Abstract
The clinical course of B-cell chronic lymphocytic leukemia (B-CLL) is variable, and novel biologic parameters need to be added to the clinical staging systems to predict an indolent or aggressive outcome. We investigated the 70-kDa zeta-associated protein (ZAP-70), CD38, soluble CD23 (sCD23), and cytogenetics in 289 patients with B-CLL. Both a shorter progression-free survival (PFS) and overall survival (OS) were observed in ZAP-70+ (P < .001), in CD38+ (P < .001) and in sCD23+ patients (P < .001 and P = .013, respectively). ZAP-70+CD38+ or ZAP-70+ patients with an unmutated IgVH status showed both a shorter PFS (P < .001) and OS (P < .001 and P < .001, respectively) as compared with ZAP-70–/CD38– or ZAP-70– patients with mutated IgVH genes. Discordant patients showed an intermediate outcome. Note, ZAP-70+ patients even if CD38– or mutated showed a shorter PFS, whereas ZAP-70– patients even if CD38+ or unmutated had a longer PFS. Furthermore, ZAP-70 positivity was associated with a shorter PFS both within normal karyotype (P < .001) and within the poor-risk cytogenetic subset (P = .02). The predictive value of ZAP-70 expression was confirmed in multivariate analysis. Thus, ZAP-70 protein determined by flow cytometry improves the prognostic significance of cytogenetics and appears to be a better predictor of outcomes than IgVH gene mutational status. On this line, we recommend and are also interested in conducting a prospective randomized trial of early intervention versus observation for ZAP-70+ patients.
Introduction
Chronic lymphocytic leukemia (CLL) is the most common leukemia in the Western world and is characterized by the accumulation of monoclonal CD5+ B cells with the appearance of small mature lymphocytes.3 One of the most intriguing features of the disease is its clinical heterogeneity with patients progressing rapidly with early death, whereas others exhibit a more stable nonprogressive disease lasting many years. Thus, it is more important than ever to develop sensitive stratification parameters to identify patients with poor prognosis.4,5
About 50% to 70% of patients with CLL have evidence of somatic hypermutation in the immunoglobulin heavy-chain variable region genes (IgVH) of the leukemic cells.6 B-cell chronic lymphocytic leukemias (B-CLLs) with mutated (M) or unmutated (UM) IgVH gene configurations may represent the neoplastic counterparts of mature B cells either in different stages of normal B-cell differentiation or, more likely, selected by different, yet unidentified, antigens.7 On average, patients with M IgVH genes have a more indolent clinical course than those with the unmutated phenotype and have a longer survival.8,9
Investigations using DNA microarrays have shown that CLL cells exhibit a characteristic gene-expression profile in which the expression of small subgroup of genes, including those encoding ZAP-70, IM1286077, and C-type lectin, correlates with the mutational status of IgVH genes.10,11 ZAP-70, a member of the Syk–ZAP-70 protein tyrosine kinase family, is normally expressed in T and natural killer cells and has a critical role in initiation of T-cell signaling.12,13 Recent studies have found that ZAP-70 is associated with enhanced signaling by the cell-surface immunoglobulin receptor in CLL B cells14 and that measurement of ZAP-70 can serve as a surrogate for mutational status of IgVH.15 Moreover, ZAP-70 protein may be conveniently measured by flow cytometry, in contrast to the technically demanding IgVH analysis.16 In a larger series of patients, Rassenti et al17 have shown that an increased expression of ZAP-70 by CLL is a more significant predictor of need for treatment than the presence of a UM IgVH gene. Moreover, the expression of ZAP-70 appears to be constant over time.17
CD38 expression also has been suggested as a surrogate marker for the mutational status in B-CLL.8 At present, both parameters are regarded as independent prognostic variables in B-CLL.18 Detection of CD38 cell-surface expression can be easily performed by flow cytometry in the general laboratory.
Genetic studies using chromosome analysis or interphase fluorescence in situ hybridization (FISH) have identified recurring abnormalities with prognostic significance. Patients with a normal karyotype or deletion of 13q14 as the sole genetic abnormality have a better prognosis than those with a complex karyotype or deletion 11q23 or 17p13.19 In a large study, these deletions were associated with a shorter overall survival.20
In this study, ZAP-70, CD38, soluble CD23 (sCD23), mutational status, and genomic aberrations in relation to other laboratory parameters and clinical information were examined in a large series of patients with B-CLL.
In particular, we aimed to develop a new flow cytometric assay for ZAP-70 protein determination; to assess the prognostic impact of ZAP-70, CD38, sCD23, mutational status, and cytogenetic groups; whether combined analysis of ZAP-70 and CD38 or ZAP-70 and IgVH mutational status could allow us to identify new prognostic subsets; whether ZAP-70 could predict varied outcome within interphase cytogenetic groups; and, finally, to suggest preliminarily whether ZAP-70 could replace IgVH gene mutational analysis for prognosis.
Patients, materials, and methods
Patients
Approval for this study was obtained from the institutional review board of the Cattedra di Ematologia, Ospedale S. Eugenio, Universitá Tor Vergata (Roma, Italy). Informed consent was provided according to the Declaration of Helsinki. Two hundred eighty-nine consecutive and unselected patients with B-CLL were enrolled in this study from 1990 to 2004, all fulfilling the recommended diagnostic criteria with dim surface immunoglobulins and CD5+CD23+ immunologic pattern. There were 148 men and 141 women with a median age of 65 years (range, 37-84 years) at the time of diagnosis. Fresh or cryopreserved B-CLL cells were available for ZAP-70 and CD38 analysis in 289 patients. The median age of the patients with greater than 20% ZAP-70+ (median, 65 years; range, 37-84 years) and lower than 20% ZAP70+ (median, 65 years; range, 37-83 years) was the same. Fresh or frozen serum samples were obtained for sCD23 from 256 patients. All the samples were collected on a single day for each patient and were evaluated at diagnosis or before disease progression or before any chemotherapeutic approach. We tried to set empirically various cutoff points for each biologic variable (ZAP-70, CD38, sCD23), and the selected thresholds were sufficient to predict progression and survival, identifying accurately patients at poor prognosis. Moreover, we applied a discriminant function analysis, based on the squared Mahalanobis distances of each case from its group centroids (ZAP-70, CD38, sCD23) to verify the selected cut-points. The percentage correct (observed classification vs predicted classification) was about 90% for all variables. Eighty-seven patients had a low-modified Rai stage, 189 had intermediate stage, and 13 had high stage. One hundred forty-nine of the 289 patients received chemotherapy for their disease. Fifty-six were intermittently treated with a combination of chlorambucil at conventional doses and prednisone. The remaining 93 patients received 6 courses of fludarabine monophosphate (Fludara; Schering AG, Berlin, Germany) at 25 mg/m2 per day for 5 days every 28 days. A total of 34 patients had died of causes related to B-CLL at the time of analysis.
Cellular immunophenotypic analysis
The following antibody conjugates were used: anti–CD23-PE, anti–CD5-FITC, anti–CD38-PE, anti–CD19-APC, anti–CD45-FITC, anti–CD14-PE, anti–CD95-PE, and anti–CD10-FITC (Becton Dickinson Immunocytometry Systems, San Jose, CA). Peripheral blood mononuclear cells were analyzed for surface expression of CD19/CD5/CD38 and CD19/CD5/CD23 by triple-color immunofluorescence, as described elsewhere.21
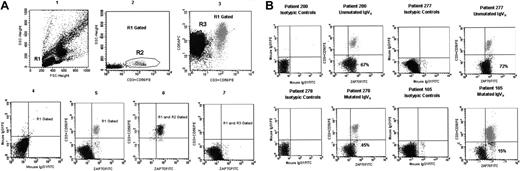
ZAP-70 protein determination was performed by flow cytometry. Peripheral blood mononuclear cells were fixed and permeabilized with Fix & Perm kit (Caltag Laboratories, Burlingame, CA). Then the cells were placed into 2 tubes and incubated, the first with 10 μL mouse IgG1 isotypic control conjugated to Alexa Fluor 488 dye (MG120; Caltag Laboratories) and the second with 10 μL monoclonal antibody (MoAb) anti–ZAP-70 (clone 1E7.2, isotype IgG1) conjugated to Alexa Fluor 488 dye (MHZAP7020; Caltag Laboratories) for 20 minutes at room temperature in the dark. Alexa Fluor 488 dye is excited at 488 nm and presents a peak emission at 519 nm as FITC. After 2 washings with PBS, the cells in the first tube were stained with mouse IgG1 PerCP, APC, PE isotypic controls, and the cells in the second tube were incubated with 10 μL CD19 PerCP, 10 μL CD5 APC, and 3 μL CD3 PE plus 3 μL CD56 PE (Becton Dickinson), for 15 minutes at room temperature. Finally, the samples were analyzed with a FACSCalibur flow cytometer (Becton Dickinson) with a gate on the fluorescence 2 detector to ensure that at least 5000 T and natural killer cells were analyzed in each sample. The ZAP-70 analysis, performed with the CellQuest software, as described elsewhere,15 is shown in Figure 1A. Figure 1B shows the level of expression of ZAP-70 by lymphocytes from 4 representative patients with CLL according to the mutational status of IgVH genes.
Enzyme-linked immunosorbent assays
Soluble CD23 (sCD23) immunoenzymometric assay was performed as described elsewhere.21 The threshold of positivity was set at sCD23 value of higher than 70 U/mL.
Interphase FISH
Separate hybridizations were carried out for loci on chromosomes 11, 12, 13, and 17. For chromosomes 11 (q23), 13, and 17, commercial probes (ATM-2, Rb-1, and p53, respectively) were used (Vysis, London, United Kingdom). An alpha-satellite DNA probe CEP12, directly labeled with SpectrumGreen, was used to detect aneuploidy of chromosome 12. LSIp53, labeled with SpectrumOrange (Vysis), was used to evaluate chromosome deletion at 17p13.1. We used peripheral blood lymphocytes, which were separated by density gradient centrifugation, treated with hypotonic solution (KCl), and fixed with methanol-acetic acid. Then the slides were aged for 20 minutes at 80°C on a hot plate and dehydrated for 2 minutes in 70%, 80%, and 100% ethanol and air dried. Gene frames were applied to dried slides to mark and separate the hybridization areas of single probes. Slides were placed on a hot plate at 37°C, and 5 μL of each probe buffer solution was applied inside the area of the slides delineated by the frame. After, the slides were sealed with a 22 × 22-mm gene-frame plastic coverslip and placed in the Vysis Hybrite machine. Co-denaturation was carried out at 68°C for 5 minutes and hybridization at 37°C overnight. Then the slides were washed in 0.4 × SSC/0.3% NP-40 for 2 minutes at 71°C, followed by 1 minute of washing in 2 × SSC at room temperature. Finally, the nuclei were counterstained with 4′, 6′-diamidino-2-phenylindole (DAPI), and signals were visualized using an Olympus BX51 microscope (Olympus Italia, Milan, Italy). Two hundred interphase cells with well-delineated fluorescent spots were examined.
IgVH mutation analysis
Total RNA was extracted and reverse-transcribed as previously reported.22 The resulting cDNAs, checked for first-strand synthesis,23 were amplified using a mixture of sense primers annealing either to the VH1 through VH6 leader sequences or to the 5′ end of VH1 through VH6 FR1, as reported.6,24-26 These primers were used in conjunction with a mixture of antisense primers complementary to the germ line JH regions. The purified amplified products, inserted into the PCR2.1-TOPO vector (Invitrogen, Milan, Italy) were expanded in TOP10 One Shot competent cells (Invitrogen) and cloned. Plasmid DNAs were isolated from overnight cultures of randomly selected colonies and sequenced by using an automatic DNA sequencer (ABI PRISM 3100; Applied Biosystem, Foster City, CA). Comparisons between the obtained sequences and those of the various germ line IgVH genes were performed with the IgBLAST directory (http://www.ncbi.nlm.nih.gov/igblast) using the Mac Vector 7.1 sequence analysis software (Accelerys; Symantec, San Diego, CA). Only when the same VHDJH rearrangement was identified in at least 5 to 10 clones, a given IgVH sequence was further analyzed. Alignment of the IgVH sequences available for each patient often revealed, along with mutations shared by all the transcripts analyzed, a number of unique or partially shared mutations.24,25 For this reason, all mutational analyses were carried out in each IgVH transcript separately, and the percentage of mutation assigned to a given B-CLL was the mean value of the percentage of mutations found in each transcript. VH gene sequences deviating greater than 2% from the corresponding germ line gene were defined as mutated.
Flow cytometric analysis of ZAP-70 expression and flow cytometric profiles of ZAP-70 protein on 4 B-CLL cases. (A) The method used to quantify the expression of ZAP-70 by B-CLL cells is shown. In plot 1, forward and side scatter of mononuclear cells is represented with a region (R1) drawn around the lymphocytes. Lymphocytes were R1 gated, and then T cells and natural killer (CD3+CD56+) cells (the R2 region in plot 2) and CLL cells (CD5+CD3–CD56–) cells (the R3 region in plot 3) were selected according to their phenotype. The plot 4 shows the expression of mouse IgG1 isotypic controls after lymphocyte (R1) gating. The plot 5 shows the expression of ZAP-70 after lymphocyte (R1) gating. For the purpose of quantification, markers were placed so that the T cells and natural killer cells (R1 and R2 gated) with a high level of expression of ZAP-70 would appear in the upper-right quadrant (plot 6). Mouse IgG1 isotypic antibody conjugated with Alexa Fluor was used as control marker for ZAP-70 positivity. Then, B-CLL cells were plotted, and the same marker that included T cells and natural killer cells in the upper-right quadrant was used to calculate the percentage of CLL cells that were positive for ZAP-70, as shown in the plot 7. Panel B shows the level of ZAP-70 expression by lymphocytes from 4 representative patients with CLL according to the mutational status of IgVH status. In each case, mouse IgG1 isotypic antibody is used as control for ZAP-70 positivity. The percentage of CLL cells with a high level of ZAP-70 expression is shown in the lower-right quadrant of each plot, after the exclusion of T and natural killer cells. FITC denotes Alexa Fluor 488.
Flow cytometric analysis of ZAP-70 expression and flow cytometric profiles of ZAP-70 protein on 4 B-CLL cases. (A) The method used to quantify the expression of ZAP-70 by B-CLL cells is shown. In plot 1, forward and side scatter of mononuclear cells is represented with a region (R1) drawn around the lymphocytes. Lymphocytes were R1 gated, and then T cells and natural killer (CD3+CD56+) cells (the R2 region in plot 2) and CLL cells (CD5+CD3–CD56–) cells (the R3 region in plot 3) were selected according to their phenotype. The plot 4 shows the expression of mouse IgG1 isotypic controls after lymphocyte (R1) gating. The plot 5 shows the expression of ZAP-70 after lymphocyte (R1) gating. For the purpose of quantification, markers were placed so that the T cells and natural killer cells (R1 and R2 gated) with a high level of expression of ZAP-70 would appear in the upper-right quadrant (plot 6). Mouse IgG1 isotypic antibody conjugated with Alexa Fluor was used as control marker for ZAP-70 positivity. Then, B-CLL cells were plotted, and the same marker that included T cells and natural killer cells in the upper-right quadrant was used to calculate the percentage of CLL cells that were positive for ZAP-70, as shown in the plot 7. Panel B shows the level of ZAP-70 expression by lymphocytes from 4 representative patients with CLL according to the mutational status of IgVH status. In each case, mouse IgG1 isotypic antibody is used as control for ZAP-70 positivity. The percentage of CLL cells with a high level of ZAP-70 expression is shown in the lower-right quadrant of each plot, after the exclusion of T and natural killer cells. FITC denotes Alexa Fluor 488.
Statistical analysis
All statistical analyses were performed at the end of data collection. Correlations between modified Rai stages or lymphadenopathy/splenomegaly or β2-microglobulin and ZAP-70 percentages were based on the 2-tailed Fisher exact test. Associations between ZAP-70 and mutational status or cytogenetic subgroups were analyzed by the 2-tailed Fisher exact test. The correlations between ZAP-70 or CD38 percentages and response to fludarabine were assessed by the proportional odds ordered logistic regression univariate model for categorical variables. To quantify the degree of association between ZAP-70 and CD38 percentages or sCD23 levels, the Spearman coefficient was calculated. The assessment of response was based on the National Cancer Institute Working Group criteria.27 Progression-free survival (PFS) and overall survival (OS), measured from diagnosis, were estimated according to the method of Kaplan and Meier and compared between groups by means of log-rank test. Cox proportional hazards regression model was used to assess the independent effect of covariables, treated as dichotomous, on the PFS or OS.
Results
Profiles of ZAP-70, CD38, soluble CD23, IgVH mutational status, and FISH
As currently reported in literature,15,17,28 a CLL population was considered ZAP-70 positive when at least 20% of the CD19+ B cells expressed the antigen. The B-CLL cells were ZAP-70+ in 104 patients (36%) and ZAP-70– in 185 patients (64%). To confirm the stability of ZAP-70 expression during the disease course, sequential samples from 32 patients (2-3 samples per patient) over time periods ranging from 4 to 36 months were analyzed to investigate whether ZAP-70 expression remains constant over time. In 28 (87.5%) of 32 cases, including 15 untreated and 13 treated patients, ZAP-70 expression differences of lower than 10% were detected. Although 4 cases (12.5%) showed a variation greater than 10%, in none of these patients the change of ZAP-70 expression crossed the 20% cutoff.
The proportion of B cells expressing CD38 varied from 0% to 87%, and the threshold was set at 30%, as previously reported.21 On the basis of this cutoff value, 67 patients (23%) were CD38+ and 222 patients (77%) were CD38–. To test the stability of CD38 over time, we further analyzed sequential samples from 65 patients (range, 3-8 samples per patient) over periods ranging from 2 to 45 months. In 58 (89%) of 65 cases, slight differences (< 10%) were detected. A total of 7 cases (11%) showed a variation higher than 10%. However, no patient crossed the used 30% cutoff. Finally, sCD23 was higher than 70 U/mL in 82 patients (32.2%) and lower than 70 U/mL in 172 patients (67.7%). Considering the possibility that sCD23 levels also may be unstable during disease, we analyzed samples from 85 patients over periods ranging from 2 to 66 months. In 75 (88%) of 85 cases, we detected ignorable differences (< 15%). A total of 10 cases (12%) showed variations higher than 15%, but the selected threshold (70 U/mL) was never crossed.
The Spearman correlation between the percentages of ZAP-70+ cells and those of CD38+ cells or the serum levels of sCD23 was r = 0.35 (P < .001) and r = 0.32 (P < .001), indicating moderate direct relationships.
Moreover, in 140 patients analyzed for both IgVH mutations and ZAP-70 expression, 79 (93%) of 85 patients with lower ZAP-70 had IgVH mutations greater than 2% (P < .001), confirming the close correlation currently described.15-17
Also a relationship between CD38 and IgVH mutational status was demonstrated (85 of 101 patients with CD38 < 30% showed IgVH mutations > 2%, P < .001).
Finally, lower sCD23 was also significantly correlated with mutated IgVH status (66 of 76 patients with sCD23 < 70 U/mL had IgVH mutations > 2%, P < .001).
One hundred fifty-seven patients were analyzed by interphase FISH to evaluate deletions in chromosome bands 17p13, 11q23, 13q14, and trisomy of band 12q13. With regard to cytogenetic groups, 85 patients (54%) had a normal karyotype and 42 (27%) had 13q–. Thirty patients (19%) had trisomy 12 (n = 16), 11q– (n = 11), 17p– (n = 3). There was a significant correlation between ZAP-70 greater than 20% and the poor-risk cytogenetic subset, encompassing trisomy 12, 11q–, and 17p– (22 of 30, 73%, P = .002). However, 13q14 deletion was significantly correlated with CD38 lower than 30% (34 of 42, 81%, P = .01), whereas normal karyotype was very frequent in patients with lower sCD23 levels (62 of 83, 75%, P = .001).
Clinical course and outcome
No significant correlation was found between sex and ZAP-70 positivity. However, there was a trend of association between female sex and low Rai stage (51 of 87, P = .07).
With regard to the clinical course, we found significant associations between higher ZAP-70 and intermediate/high modified Rai stage (P < .001; Table 1) or the presence of multiple (3 or more) intrathoracic/abdominal lymphadenopathies (> 3 cm in diameter) and/or splenomegaly (P < .001; Table 1) or β2-microglobulin serum levels higher than 2.2 mg/mL (P < .001; Table 1).
Modified Rai stages or lymphadenopathy/splenomegaly or β2-microglobulin and ZAP-70 percentages
. | Rai stage . | . | Lymphadenopathy/splenomegaly* . | . | β2-microglobulin* . | . | |||
|---|---|---|---|---|---|---|---|---|---|
. | Low . | Int/high* . | Present . | Absent . | Greater than 2.2 mg/mL . | Less than 2.2 mg/mL . | |||
| Total, no. (%) | 87 (30) | 202 (70) | 101 (35) | 188 (65) | 121 (46) | 144 (54) | |||
| ZAP-70, no. (%)† | |||||||||
| Greater than 20% | 12 (12) | 92 (88) | 66 (63) | 38 (37) | 56 (46) | 31 (22) | |||
| Lower than 20% | 75 (41) | 110 (59) | 35 (19) | 150 (81) | 65 (54) | 113 (78) | |||
. | Rai stage . | . | Lymphadenopathy/splenomegaly* . | . | β2-microglobulin* . | . | |||
|---|---|---|---|---|---|---|---|---|---|
. | Low . | Int/high* . | Present . | Absent . | Greater than 2.2 mg/mL . | Less than 2.2 mg/mL . | |||
| Total, no. (%) | 87 (30) | 202 (70) | 101 (35) | 188 (65) | 121 (46) | 144 (54) | |||
| ZAP-70, no. (%)† | |||||||||
| Greater than 20% | 12 (12) | 92 (88) | 66 (63) | 38 (37) | 56 (46) | 31 (22) | |||
| Lower than 20% | 75 (41) | 110 (59) | 35 (19) | 150 (81) | 65 (54) | 113 (78) | |||
For ZAP-70 greater than 20%, n = 104 (36%); for ZAP-70 lower than 20%, n = 185 (64%)
n indicates the number of samples.
As determined by the 2-tailed Fisher exact test.
P < .001
Similarly, higher CD38 and sCD23 levels were significantly correlated with intermediate/high modified Rai stages, multiple intrathoracic/abdominal lymphadenopathies, and/or splenomegaly or higher than 2.2 mg/mL β2-microglobulin (data not shown).
Lymphocyte doubling time (LDT) of less than 12 months was observed in 27 patients: 19 (70%) of 27 showed higher ZAP-70 expression (P < .001).
Moreover, although 80 (77%) of 104 of the ZAP-70+ patients had received chemotherapy at the time of analysis, only 64 (34.6%) of 185 ZAP-70– patients had been treated for B-CLL (P < .001).
Ninety-three patients (87 cases classified as low or intermediate Rai stage) underwent 6 monthly courses of fludarabine monophosphate at 25 mg/m2 for 5 days as first-line chemotherapy, achieving a global complete remission (CR) rate of 46% (43 of 93 patients, all belonging to low or intermediate Rai stage). A higher CR rate was found both in ZAP-70– (64% vs 31%; P = 0.003; Table 2) and in CD38– patients (61% vs 19%; P < .001; Table 2).
Complete, partial, or no response to fludarabine and ZAP-70 or CD38 expression
Variable . | CR, % (95% Cl) . | PR, % (95% Cl) . | NR, % (95% Cl) . |
|---|---|---|---|
| All observations | 43 | 36 | 14 |
| ZAP-70* | |||
| Greater than 20% | 31 (13-55) | 43 (23-64) | 25 (12-44) |
| Lower than 20% | 64 (34-82) | 33 (14-57) | 2 (0.5-8) |
| CD38† | |||
| Greater than 30% | 19 (6-36) | 50 (26-72) | 31 (11-53) |
| Lower than 30% | 61 (35-83) | 33 (13-51) | 6 (1-21) |
Variable . | CR, % (95% Cl) . | PR, % (95% Cl) . | NR, % (95% Cl) . |
|---|---|---|---|
| All observations | 43 | 36 | 14 |
| ZAP-70* | |||
| Greater than 20% | 31 (13-55) | 43 (23-64) | 25 (12-44) |
| Lower than 20% | 64 (34-82) | 33 (14-57) | 2 (0.5-8) |
| CD38† | |||
| Greater than 30% | 19 (6-36) | 50 (26-72) | 31 (11-53) |
| Lower than 30% | 61 (35-83) | 33 (13-51) | 6 (1-21) |
For ZAP-70 greater than 20%, n = 104; for ZAP-70 lower than 20%, n = 185.
CR indicates complete remission; PR, partial remission; and NR, no response or progression.
P = .003. P values are determined by the proportional odds ordered logistic regression univariate model.
P < .001.
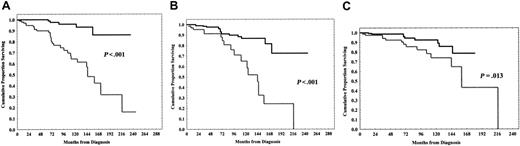
A significant shorter PFS was observed in ZAP-70+ patients (0% vs 59% at 13 years; P < .001; Figure 2A), in CD38+ patients (8% vs 42% at 14 years; P < .001; Figure 2B) and in sCD23 higher than 70 U/mL cases (0% vs 55% at 13 years; P < .001; Figure 2C).
Likewise, a shorter OS was found in ZAP-70+ patients (16% vs 86% at 18 years; P < .001; Figure 3A), in CD38+ patients (0% vs 73% at 18 years; P < .001; Figure 3B) and, less significantly, in sCD23+ cases (43% vs 79% at 14 years; P = .013; Figure 3C).
These significant differences in terms of PFS and OS were maintained when the analysis was restricted to Rai intermediate-risk group (189 patients) with regard to ZAP-70, CD38, and sCD23 (data not shown).
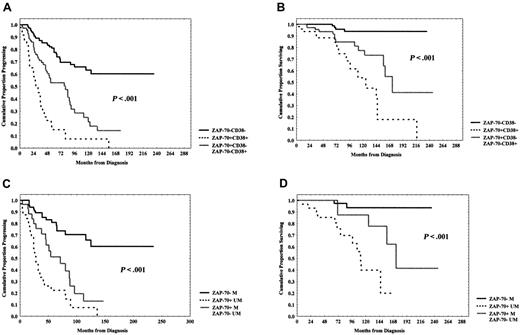
Interestingly, the simultaneous positivity or negativity for ZAP-70 and CD38 identified 2 subsets of patients, the former with a worse prognosis and the latter with a better prognosis with regard to both PFS (8% vs 60% at 12 years; P < .001; Figure 4A) and OS (18% vs 94% at 16 years; P < .001; Figure 4B). Discordant patients (n = 77), ZAP-70+CD38– (n = 63, 82%), or ZAP-70–CD38+ (n = 14, 18%) showed an intermediate outcome (PFS = 14% at 12 years; OS = 41% at 16 years; Figure 4A-B). No significant association between ZAP-70/CD38 discordant patients (n = 43) and IgVH mutational status was observed. In detail, 12 (35%) of 34 ZAP-70+CD38– had UM IgVH sequences, whereas 22 (65%) ZAP-70+CD38– patients had M IgVH genes. However, 2 of (22%) of 9 cases ZAP-70–CD38+ were UM IgVH and 7 (78%) ZAP-70–CD38+ were M IgVH.
Moreover, ZAP-70 expression and IgVH mutational status showed additive properties; in fact, ZAP-70 positivity and UM IgVH status identified a subset of patients at worst prognosis with regard to PFS (0% vs 60% at 11 years; P < .001; Figure 4C) and OS (20% vs 94% at 13 years; P < .001; Figure 4D). Discordant patients (ZAP-70+ M or ZAP-70– UM, n = 28) showed an intermediate outcome (PFS = 13% at 11 years; OS = 62% at 13 years; Figure 4C-D).
Progression-free survival (PFS) curves based on ZAP-70, CD38 expression, and soluble CD23 (sCD23) levels. (A) Kaplan-Meier plot comparing progression-free survival based on the detection of greater than 20% or lower than 20% ZAP-70+ B cells. Lower than 20% ZAP-70+ patients experienced a longer PFS (P < .001). Black line indicates ZAP-70 < 20%; gray line, ZAP-70 > 20%. (B) Equally, patients with B-CLL with CD38+ lower than 30% showed a significantly longer PFS (P < .001). Black line indicates CD38 < 30%; gray line, CD38 > 30%. (C) sCD23 levels lower than 70 U/mL characterized patients with a longer PFS (P < .001). Black line indicates sCD23 < 70 U/mL; gray line, sCD23 > 70 U/mL.
Progression-free survival (PFS) curves based on ZAP-70, CD38 expression, and soluble CD23 (sCD23) levels. (A) Kaplan-Meier plot comparing progression-free survival based on the detection of greater than 20% or lower than 20% ZAP-70+ B cells. Lower than 20% ZAP-70+ patients experienced a longer PFS (P < .001). Black line indicates ZAP-70 < 20%; gray line, ZAP-70 > 20%. (B) Equally, patients with B-CLL with CD38+ lower than 30% showed a significantly longer PFS (P < .001). Black line indicates CD38 < 30%; gray line, CD38 > 30%. (C) sCD23 levels lower than 70 U/mL characterized patients with a longer PFS (P < .001). Black line indicates sCD23 < 70 U/mL; gray line, sCD23 > 70 U/mL.
Note, we identified 2 small subsets of discordant patients ZAP-70–CD38+ (n = 14) and ZAP-70– UM (n = 5) that showed a significant better outcome than that of ZAP-70+CD38– and ZAP-70+ M patients with regard to PFS (Figure 5A-B).
Interestingly, within the ZAP-70– subset (185 of 289 patients, 64%) patients with sCD23 higher than 70 U/mL levels had a significant shorter PFS, as compared with cases with sCD23 lower than 70 U/mL cutoff (21% vs 76% at 10 years; P < .001; Figure 5C).
With regard to clonal genomic aberrations, a significant shorter PFS was observed in patients with prognostically unfavorable aberrations, such as 17p–, 11q–, and trisomy 12, which were pooled together and defined as a poor-risk cytogenetic subset (n = 30), versus normal karyotype patients (n = 85, 7% vs 39% at 12 years; P = .004; Figure 6A). The patients with 13q– (n = 42) showed an intermediate outcome (22% at 12 years).
To further explore the clinical impact of ZAP-70 expression among different cytogenetic groups, we investigated its expression within the normal karyotype and the poor-risk cytogenetic subsets. As a matter of fact, ZAP-70 positivity was significantly associated with a shorter PFS both within normal karyotype (14% vs 67% at 10 years; P < .001; Figure 6B) and within the poor-risk subset (0% vs 29% at 10 years, P = .02; Figure 6C).
Survival curves according to ZAP-70, CD38 expression, and sCD23 levels. (A) Kaplan-Meier plot comparing numbers of ZAP-70+ B-CLL cells with overall survival (OS). The difference between patients with ZAP-70 greater than 20% and patients with ZAP-70 lower than 20% was highly significant (P < .001). Black line indicates ZAP-70 < 20%; gray line, ZAP-70 > 20%. (B) CD38 expression lower than 30% identified patients with a longer OS (P < .001). Black line indicates CD38 < 30%; gray line, CD38 > 30%. Less significantly, a longer OS was found in patients with sCD23 levels lower than 70 U/mL (C) (P = .013). Black line indicates sCD23 < 70 U/mL; gray line, sCD23 > 70 U/mL.
Survival curves according to ZAP-70, CD38 expression, and sCD23 levels. (A) Kaplan-Meier plot comparing numbers of ZAP-70+ B-CLL cells with overall survival (OS). The difference between patients with ZAP-70 greater than 20% and patients with ZAP-70 lower than 20% was highly significant (P < .001). Black line indicates ZAP-70 < 20%; gray line, ZAP-70 > 20%. (B) CD38 expression lower than 30% identified patients with a longer OS (P < .001). Black line indicates CD38 < 30%; gray line, CD38 > 30%. Less significantly, a longer OS was found in patients with sCD23 levels lower than 70 U/mL (C) (P = .013). Black line indicates sCD23 < 70 U/mL; gray line, sCD23 > 70 U/mL.
Finally, we performed a multivariate Cox regression analysis of PFS and OS, including as covariates, age younger than or older than 60 years, modified Rai stages, β2-microglobulin, sCD23, CD38, and ZAP-70. With regard to PFS, ZAP-70, sCD23, and modified Rai stages were confirmed to be independent prognostic factors (Table 3). Concerning OS, only ZAP-70 was an independent prognostic factor, whereas CD38 showed a trend toward a statistical significance (Table 4).
Cox regression analysis of progression-free survival
Variable . | Hazard ratio . | P . | 95% confidence interval . |
|---|---|---|---|
| ZAP-70 | 16.05 | < .001 | 5.68-49.23 |
| sCD23 | 7.21 | .007 | 2.55-22.35 |
| Modified Rai stages | 7.04 | .008 | 1.98-21.43 |
Variable . | Hazard ratio . | P . | 95% confidence interval . |
|---|---|---|---|
| ZAP-70 | 16.05 | < .001 | 5.68-49.23 |
| sCD23 | 7.21 | .007 | 2.55-22.35 |
| Modified Rai stages | 7.04 | .008 | 1.98-21.43 |
Discussion
ZAP-70 expression, determined by flow cytometry, has already been identified as a significant predictor of disease progression and overall survival in B-CLL.1,15-17,28
We used a well-standardized flow cytometric methodology15 for ZAP-70 determination, but the use of the novel fluorochrome Alexa Fluor directly conjugated with the MoAb anti–ZAP-70 clone 1E7.2 allowed us to obtain, as in the work of Rassenti et al,17 a signal clearer than that currently performed with unconjugated antibodies, such as clone 2F3.2, as shown in Figure 1. Gibbs et al29 investigated the measurement of ZAP-70 expression in CLL using 2 different antibodies and 2 different staining methods. Those researchers demonstrated that ZAP-70 antibody clone 1E7.2 and Fix & Perm kit staining method were the easiest to use and the most sensitive and specific combination. Therefore, this combination may provide a standardized flow cytometric method that could be introduced into a routine CLL immunophenotyping panel in a clinical diagnostic laboratory. Moreover, this technologic improvement was important to define the optimal cutoff, in terms of percentage of ZAP-70+ cells, capable to split patients with B-CLL into 2 subsets with different clinical features and outcomes. As stability of ZAP-70 expression by the leukemic cell clone over time is an important precondition for a reliable use of this protein as prognostic marker, we analyzed ZAP-70 levels in 32 patients from whom sequential samples were available. ZAP-70 expression levels were stable in the majority of patients and, notably, in none of these patients the change of ZAP-70 expression crossed the 20% cutoff. Even though data from the literature17,30 indicate a certain degree of variability in ZAP-70 expression over time, the prognostic prediction made at diagnosis does not change in the majority of cases. Surely, further ZAP-70 time course analyses and standardization of flow cytometry protocols are needed to resolve this issue.
PFS and OS curves in relation either to combined ZAP-70 and CD38 expression or ZAP-70 and IgVHmutational status. (A-B) PFS and OS were significantly longer within the ZAP-70–CD38– subgroup (P < .001 and P < .001, respectively). (C-D) Equally, ZAP-70– M patients experienced both a longer PFS (P < .001) and OS (P < .001). Discordant patients (ZAP-70+CD38–/ZAP-70–CD38+ or ZAP-70+ M/ZAP-70– UM) showed an intermediate outcome.
PFS and OS curves in relation either to combined ZAP-70 and CD38 expression or ZAP-70 and IgVHmutational status. (A-B) PFS and OS were significantly longer within the ZAP-70–CD38– subgroup (P < .001 and P < .001, respectively). (C-D) Equally, ZAP-70– M patients experienced both a longer PFS (P < .001) and OS (P < .001). Discordant patients (ZAP-70+CD38–/ZAP-70–CD38+ or ZAP-70+ M/ZAP-70– UM) showed an intermediate outcome.
PFS curves in discordant patients (ZAP-70+CD38–/ZAP-70–CD38+and ZAP-70+M/ZAP-70–UM) and in ZAP-70–patients by sCD23 levels. (A-B) Interestingly, ZAP-70– either CD38+ or UM patients showed a longer PFS in comparison with ZAP-70+ either CD38– or M patients (P < .001 and P = .002, respectively). In panel A, the gray line indicates ZAP-70+CD38–; the black line, ZAP-70–CD38+. In panel B, the gray line indicates ZAP-70+ M; the black line, ZAP-70– UM. (C) Levels lower than 70 U/mL of sCD23 identified patients with a longer PFS within the ZAP-70– subset (P < .001). Black line indicates sCD23 < 70 U/mL; gray line, sCD23 > 70 U/mL.
PFS curves in discordant patients (ZAP-70+CD38–/ZAP-70–CD38+and ZAP-70+M/ZAP-70–UM) and in ZAP-70–patients by sCD23 levels. (A-B) Interestingly, ZAP-70– either CD38+ or UM patients showed a longer PFS in comparison with ZAP-70+ either CD38– or M patients (P < .001 and P = .002, respectively). In panel A, the gray line indicates ZAP-70+CD38–; the black line, ZAP-70–CD38+. In panel B, the gray line indicates ZAP-70+ M; the black line, ZAP-70– UM. (C) Levels lower than 70 U/mL of sCD23 identified patients with a longer PFS within the ZAP-70– subset (P < .001). Black line indicates sCD23 < 70 U/mL; gray line, sCD23 > 70 U/mL.
We show here that ZAP-70 expression was significantly correlated with CD38 antigen; both of these 2 markers are more highly expressed in UM IgVH CLL and are consistent with a more activated phenotype. Ongoing antigen-mediated activation of the leukemic cells through the B-cell receptor (BCR) is suggested by the presence of cellular activation markers on the cell surface.31 Probably, the B-CLL subset expressing ZAP-70 and CD38 may reflect ongoing in vivo stimulation, thereby explaining the more aggressive disease course observed in these patients.10 Chen et al11 demonstrated that the expression of ZAP-70 is associated with an increased BCR signaling in CLL cells. In that report, the researchers further suggest that global tyrosine phosphorylation is increased in ZAP-70+ cells. Such increased intracellular signaling could influence the survival or proliferation of CLL cells, leading to a tendency toward disease progression. Moreover, Zupo et al32 have shown that CD38-expressing B cells appeared to be more responsive to BCR ligation, thereby supporting a potential link between CD38 and ZAP-70 expression.
PFS curves based on cytogenetics and ZAP-70 expression within cytogenetic subsets. (A) Patients with B-CLL with a normal karyotype showed a longer PFS (P = .004) as compared with high-risk genetic aberrations. (11q–,17p–, and +13). Black line indicates normal; dashed line, poor; and gray line, del 13q. (B-C) ZAP-70 protein overexpression distinguished patients at worse prognosis both within normal karyotype (P < .001) and within the poor-risk B-CLL subset (P = .02). Black line indicates ZAP-70 < 20%; gray line, ZAP-70 > 20%.
PFS curves based on cytogenetics and ZAP-70 expression within cytogenetic subsets. (A) Patients with B-CLL with a normal karyotype showed a longer PFS (P = .004) as compared with high-risk genetic aberrations. (11q–,17p–, and +13). Black line indicates normal; dashed line, poor; and gray line, del 13q. (B-C) ZAP-70 protein overexpression distinguished patients at worse prognosis both within normal karyotype (P < .001) and within the poor-risk B-CLL subset (P = .02). Black line indicates ZAP-70 < 20%; gray line, ZAP-70 > 20%.
Our study confirms the significant association between the expression of ZAP-70 in CLL cells and UM IgVH genes in agreement with the results of other studies15-17 even though the mechanisms accounting for the relation between ZAP-70 expression and IgVH mutational status are still unknown.
However, the simultaneous information of both ZAP-70 level and IgVH mutational status still provides more useful prognostic information given a certain degree of discordance between these 2 prognosticators (from 5% to 23%), as underscored in recent studies.33 We obtained that 20% of the results of the 2 methods were discordant. Our discordance rate was greater than 5% to 6% reported by Crespo et al15 and Orchard et al,16 but similar to that reported (23%) by Rassenti et al.17 Interestingly, we used the same monoclonal antibody (1E7.2) and fluorochrome (Alexa-488 dye) reported by Rassenti et al17 and surely identical antibodies may give similar results.33
In our study, we performed chromosome analysis by FISH in 157 patients with B-CLL, and a significant correlation between unfavorable cytogenetic aberrations such as trisomy 12, 11q–, 17 p–, and ZAP-70 expression was demonstrated.
From a clinical point of view, in our series of consecutive unselected patients with CLL there was a large prevalence of low and intermediate Rai stages accounting for about 95% of all patients. It is our opinion that this situation reflects the actual recruitment of patients, because it happens in a first-level hematology center such as our institution; this may at least in part explain both the higher number of women and the lower frequency of ZAP-70 positivity observed in our series.
Higher ZAP-70 expression was significantly correlated with more advanced Rai stages, with large intrathoracic/abdominal lymphadenopathies, splenomegaly, and a shorter LDT, all hallmarks of an active and aggressive disease. Moreover, significant differences in treatment histories between ZAP-70+ and ZAP-70– cases were found because a larger number of ZAP-70+ patients received treatment at the time of analysis (P < .001).
Besides, the high global CR rate (46%) of patients treated with fludarabine was to be attributed to the fact that almost all (87 of 93) treated patients as progressing belonged to low or intermediate Rai stages. In fact, in literature, considering only this subset of patients, CR percentages are higher than the 15% to 20% range, currently reported in most large series.34,35
Furthermore, complete response to fludarabine as initial therapy was significantly correlated with ZAP-70 percentages (P < .001), as well as with CD38 expression (P < .001), confirming that these biologic markers may be used to predict the chemosensitivity of patients with B-CLL.
We demonstrated that ZAP-70 positivity was significantly related to an unfavorable clinical course in regard to both PFS (P < .0001) and OS (P < .001) on a large series of patients with B-CLL in accordance with the studies by Crespo et al,15 Orchard et al,16 and Rassenti et al.17 These important results were also confirmed by restricting the analysis to patients initially presenting in the Rai intermediate stage, which includes clinically heterogeneous cases. Moreover, also higher CD38 expression and sCD23 levels were significantly associated with a shorter PFS and OS in this large cohort of patients, confirming our previous results.21 Interestingly, combined analysis of ZAP-70 and CD38 allowed us to separate our patients into 3 subgroups: ZAP-70–CD38– (n = 161) presenting good clinical features, ZAP-70+CD38+ (n = 51) with a poor prognosis, and discordant ZAP-70/CD38 (n = 77) with an intermediate outcome (Figure 4).
Of note, almost all discordant patients (63 of 77, 82%) were ZAP-70+ and CD38–, suggesting the superior clinical impact of ZAP-70 over CD38. The phenomenon of discordant ZAP-70/CD38 expression was further explored by IgVH mutation analysis in this subset of patients. Interestingly, we found a high proportion of M IgVH patients (21 of 32, 64.5%) in the ZAP-70+CD38– subgroup, indicating that ZAP-70 could be prognostically even more important than Ig-mutational status. Our results are in disagreement with the observations of Schroers et al30 who reported a high proportion of UM cases among discordant patients.
Furthermore, the combined analysis of ZAP-70 and IgVH mutational status allowed us to identify a discordant group ZAP-70/IgVH presenting an intermediate outcome, as described in Figure 4. Again, almost all discordant patients were ZAP-70+ and M IgVH, suggesting that ZAP-70 may be a better predictor of outcomes than Ig-mutation status.17,36
Interestingly, in our study we described a novel clinical preliminary observation that the 2 small discordant subsets ZAP-70–CD38+ (n = 14) and ZAP-70– UM IgVH (n = 5) presented a much more favorable outcome than that of ZAP-70+CD38– or ZAP-70+ M IgVH patients, as reported in Figure 5. This result suggests that the prognostic impact both of IgVH mutational status and CD38 can be dependent mainly by the ZAP-70 contemporary positivity, even if it should be confirmed both on larger numbers of patients and in the setting of a prospective controlled trial. Concerning that, Chen et al14 observed that the enhancement of IgM signaling with an increased tyrosine phosphorylation and increased calcium flux, responsible for an aggressive clinical behavior, was more tightly higher in UM or M ZAP-70+ CLL as compared with UM ZAP-70– CLL.
Another interesting point of discussion is how to evaluate the disease progression and to establish the time to treatment within ZAP-70 negative patients who represent a large subgroup (185 of 289 patients) with variable outcome also in our experience. Interestingly, sCD23 levels varied within ZAP-70–negative patients, allowing us to distinguish different prognostic subsets with regard to PFS. Therefore, this biologic marker reveals prognostic capabilities37 and has to be added to the other well-known factors (ZAP-70, CD38, mutational status, clinical stage) to monitor the clinical outcome of patients with B-CLL.
Moreover, our study confirmed the prognostic significance of chromosome analysis by FISH in B-CLL, demonstrating a longer PFS in normal karyotype and 13q deletion in comparison with high-risk genomic aberrations such as trisomy 12, 11q–, and 17p–, as previously reported.19,20
Today, there are not large studies on the combined prognostic significance of ZAP-70 and cytogenetics in B-CLL.2 In our study, ZAP-70 protein confirmed a predictive power, distinguishing patients who have early PFS or short OS within normal karyotype and poor-risk cytogenetic subsets, whereby progression was heterogeneous.
The independent prognostic effect of ZAP-70 expression on the clinical outcome of patients with B-CLL was finally corroborated by the multivariate analysis with regard both to PFS and OS (Tables 3, 4).
In conclusion, our study demonstrates that the increased expression of ZAP-70 by CLL cells is a more significant predictor of disease progression than the presence of CD38 and sCD23. Moreover, ZAP-70 was able to predict a different outcome within interphase cytogenetic groups. In addition, combined analysis of ZAP-70 and CD38 or ZAP-70 and IgVH status allowed us to identify discordant subsets of patients in which the presence of ZAP-70 alone was sufficient to define a poor prognosis. These results suggest a superior prognostic role of ZAP-70 over CD38 and IgVH mutational status. Importantly, this parameter can be determined easily and rapidly by flow cytometry, even though further studies are required to develop a standardized flow cytometry protocol. Finally, because ZAP-70 expression appears to be stable over time, it should be used at the time of diagnosis to identify patients at increased risk for early disease progression. Noteworthy, the large prevalence of the intermediate Rai stage found in our series (189 of 289, 65%) reinforces the importance of ZAP-70 determination; because patients belonging to this stage may have either an indolent course or a rapid aggressive outcome, information on ZAP-70 expression levels may be of great help for clinicians to select which patients are eligible or not for treatment. Clinical trials of effective treatment stratified by more reliable prognostic markers, such as ZAP-70, are surely now warranted.38
Prepublished online as Blood First Edition Paper, April 6, 2006; DOI 10.1182/blood-2005-12-4986.
Supported in part by Ministero dell'Università e della Ricerca Scientifica e Tecnologica (MURST), Programmi di Ricerca di Interesse Nazionale, 2003, and by Ministero della Salute (Ricerca Finalizzata Istituto di Ricovero e Cura a Carattere Scientifico [IRCCS] and “Alleanza contro il Cancro”), Rome, Italy.
G.D.P., F.L.C., D.D.P., and S.A. provided the original concept and design for the study. F.B. and M.I.C. obtained ZAP-70 and CD38 flow cytometry data. V.G., A.Z., R.B., and M.D. investigated IgVH mutational status and generated part of the ZAP-70 flow cytometry data. P.N. and A.B. did the statistical analyses. M.I.D.P., G.D.P., and L.M. drafted the manuscript. P.P. obtained the interphase FISH cytogenetics data. R.M. and G.S. obtained the soluble CD23 data. C.M., L.O., A.V., M.C., and P.d.F. provided clinical care and recorded the clinical data.
M.I.D.P and G.D.P. contributed equally to this study.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal