2–12.0), 6.5 (2.5–9.0), 274 (3.5–651), 48.3 (11.3–75.2), and 6.7 (3.2–10.8). The CTR adsorption considerably reduced the concentrations of these cytokines except for IL-18 (Figure 1). The adsorption rates of these cytokines were 64% for TNFα, 48% for TNFR1, 59% for IL-6, more than 94% for IL-8, and 0% for IL-18. The efficient removal of inflammatory cytokines suggests that extracorporeal blood purification with CTR column may be effective in the treatment of aGVHD. This treatment strategy may be promising because it essentially has no deleterious effects on immune functions of SCT recipients unlike other GVHD treatments.
Author notes
Disclosure: No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal