Abstract
von Willebrand disease (VWD), the most common inherited bleeding disorder in the U.S. population, is caused by defects in the expression and processing of von Willebrand factor (VWF), a blood glycoprotein required for normal hemostasis that mediates the adhesion of platelets to sites of vascular damage by binding to specific platelet glycoproteins and to constituents of exposed connective tissue. To assess whether VWF deficiency can be corrected by gene transfer, a plasmid expressing the intact 8.4-kb murine VWF coding sequence, directed by the cyto-megalovirus immediate/early promoter/enhancer, was delivered through hydrodynamic tail vein injection into VWF knockout mice (VWF–/–) that exhibit defects in hemostasis, including highly prolonged bleeding time and spontaneous bleeding events, closely mimicking severe human VWD. VWF antigen levels in plasma from animals receiving VWF cDNA, but not control animals, revealed normalized levels of circulating VWF that persisted for at least 1 week after injection. Western blot analysis of plasma from animals receiving VWF cDNA, but not control animals, revealed high molecular–weight multimers with patterns similar to those observed in wild-type mice. Reverse transcription–polymerase chain reaction (RT-PCR) on RNA isolated from the livers of animals receiving VWF cDNA, but not control animals, demonstrated that VWF was expressed in the liver, and immunohistochemical analysis of the livers of treated VWF–/– mice revealed VWF-specific staining throughout the liver parenchyma but not in endothelial cells. Plasma from treated VWF–/– mice, but not control VWF–/– mice, supported the hypothesis that murine platelets aggregate in the presence of botrocetin. Although levels of circulating factor VIII in untreated VWF–/– mice were less than 10% those in wild-type mice, levels of factor VIII in VWF–/– animals treated with VWF cDNA, but not in control animals, were normalized to values in wild-type mice, indicating the restoration of factor VIII carrier function for VWF in treated mice that persisted for at least 1 week at higher doses of VWF cDNA. Most important, bleeding time was normalized by 48 hours after the delivery of VWF cDNA, but not by the control plasmid. These data suggest that with the use of gene transfer of VWF cDNA, VWF protein can be expressed, processed, and secreted in a physiologically active form; thus, it may be possible to correct VWD using gene transfer.
Introduction
von Willebrand disease (VWD), the most common inherited bleeding disorder in the United States, affects 1% to 3% of the population.1-7 The disease is caused by defects in the expression and processing of von Willebrand factor (VWF), a blood glycoprotein required for normal hemostasis.8,9 VWF mediates the adhesion of platelets to collagen at sites of vascular damage through an interaction requiring the formation of a glycoprotein Ib-IX-V complex on activated blood platelets, exposed collagen fibrils, and fluid shear stress. VWF also binds to and stabilizes coagulation factor VIII.5,8,10,11 The phenotype of VWF deficiency varies with the mutations in the VWF gene.5,10 Most forms are inherited as dominant traits. In mild cases, problems with abnormal homeostasis develop only after trauma or surgery. In more severe cases, spontaneous bleeding can develop from the nasal, oral, gastrointestinal, and genitourinary mucosa, and joint bleeding can also develop. In the laboratory, VWF deficiency is characterized by prolonged bleeding time, reduced VWF plasma levels, abnormal ristocetin-induced platelet aggregation, and reduced factor VIII levels.5,10,12,13
VWF is normally produced in endothelial cells and megakaryocytes.11,14-16 The large murine VWF gene is transcribed to produce a mature mRNA of 8.4 kb.17 VWF biosynthesis involves a complex series of posttranslational events that include propeptide cleavage, disulfide bonding of VWF subunits to form dimers, and subsequent assembly of dimers into a series of disulfide-linked multimers (ranging in size from 540 kDa to approximately 20 000 kDa in healthy individuals) that are glycosylated and sulfated during transit through the Golgi apparatus and the endoplasmic reticulum.6,9,11,18,19 A portion of the VWF produced by endothelial cells is secreted constitutively into the bloodstream, whereas the remainder is packaged into Weibel-Palade bodies—specialized intracellular organelles that serve as a reservoir for release on stimulation.18,20-23 The focus of the present study was to develop a strategy to treat VWD using gene transfer. Because VWD can be ameliorated with VWF levels greater than 0.5 μg/mL (greater than 5% of the normal plasma levels of 10 μg/mL), theoretically it should be possible to treat the disease with existing gene transfer technology.24,25 To this end, the present study was designed to determine whether a murine model of VWF deficiency can be corrected by gene transfer of the normal murine VWF cDNA. We used a murine knockout model of VWD, created by disrupting the naturally occurring VWF gene (VWF–/– mice).12,26 These homozygous VWF–/– mice exhibit defects in hemostasis with highly prolonged bleeding time and spontaneous bleeding events that closely mimic those of severe human VWD. Transected-tail wounds in untreated VWF–/– mice bleed continuously unless cauterized, whereas similar wounds in wild-type mice stop bleeding in 2 to 3 minutes. We investigated the impact of murine VWF cDNA transfer on untreated VWF–/– mice with the use of high-volume intravenous administration, a technique known to elicit efficient transient in vivo expression from plasmids.27,28 In the present study, the data demonstrate that after in vivo delivery of normal VWF cDNA, VWF antigen and high molecular–weight VWF multimers appear in plasma in patterns similar to those observed in wild-type mice, VWF-specific reverse transcription–polymerase chain reaction (RT-PCR) can be demonstrated from liver RNA of treated animals, VWF protein is detected in the liver parenchyma by immunohistochemistry, botrocetin-mediated platelet aggregation is corrected, plasma levels of factor VIII are corrected, and, most important, bleeding time is normalized. These data from the present study suggest that genetic correction of VWF deficiency is feasible and can be achieved by gene transfer of the normal VWF cDNA with the consequent manufacture, processing, and secretion of normal VWF protein.
Materials and methods
Plasmids
Murine VWF cDNA (http://www.sheffield.ac.uk/VWF/murinecdna.html) and eGFP (control) cDNA expression cassettes were cloned into pcDNA3.1(+) (Invitrogen, Carlsbad, CA) under the control of a cytomegalovirus immediate/early promoter/enhancer.
Experimental murine model
The murine model for VWD used in this study was developed by disruption of the murine VWF gene by gene targeting (VWF knockout mice; VWF–/–).12,26 The mice appear normal at birth; they are viable and fertile. VWF protein is not detectable in plasma, platelets, or endothelial cells of the homozygous mutant mice. The mice exhibit defects in hemostasis with highly prolonged bleeding times and spontaneous bleeding events. As in the human form of the disease, factor VIII levels are markedly reduced because of the lack of the protective carrier function provided by VWF. Overall, the phenotype of these mice very closely mimics that of severe VWD in humans.
To demonstrate the VWF expression cassette in vivo, plasmids were administered to mice through hydrodynamic tail vein injection.27,28 As controls, some animals received human VWF. Experiments were performed with male or female VWF–/– mice that weighed 20 to 24 g each. Mice were anesthetized with intraperitoneal injection of ketamine HCl (Rhône Mérieux, Brussels, Belgium). Plasmid DNA (10-250 μg, VWF; eGFP, control) or human VWF protein (10 μg; factor VIII-free; Haematologic Technologies, Essex Junction, VT) in 1.6 mL of 150 mM NaCl was injected into tail veins through a 28-gauge needle in less than 10 seconds.
Assessment of plasma VWF antigen levels
Blood was obtained by retro-orbital venous plexus sampling in polypropylene tubes containing 0.1 vol of 38 mM citric acid, 75 mM trisodium citrate, and 100 mM dextrose. Plasma was prepared by centrifugation of the blood at 2500g for 15 minutes at 23°C. Microtiter plates were coated overnight at 4°C with 3 μg/mL rabbit anti–human VWF in 50 mM sodium carbonate buffer (pH 9.6). Plates were washed 3 times with 0.14 M NaCl, 20 mM Tris (pH 7.4), 0.1% Tween 20, 0.3% bovine serum albumin (BSA; Sigma, St Louis, MO), and plasma, diluted 1:1 to 1:5000 in 0.1% Tween 20 Tris-buffered saline containing 3% BSA, and incubated in the wells for 2 hours at 37°C. After 3 washes, the plates were incubated with polyclonal anti–human VWF coupled to peroxidase (DAKO, Glostrup, Denmark) diluted 1:3000 in 0.1% Tween 20, Tris-buffered saline (pH 7.4), and 3% BSA for 2 hours at 37°C. After washing, o-phenylenediamine dihydrochloride (Sigma) 0.5 mg/mL in 50 mM citric acid and 100 mM Na2HPO4 (pH 5.0) in the presence of 0.015% H2O2 were added to the wells. After 10 minutes, the reaction was stopped by the addition of 2 M H2SO4. Absorbance was read at 490 nm in an ELISA reader (BioWhittaker, Walkersville, MD).
Assessment of multimers in plasma
To demonstrate that VWF produced by gene transfer was expressed and processed in a manner physiologically similar to that of natural VWF, plasma from controls and treated VWF–/– mice was analyzed for the presence of VWF multimers.29 Agarose (Sea Kem HGT, VWF grade; FMC Bioproducts, Rockland, ME) was dissolved in 40 mM Tris-acetate, pH 7.8, 0.1% sodium dodecyl sulfate, 1 mM ethylenediamine tetraacetate (EDTA), poured to form a 4-mm–thick 0.8% gel, and solidified at 4°C. Titrated plasma was diluted 1:50 in 50 mM Tris-HCl, pH 7.3, and 10 mM EDTA. Plasma samples were mixed at a 1:1 ratio with 10 mM NaH2PO4, pH 7.0, 37 mM iodoacetamide, and 1.0% sodium dodecyl sulfate and were incubated at 37°C for 60 minutes. After incubation, 0.1 vol 50% glycerol and 1% bromophenol blue were added to the samples. Electrophoresis was carried out for 6 hours at 4°C at 50 mA (constant amperage). After electrophoresis, the gel was immersed in 2.5 mM Tris-HCl, pH 8.8, 19.2 mM glycine, 20% methyl alcohol, and 0.01% sodium dodecyl sulfate and was equilibrated for transfer. Polyvinylidene fluoride (PVDF) 0.45-μm membranes (Immobilon-P; Millipore, Bedford, MA) were presoaked for 2 minutes in methyl alcohol and rinsed in 2.5 mM Tris, pH 8.8, 19.2 mM glycine, 20% methyl alcohol, and 0.01% sodium dodecyl sulfate. Transfer was carried out in a Trans-Blot Cell (Bio-Rad Laboratories, Hercules, CA) in 2.5 mM Tris, pH 8.8, 19.2 mM glycine, 20% methyl alcohol, and 0.01% sodium dodecyl sulfate. Transfer was performed at 70 mA (constant amperage) at 4°C. After transfer, the membrane was blocked in 5% nonfat dry milk (Bio-Rad Laboratories) diluted in 50 mM Tris-HCl, pH 7.4, 100 mM NaCl, and 0.1% Tween-20 for 1 hour. Detection was accomplished with a primary rabbit anti-VWF antibody (DAKO) diluted 1:4000 in 50 mM Tris-HCl, pH 7.4, 100 mM NaCl, and 0.1% Tween-20 for 1 hour at 23°C with mild shaking. After washing, the membrane was incubated with anti–rabbit immunoglobulin horseradish peroxidase (Amersham Life Science, Buckinghamshire, United Kingdom) diluted 1:1000 under the same conditions used for the primary antibody and was washed several times in 50 mM Tris, pH 7.4, 100 mM NaCl, and 0.1% Tween-20. VWF multimers were visualized with enhanced chemiluminescence (ECL) Western blot analysis reagents (Amersham Life Science, Piscataway, NJ) and x-ray film.
Localization of VWF expression
To evaluate the expression in liver of VWF cDNA after high-volume injection into the tail vein, RT-PCR was performed with VWF-specific primers. From 24 to 72 hours after injection, the mice were deeply anesthetized with ketamine (100 mg/kg) and xylazine (10 mg/kg) and were killed by carbon dioxide inhalation. Livers were excised, and total RNA was isolated with TRIzol (Invitrogen, Carlsbad, CA) and was reverse transcribed (SuperScript First-Strand Synthesis System for RT-PCR; Invitrogen) after oligo-dT priming. PCR was performed with Platinum Taq DNA Polymerase (Invitrogen) with VWF-specific forward (5′-GGACATTTTCTCAGACCACCATA-3′) and reverse (5′-TGTGGAGGCTGAGTTGGG-3′) primers. PCR amplification (30 cycles) was carried out at 94°C for 0.5 minute, 62°C for 0.5 minute, and 72°C for 1 minute, followed by final extension at 72°C for 5 minutes, and was analyzed by agarose gel electrophoresis.
For immunohistochemical localization of VWF expression, livers were excised 24 to 72 hours after cDNA injection, and slices were fixed with 4% paraformaldehyde (EM Sciences, Hatfield, PA) in PBS for 1 hour at 23°C, blocked with 5% goat serum, treated with primary rabbit monoclonal anti-VWF antibody (Sigma) in block (2 hours, 22°C), washed, treated with secondary horseradish peroxidase-goat anti–rabbit Fab 2 (1 hour, 22°C), counterstained with Mayer hematoxylin (Sigma), and viewed through a Nikon Microphot SA microscope equipped with a Plan 10 ×/0.3 NA objective lens (Nikon Instruments, Melville, NY) and a DP70 color CCD camera (Olympus America, Center Valley, PA), using DP Controller software version 2.1.1.183 (Olympus America). Isotype-matched irrelevant primary antibodies were used to confirm specific labeling in immunofluorescence experiments.
Platelet aggregation
To further ascertain whether gene-transferred VWF behaved physiologically like the wild-type endogenous factor, plasma from the treated VWF–/– was evaluated for the capacity to induce platelet aggregation.30,31 To evaluate stimulated aggregation of murine platelets, blood (500 μL) was collected in plastic tubes containing 50 μL of 106 mM sodium citrate. Platelet-rich plasma was prepared by centrifuging plasma for 15 minutes at 360g. The supernatant was discarded, and the platelets were resuspended in 500 μL of 250 mM Tris-HCl, pH 7.3, 50 mM EDTA. Platelets were washed 3 times and finally resuspended in the same buffer at a concentration of 250 × 103/μL. Platelet aggregation was assessed by mixing 50 μL platelet-rich plasma with 20 μL test plasma and 25 μL botrocetin (2 μg/mL; Diamed, Cressiersur-Morat, Switzerland) on a clean, silanized glass plate. After 2 hours at 23°C with gentle rocking, aggregation was photographed under dark-field microscopy.
Factor VIII levels in plasma
As in VWD in humans, factor VIII levels in VWF–/– mice are markedly reduced because of the lack of the protective carrier function provided by VWF.12 The ability of the VWF cDNA to protect and normalize factor VIII levels in VWF–/– mice after the administration of the VWF plasmid was assessed. Factor VIII activity (FVIII:C) levels in murine plasma were quantitated in 96-well microtiter plates by a fluid-phase assay (Coatest VIII:C/4; DiaPharm, Franklin, OH) according to a modification of the manufacturer's protocol.22,32 Briefly, plasma was mixed with buffer (5 mM Tris-HCl pH 7.3, 0.02% bovine serum albumin) in triplicate to uncoated wells at a ratio of 25:1 (buffer to plasma). Premixed assay reagent, including phospholipid, factor IXa, factor X, and 25 mM calcium chloride, was added, and the plates were incubated for 10 minutes at 37°C. The chromogenic substrate for factor Xa (S-2222; 4 mM) was added, and the plate was transferred to a microplate reader (Molecular Devices, Menlo Park, CA) preset at 37°C. Finally, 25 μL of 20% acetic acid was added to stop the reaction, and the reaction was read on a microtiter plate reader at 405 nm. Factor Xa–dependent conversion of S-2222 is directly related to the concentration of factor VIII C in each well. Standard curves were constructed by plotting the results of the chromogenic substrate assay as a function of known amounts of recombinant human factor VIII (rhFVIII; Baxter, Glendale, CA) and by using human normal plasma calibrated against the supplied international standard for plasma factor VIII with the pooled plasma factor VIII level.33,34 Results are reported as a percentage of normal murine factor VIII level.
Correction of bleeding time
The most direct way to assess the capacity of gene-transferred VWF to act in a manner indistinguishable from that of naturally occurring endogenous VWF is to determine whether it is able to support hemostasis after a standard wound. Transected-tail wounds in untreated VWF–/– mice do not stop bleeding spontaneously, whereas similar wounds in wild-type mice stop bleeding in less than 3 minutes.12,35,36 To assess the ability of the VWF cDNA to correct the bleeding time, 10 to 250 μg VWF cDNA or eGFP cDNA plasmids were administered as described in “Experimental murine model,” and bleeding time was quantified as a phenotypic end point. Tails of restrained mice were placed under a heating lamp at a controlled temperature (37°C). The tail was cut 1 cm from the tip, and the tail was immersed in saline at 37°C. Bleeding time was recorded as the time at which the flow of blood stopped.
Statistical analysis
Murine experiments were performed with 5 or more animals per data point. Effects of single parameters were assessed with 2-tailed Student t tests adjusting for multiple groups when appropriate. Multiple parameters were compared according to analysis of variance (ANOVA; StatView; SAS Institute, Cary, NC).
Results
Plasma VWF antigen and multimers
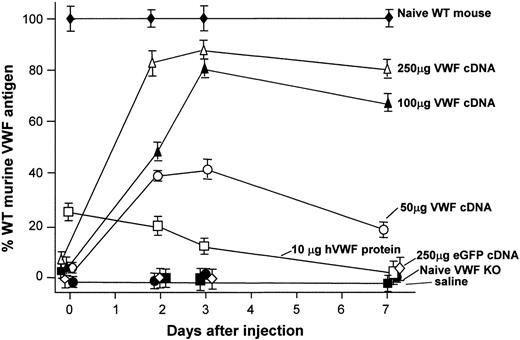
The VWF–/– mice used in this study have been previously shown to have no detectable plasma VWF antigen.12 No VWF antigen was detected in the plasma of the VWF–/– in this study (Figure 1), whereas VWF–/– mice treated with 50, 100, or 250 μg murine VWF cDNA by hydrodynamic tail vein injection revealed a dose-dependent increase in VWF antigen at 48 hours, 72 hours, and 1 week. Injection with human recombinant VWF protein (10 μg) also resulted in detectable levels of circulating VWF antigen in the VWF–/– mice, but eGFP cDNA or saline injection did not. VWF antigen arising from all dose levels of VWF cDNA injection was still detectable after 1 week.
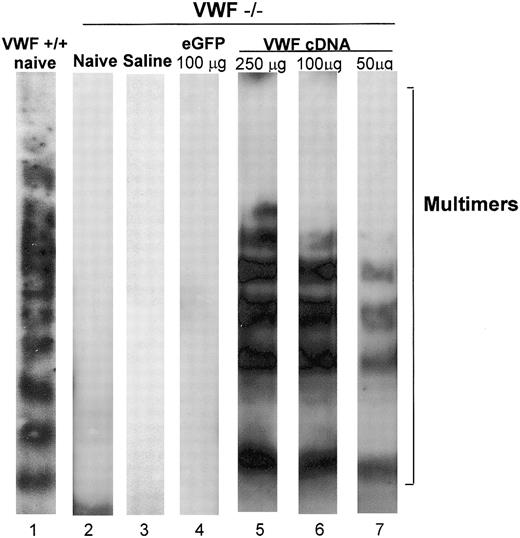
Plasma VWF from healthy mice or humans appears on Western blot analysis as a series of multimers forming a ladder of increasing molecular weight.29 The highest molecular weight multimers are the most effective in supporting interaction with collagen and platelet receptors, and they participate to the greatest extent in wound healing.6,37 The pattern of VWF multimers was assessed in the plasma of wild-type and VWF–/– mice and in VWF–/– mice 48 hours after the injection of saline or VWF cDNA by tail vein. Plasma of wild-type mice showed the pattern of multimers (Figure 2, lane 1). These multimers were absent in the plasma of naive VWF–/– mice (lane 2), VWF–/– mice 48 hours after the administration of saline (lane 3), and VWF–/– mice given injections of 10 μg VWF cDNA (not shown). Similarly, no VWF multimers were observed in VWF–/– mice receiving 100 μg eGFP-cDNA (lane 4). In contrast, the administration of 50, 100, or 250 μg VWF cDNA resulted in the appearance of VWF multimers similar to those of wild-type VWF (lanes 5-7), demonstrating that the appearance of multimers was specific to VWF cDNA administration.
VWF antigen levels in plasma after tail vein administration of murine VWF cDNA to VWF–/– mice. VWF antigen levels at 48 hours, 72 hours, and 1 week after VWF cDNA injection, respectively. Results were determined by ELISA, as described in “Materials and methods,” and are reported as percentages of normal murine VWF. Profiles are shown for plasma VWF antigen level from untreated wild-type mice, plasma VWF antigen level from untreated VWF–/– mice, plasma VWF antigen level after injection of 50, 100, or 250 μg murine VWF cDNA, plasma VWF antigen level after injection of 10 μg recombinant human VWF protein, plasma VWF antigen level after injection of 250 μg eGFP cDNA (negative control), and plasma VWF antigen level after hydrodynamic injection of saline (negative control).
VWF antigen levels in plasma after tail vein administration of murine VWF cDNA to VWF–/– mice. VWF antigen levels at 48 hours, 72 hours, and 1 week after VWF cDNA injection, respectively. Results were determined by ELISA, as described in “Materials and methods,” and are reported as percentages of normal murine VWF. Profiles are shown for plasma VWF antigen level from untreated wild-type mice, plasma VWF antigen level from untreated VWF–/– mice, plasma VWF antigen level after injection of 50, 100, or 250 μg murine VWF cDNA, plasma VWF antigen level after injection of 10 μg recombinant human VWF protein, plasma VWF antigen level after injection of 250 μg eGFP cDNA (negative control), and plasma VWF antigen level after hydrodynamic injection of saline (negative control).
Western blot analysis of VWF multimers in plasma after tail vein administration of the murine VWF cDNA to VWF–/– mice. Murine VWF cDNA (100 μg) was administered to VWF–/– mice with saline as a negative control. After 48 hours, plasma was collected from mice that had received injections and was assessed by Western blot analysis. Relative gel positions of VWF multimer bands are indicated on the right. Lane 1, plasma of naive wild-type mice showing normal VWF multimer pattern; lane 2, plasma from naive VWF–/– mice showing the absence of VWF multimers; lane 3, plasma from VWF–/– mice 48 hours after administration of saline showing the monomer/dimer pattern; lane 4, VWF–/– mice 48 hours after administration of 100 μg eGFP cDNA showing the absence of VWF multimers; lane 5, plasma from VWF–/– mice 48 hours after administration of 250 μg VWF cDNA; lane 6, plasma from VWF–/– mice 48 hours after administration of 100 μg VWF cDNA; lane 7, plasma from VWF–/– mice 48 hours after administration of 50 μg VWF cDNA. Lanes 5 to 7 reveal a VWF pattern similar to that seen in WT mice.
Western blot analysis of VWF multimers in plasma after tail vein administration of the murine VWF cDNA to VWF–/– mice. Murine VWF cDNA (100 μg) was administered to VWF–/– mice with saline as a negative control. After 48 hours, plasma was collected from mice that had received injections and was assessed by Western blot analysis. Relative gel positions of VWF multimer bands are indicated on the right. Lane 1, plasma of naive wild-type mice showing normal VWF multimer pattern; lane 2, plasma from naive VWF–/– mice showing the absence of VWF multimers; lane 3, plasma from VWF–/– mice 48 hours after administration of saline showing the monomer/dimer pattern; lane 4, VWF–/– mice 48 hours after administration of 100 μg eGFP cDNA showing the absence of VWF multimers; lane 5, plasma from VWF–/– mice 48 hours after administration of 250 μg VWF cDNA; lane 6, plasma from VWF–/– mice 48 hours after administration of 100 μg VWF cDNA; lane 7, plasma from VWF–/– mice 48 hours after administration of 50 μg VWF cDNA. Lanes 5 to 7 reveal a VWF pattern similar to that seen in WT mice.
Localization of VWF expression
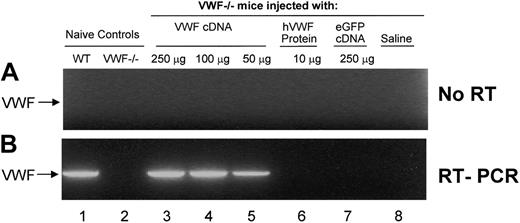
Because previous studies had demonstrated that most gene expression occurs in the liver after hydrodynamic tail vein injection of cDNA27,28 we evaluated liver-specific VWF expression by RT-PCR 48 hours after hydrodynamic injection of VWF cDNA into VWF–/– mice visualized on ethidium bromide agarose gels (Figure 3). To demonstrate that expressed VWF RNA, not VWF plasmid DNA, was amplified, control reactions were performed without the addition of reverse transcriptase (Figure 3A, no RT). The lower panel (Figure 3B, RT-PCR) shows identical reactions with reverse transcriptase included. The data revealed VWF RNA expression in untreated WT mice (lane 1) but not in RNA from untreated VWF–/– mice (lane 2). However, VWF–/– mice were strongly positive for VWF expression 48 hours after the injection of 250 μg (lane 3), 100 μg (lane 4), or 50 μg (lane 5) murine VWF cDNA. No amplification was observed from VWF–/– mice given injections of 10 μg recombinant human VWF protein (negative control, lane 6), 250 μg eGFP cDNA (negative control, lane 7), or saline (negative control, lane 8). These data indicate that gene-transferred VWF was expressed in the liver.
Evaluation of liver-specific VWF expression by RT-PCR after hydrodynamic injection of VWF cDNA. Analysis was carried out on total RNA from liver with VWF-specific primers (forward, 5′-GGACATTTTCTCAGACCACCATA-3′; reverse, 5′-TGTGGAGGCTGAGTTGGG-3′) 48 hours after hydrodynamic injection with murine VWF cDNA. To demonstrate that VWF RNA and not plasmid DNA was amplified, control reactions were performed without the addition of (A) reverse transcriptase. The lower panel shows identical reactions with (B) reverse transcriptase included. Lane 1, RT-PCR of RNA from untreated WT mice; lane 2, RT-PCR of RNA from untreated VWF–/– mice; lane 3, RT-PCR of RNA from VWF–/– mice 48 hours after hydrodynamic injection of 250 μg murine VWF cDNA; lane 4, RT-PCR of RNA from VWF–/– mice 48 hours after hydrodynamic injection of 100 μg murine VWF cDNA; lane 5, RT-PCR of RNA from VWF–/– mice 48 hours after hydrodynamic injection of 50 μg murine VWF cDNA; lane 6, RT-PCR of RNA from VWF–/– mice 48 hours after hydrodynamic injection of 10 μg recombinant human VWF protein (rhVWF; negative control); lane 7, RT-PCR of RNA from VWF–/– mice 48 hours after hydrodynamic injection of 250 μg eGFP cDNA (negative control); lane 8, RT-PCR of RNA from VWF–/– mice 48 hours after hydrodynamic injection of saline (negative control). Using the described primers, the RT-PCR product of VWF mRNA is a 2.2-kb band, marked by an arrow in the left of both panels. RT-PCR products were visualized on ethidium bromide agarose gels.
Evaluation of liver-specific VWF expression by RT-PCR after hydrodynamic injection of VWF cDNA. Analysis was carried out on total RNA from liver with VWF-specific primers (forward, 5′-GGACATTTTCTCAGACCACCATA-3′; reverse, 5′-TGTGGAGGCTGAGTTGGG-3′) 48 hours after hydrodynamic injection with murine VWF cDNA. To demonstrate that VWF RNA and not plasmid DNA was amplified, control reactions were performed without the addition of (A) reverse transcriptase. The lower panel shows identical reactions with (B) reverse transcriptase included. Lane 1, RT-PCR of RNA from untreated WT mice; lane 2, RT-PCR of RNA from untreated VWF–/– mice; lane 3, RT-PCR of RNA from VWF–/– mice 48 hours after hydrodynamic injection of 250 μg murine VWF cDNA; lane 4, RT-PCR of RNA from VWF–/– mice 48 hours after hydrodynamic injection of 100 μg murine VWF cDNA; lane 5, RT-PCR of RNA from VWF–/– mice 48 hours after hydrodynamic injection of 50 μg murine VWF cDNA; lane 6, RT-PCR of RNA from VWF–/– mice 48 hours after hydrodynamic injection of 10 μg recombinant human VWF protein (rhVWF; negative control); lane 7, RT-PCR of RNA from VWF–/– mice 48 hours after hydrodynamic injection of 250 μg eGFP cDNA (negative control); lane 8, RT-PCR of RNA from VWF–/– mice 48 hours after hydrodynamic injection of saline (negative control). Using the described primers, the RT-PCR product of VWF mRNA is a 2.2-kb band, marked by an arrow in the left of both panels. RT-PCR products were visualized on ethidium bromide agarose gels.
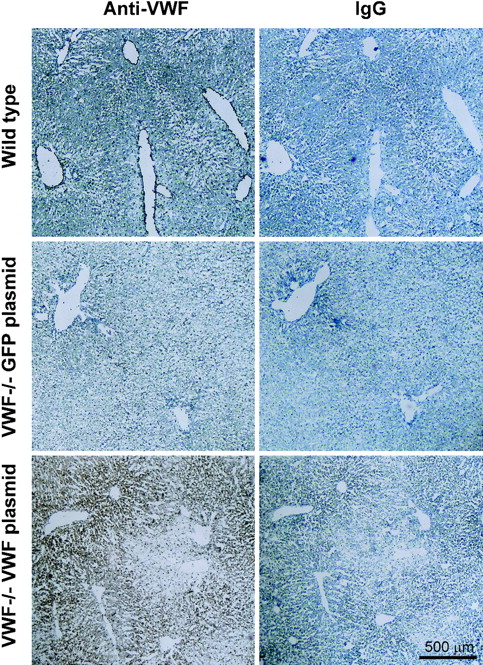
To further assess the source of gene-transferred VWF secretion in vivo, immunohistochemical staining of liver sections from VWF–/– mice was performed 48 hours after administration of the plasmids. Immunohistochemical assessment of VWF expression in the liver showed VWF expression throughout the parenchyma, but not in the endothelial cells lining the vessels (Figure 4). As a positive control, livers from untreated wild-type mice revealed the expected endothelial staining with anti-VWF antibody (Figure 4A) but no staining with nonspecific rabbit IgG (Figure 4B, control). No VWF-specific staining was observed in VWF–/– mice receiving eGFP cDNA (250 μg) with either the anti-VWF antibody (Figure 4C) or the control antibody (Figure 4D). However, VWF–/– mice received 250 μg VWF cDNA strong VWF-specific hepatocyte staining throughout the parenchyma (Figure 4E) but no staining with the control antibody (Figure 4F). No accumulation of VWF protein was observed in the liver endothelia of these animals.
Immunohistochemical localization of VWF expression in liver sections from VWF–/– mice after intravenous administration of murine VWF cDNA. Data are presented pairwise. Sections on the left were stained with a specific anti-VWF antibody, and sections on the right (controls) were treated with an irrelevant antibody (rabbit IgG). (A) Naive wild-type C57BL/6 mouse stained with rabbit anti–human VWF antibody. Dense endothelial staining of VWF is observed. (B) Same as upper left but stained with rabbit IgG (control). (C) Liver, VWF–/–, 48 hours after administration of 250 μg eGFP cDNA plasmid (negative control), anti–human VWF antibody. (D) Same as middle left, control antibody. No staining is observed in either sample. (E) Liver, VWF–/– mouse, 48 hours after administration of 250 μg VWF cDNA plasmid, anti–human VWF antibody. (F) Same as lower left, control antibody; strong diffuse staining is observed within the liver parenchyma. Bar represents 500 μm.
Immunohistochemical localization of VWF expression in liver sections from VWF–/– mice after intravenous administration of murine VWF cDNA. Data are presented pairwise. Sections on the left were stained with a specific anti-VWF antibody, and sections on the right (controls) were treated with an irrelevant antibody (rabbit IgG). (A) Naive wild-type C57BL/6 mouse stained with rabbit anti–human VWF antibody. Dense endothelial staining of VWF is observed. (B) Same as upper left but stained with rabbit IgG (control). (C) Liver, VWF–/–, 48 hours after administration of 250 μg eGFP cDNA plasmid (negative control), anti–human VWF antibody. (D) Same as middle left, control antibody. No staining is observed in either sample. (E) Liver, VWF–/– mouse, 48 hours after administration of 250 μg VWF cDNA plasmid, anti–human VWF antibody. (F) Same as lower left, control antibody; strong diffuse staining is observed within the liver parenchyma. Bar represents 500 μm.
Induction of platelet aggregation
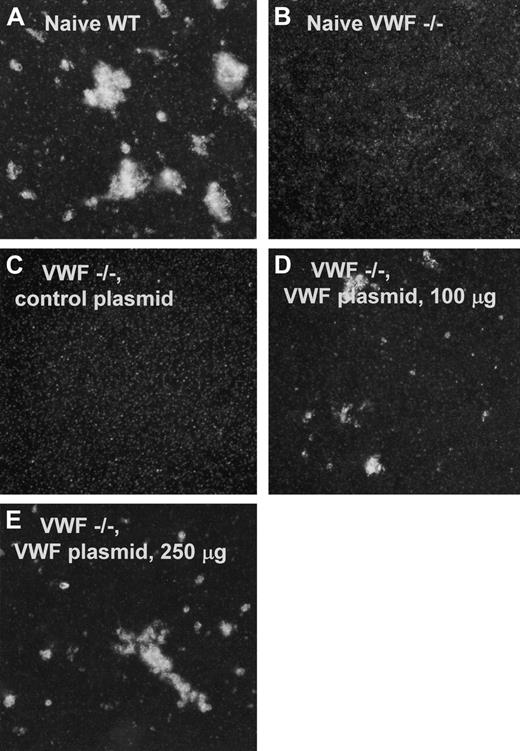
To assess the ability of plasma VWF from VWF–/– mice receiving VWF cDNA to support botrocetin-induced platelet aggregation, washed platelets were mixed with plasma and stimulated with botrocetin, a 25-kDa disulfide-linked dimeric protein–purified Bothrops jararaca snake venom that causes VWF-dependent agglutination of platelets from a variety of species, including mice.38,39 Naive wild-type plasma induced the aggregation of platelets from wild-type mice (Figure 5A). No aggregation was seen when purified platelets from VWF–/– mice were mixed with plasma from naive VWF–/– mice and botrocetin, illustrating the absence of functional VWF from the plasma of these animals (Figure 5B). Similarly, plasma from VWF–/– mice given injections of 100 μg irrelevant cDNA (eGFP) showed no evidence of botrocetin-mediated platelet aggregation (Figure 5C). However, plasma from VWF–/– mice administered 100 μg (Figure 5D) or 250 μg (Figure 5E) VWF cDNA–mediated botrocetin-stimulated aggregation of platelets indicated the presence of functional gene transfer–derived VWF in these animals.
Stimulated aggregation of murine platelets by plasma from wild-type or VWF–/– mice. Platelet aggregation was assessed by mixing platelet-rich plasma (PRP), test plasma, and botrocetin. Photographs were taken after 2 hours; 23°C with gentle rocking. (A) Naïve WT. PRP from VWF wild-type mice, mixed with plasma from VWF wild-type mice containing natural endogenous VWF (positive control) showing aggregation. (B) Naive, VWF–/–. PRP from VWF–/– mice mixed with plasma from naive VWF–/– mice and botrocetin (negative control), illustrating the absence of functional VWF from the plasma of VWF–/– mice. (C) VWF–/–, control plasmid. PRP from VWF–/– mice mixed with plasma from VWF–/– mice that had received 100 μg irrelevant cDNA (negative control) showing no aggregation. (D) VWF–/–, VWF plasmid, 100 μg. PRP from VWF–/– mice mixed with plasma from VWF–/– mice that had received 100 μg VWF cDNA shows botrocetin-stimulated aggregation of platelets indicating the presence of functional gene transfer–derived VWF. (E) VWF–/–, VWF plasmid, 250 μg. PRP from VWF–/– mice mixed with plasma from VWF–/– mice that had received 250 μg VWF cDNA shows increased botrocetin-stimulated aggregation of platelets, indicating the presence of functional gene transfer–derived VWF.
Stimulated aggregation of murine platelets by plasma from wild-type or VWF–/– mice. Platelet aggregation was assessed by mixing platelet-rich plasma (PRP), test plasma, and botrocetin. Photographs were taken after 2 hours; 23°C with gentle rocking. (A) Naïve WT. PRP from VWF wild-type mice, mixed with plasma from VWF wild-type mice containing natural endogenous VWF (positive control) showing aggregation. (B) Naive, VWF–/–. PRP from VWF–/– mice mixed with plasma from naive VWF–/– mice and botrocetin (negative control), illustrating the absence of functional VWF from the plasma of VWF–/– mice. (C) VWF–/–, control plasmid. PRP from VWF–/– mice mixed with plasma from VWF–/– mice that had received 100 μg irrelevant cDNA (negative control) showing no aggregation. (D) VWF–/–, VWF plasmid, 100 μg. PRP from VWF–/– mice mixed with plasma from VWF–/– mice that had received 100 μg VWF cDNA shows botrocetin-stimulated aggregation of platelets indicating the presence of functional gene transfer–derived VWF. (E) VWF–/–, VWF plasmid, 250 μg. PRP from VWF–/– mice mixed with plasma from VWF–/– mice that had received 250 μg VWF cDNA shows increased botrocetin-stimulated aggregation of platelets, indicating the presence of functional gene transfer–derived VWF.
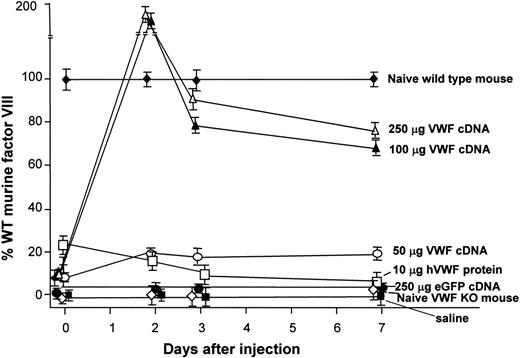
Normalization of plasma factor VIII levels after administration of the murine VWF cDNA to VWF–/– mice. Plasma factor VIII levels as percentages of normal wild-type murine factor VIII at 48 hours, 72 hours, and 1 week after injection. Data are normalized to untreated wild-type mice. Data points indicate plasma factor VIII levels from untreated VWF–/– mice; plasma factor VIII levels after injection of 50, 100, or 250 μg murine VWF cDNA; after injection of 10 μg recombinant human VWF protein; after injection of 250 μg eGFP cDNA (negative control); and after hydrodynamic injection of saline (negative control). Factor VIII levels in VWF–/– mice receiving 100 or 250 μg VWF cDNA exceeded normal levels in wild-type mice at 48 hours (160% and 185% of wild-type mouse values, respectively). Levels decreased to 70% to 80% by 1 week. VWF–/– mice receiving 50 μg VWF cDNA revealed factor VIII levels that stayed between 20% and 27% of levels in wild-type mice at all time points tested.
Normalization of plasma factor VIII levels after administration of the murine VWF cDNA to VWF–/– mice. Plasma factor VIII levels as percentages of normal wild-type murine factor VIII at 48 hours, 72 hours, and 1 week after injection. Data are normalized to untreated wild-type mice. Data points indicate plasma factor VIII levels from untreated VWF–/– mice; plasma factor VIII levels after injection of 50, 100, or 250 μg murine VWF cDNA; after injection of 10 μg recombinant human VWF protein; after injection of 250 μg eGFP cDNA (negative control); and after hydrodynamic injection of saline (negative control). Factor VIII levels in VWF–/– mice receiving 100 or 250 μg VWF cDNA exceeded normal levels in wild-type mice at 48 hours (160% and 185% of wild-type mouse values, respectively). Levels decreased to 70% to 80% by 1 week. VWF–/– mice receiving 50 μg VWF cDNA revealed factor VIII levels that stayed between 20% and 27% of levels in wild-type mice at all time points tested.
Factor VIII levels
Circulating plasma levels of factor VIII are maintained at normal physiological levels through the protective action of VWF, to which factor VIII binds.20-22 Unbound factor VIII has a very short half-life in plasma, approximately one tenth that of bound factor VIII.40 To demonstrate the restoration of normal factor VIII levels after intravenous delivery of VWF cDNA to VWF–/– mice, plasma was collected 48 hours, 72 hours, and 1 week after injection and was assayed for factor VIII (Figure 6). Low circulating levels of factor VIII were detected in naive VWF–/– mice and in VWF–/– mice given injections of saline (negative control; factor VIII levels less than 10% of normal wild-type mouse). In contrast, VWF–/– mice receiving 100 or 250 μg VWF cDNA had factor VIII levels that exceeded normal levels in wild-type mice at 48 hours (160% and 185% of values in wild-type mice, respectively), though by 1 week the levels fell to 80% of normal in wild-type mice. VWF–/– mice receiving 50 μg VWF cDNA had factor VIII levels that stayed between 20% and 27% of levels in wild-type mice at all time points tested. These data indicate that the gene-transferred VWF generated VWF protein capable of performing the normal physiological function of stabilizing factor VIII in circulation (P < .001 for both groups).
Bleeding time
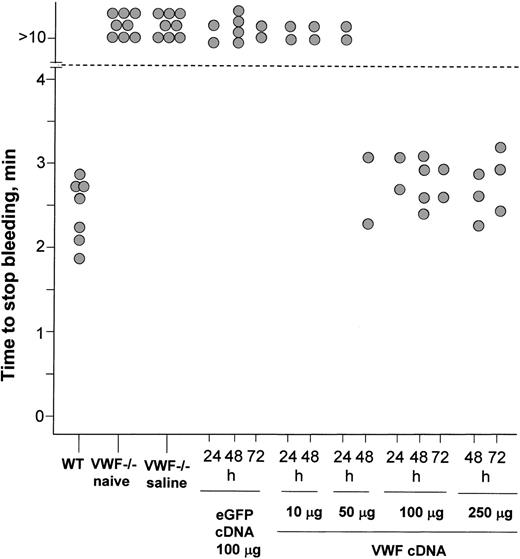
The ultimate test of gene-transferred VWF is whether it is able to participate in wound healing. Transected tail wounds cause untreated VWF–/– mice to bleed to death unless the wounds are cauterized, whereas similar wounds in wild-type mice stop bleeding in less than 3 minutes.12,35,36 To investigate the impact of VWF gene transfer on the bleeding phenotype of these mice, VWF cDNA plasmid was administered through tail vein injection into VWF–/– mice, tails were transected, and bleeding times were recorded (Figure 7). As expected, wild-type mice stopped bleeding between 2 to 3 minutes after wounding, but 48 hours after receiving saline naive VWF–/– mice and VWF–/– mice continued bleeding in episodes lasting more than 10 minutes. Injection of VWF–/– mice with 100 μg eGFP cDNA had no effect on bleeding time when tested at 24, 48, or 72 hours. Administration of 10 μg or 50 μg VWF cDNA also had no effect on bleeding time at 24 hours, but by 48 hours after injection, the bleeding time in the animals receiving 50 μg VWF cDNA was normalized (P < .001). Administration of 100 μgor250 μg VWF cDNA also resulted in the normalization of bleeding time by 24 hours and persisted until at least 72 hours (both P < .001). Thus, even by the stringent physiological test of correction of the VWF–/– bleeding phenotype, the administration of VWF cDNA is able to generate functional circulating VWF that is indistinguishable from the endogenous form.
Impact of administration of murine VWF cDNA plasmid on bleeding time of VWF–/– mice. Mice were assessed for the time required for bleeding to stop after a transected tail vein wound. After 10 minutes, bleeding was stopped by cauterization. Each data point represents a single animal. Wild-type mice stopped bleeding 2 to 3 minutes after wounding. Forty-eight hours after administration with saline, naive VWF–/– mice and VWF–/– mice continued bleeding for episodes lasting more than 10 minutes; this is the bleeding phenotype that characterizes this animal model.12 VWF–/– mice receiving 100 μg eGFP cDNA showed no effect on bleeding time when tested at 24, 48, or 72 hours. Administration of 10 μg or50 μg VWF cDNA also had no effect on bleeding time at 24 hours, but by 48 hours after injection, bleeding time in the animals receiving 50 μg VWF cDNA was normalized. Administration of 100 μg or 250 μg VWF cDNA resulted in the normalization of bleeding time by 24 hours and persisted until at least 72 hours.
Impact of administration of murine VWF cDNA plasmid on bleeding time of VWF–/– mice. Mice were assessed for the time required for bleeding to stop after a transected tail vein wound. After 10 minutes, bleeding was stopped by cauterization. Each data point represents a single animal. Wild-type mice stopped bleeding 2 to 3 minutes after wounding. Forty-eight hours after administration with saline, naive VWF–/– mice and VWF–/– mice continued bleeding for episodes lasting more than 10 minutes; this is the bleeding phenotype that characterizes this animal model.12 VWF–/– mice receiving 100 μg eGFP cDNA showed no effect on bleeding time when tested at 24, 48, or 72 hours. Administration of 10 μg or50 μg VWF cDNA also had no effect on bleeding time at 24 hours, but by 48 hours after injection, bleeding time in the animals receiving 50 μg VWF cDNA was normalized. Administration of 100 μg or 250 μg VWF cDNA resulted in the normalization of bleeding time by 24 hours and persisted until at least 72 hours.
Discussion
VWF is a large multimeric protein that supports platelet adhesion and aggregation in the initiation of hemostasis at the time of vascular injury and that functions as a carrier protein for factor VIII in the circulation.5,6,10 A number of mutations within the VWF gene contribute to the incomplete penetrance and variable expressivity of VWF, causing deficiencies in VWF and resulting in the common human bleeding disorder von Willebrand disease.3-7,12,13,41 This report demonstrates that with gene transfer of VWF cDNA, VWF protein can be expressed, processed, and secreted in a physiologically active form; thus, it may be possible to correct VWD using gene transfer. This was demonstrated by the transfer of intact murine VWF cDNA mediating the dose-dependent appearance of VWF antigen in the plasma of treated VWF–/– mice, the appearance of functional high molecular–weight VWF multimers in the plasma of treated VWF–/– mice, the appearance of murine VWF cDNA in the livers of VWF–/– mice by RT-PCR, and immunohistochemical VWF staining of hepatocytes but not endothelial cells lining the vessels in the liver. In addition, plasma containing these multimers supported the aggregation of murine platelets in the presence of botrocetin; circulating factor VIII levels in treated VWF–/– mice were normalized, indicating restoration of the carrier function for VWF; and, most significantly, bleeding time for a transected tail wound was normalized by 48 hours after the delivery of VWF cDNA above a threshold level.
Normal VWF expression
VWF is normally synthesized by megakaryocytes and endothelial cells.11,14-16 The molecule VWF is synthesized as a large precursor proprotein dimerized through disulfide bonds near the carboxy terminus and glycosylated in the endoplasmic reticulum and the Golgi apparatus, followed by multimer formation through disulfide bonds near the amino terminus in the trans-Golgi network.9,11 Final maturation is achieved by removal of the large propeptide (741 amino acids) and release of the mature VWF (2050 amino acids). Equimolar quantities of mature VWF and cleaved propeptide are copackaged into secretory granules (called Weibel-Palade bodies) in endothelial cells.18 It is unknown whether other cell types are able to perform these biochemical and secretory processes in vivo. Large multimers of VWF are the most effective at platelet aggregation.6,37 VWF forms a noncovalent complex with plasma factor VIII, protecting it from inactivation and clearance and significantly extending its half-life.5,20,22,40 In VWD, the absence of VWF leads to a secondary deficiency of factor VIII, causing defects in platelet-plug and fibrin formation. Clinical manifestations of VWD reflect this dual nature, including excessive and prolonged bleeding after surgery seen in coagulopathies and mucosal tract hemorrhages such as epistaxis and menorrhagia typical of thrombocytopathies.5,12,13
Correction by VWD by gene transfer
The VWF–/– mouse model used in this study closely mimicked severe human VWD. Neither mature VWF nor VWF propolypeptide is detectable in plasma, platelets, or endothelial cells of homozygotes.12 Mutant mice exhibited defects in hemostasis with highly prolonged bleeding times and spontaneous bleeding events. As in human VWD, factor VIII levels in these mice are greatly diminished because of the absent carrier function of VWF. Defective thrombosis is also evident in an in vivo model of vascular injury in these mice.
Hydrodynamic plasmid administration mediates transient expression and was used in this study as a model of the feasibility of correcting the knockout model. It is, therefore, difficult to draw conclusions regarding potential therapeutic levels from a given dose of cDNA. However, even at very high doses of VWF cDNA, antigen levels never rose above the level seen in wild-type mice, there was no evidence of gross hemostatic abnormalities, and bleeding time in the knockout model was never less than that seen in wild-type mice. At high VWF cDNA doses (100 or 250 μg), levels of factor VIII were elevated at 48 hours compared with levels in wild-type mice, but this was transient and decreased to 75% to 85% of wild-type levels by 72 hours despite the high volumes introduced in the hydrodynamic technique. The lowest VWF DNA concentration tested (50 μg) did not result in VWF antigen levels greater than 50% or factor VIII levels greater than 27%, and it revealed VWF multimer formation that was less robust than in the higher doses but that resulted in bleeding times identical to those of higher doses, suggesting that the lower dose might be therapeutically useful. With regard to the source of VWF expression, immunohistochemical staining of liver sections from treated VWF–/– animals indicated that hepatocytes surrounding liver sinuses were positive for VWF. There was no evidence of VWF accumulation in the endothelial cells of treated VWF–/– mice.
With regard to safety issues arising from long-term expression of VWF from the liver, only by studying animals over a much longer time period and over a wider range of doses than occurred in the present study can potential problems be completely assessed. However, because no vector-associated proteins resulted from this delivery method, the antivector immunity that has caused problems in other gene transfer studies was not an issue. We found no evidence of inflammation in the liver even at the highest dose tested, suggesting at least that no acute safety concerns are associated with expressing VWF in this manner. Although VWF protein was observed in hepatocytes from treated VWF–/– mice, we were unable to demonstrate that it accumulated in endothelial cells or in Weibel-Palade bodies, as occurs in natural expression.
VWF production arising from injected VWF cDNA appears to demonstrate a nonlinear dose response. There are several possible explanations for this, including threshold levels for the successful manufacture of VWF, multimerization, and multimer secretion from ectopic sites such as the liver. Nonlinearity apparently was not caused by the large volume used for hydrodynamic injection because it did not change VWF and FVIII levels. As an additional control in this study, we administered 10 μg purified human recombinant VWF protein intravenously. This exogenous VWF was slowly degraded over the course of a week and transiently protected factor VIII in the VWF–/– mouse. No evidence of increased multimer size was observed, suggesting that this mouse model has normal levels of ADAMTS13 activity. Further investigation, including the development of virus-based vectors to mediate persistent VWF expression, will be required to determine which, if any, of these factors is operative in gene transfer correction of the VWD phenotype.
Recombinant multimeric VWF protein can correct plasma defects in dogs with naturally occurring VWD, and plasma concentrate is used routinely in the clinical care of patients with VWD.5,42 These results suggest that a gene transfer strategy for VWF could produce clinically relevant results in VWD. This report indicates that the delivery of VWF cDNA ameliorates the symptoms of VWD in an animal model, suggesting that a strategy that will overcome the size limitations of some viral vectors for gene transfer could potentially permit long-term correction of the VWD phenotype.5
Note added in proof. The murine VWF cDNA used in this study (gift of Dr P. Lenting) was sequenced in our laboratory and was found to be identical at the amino acid level to the deposited sequence for murine VWF (GenBank accession number AAN07881; derived from the BALB/c strain) except for 2 amino acid substitutions (Arg867Cys and Tyr895Gln). Both substitutions occurred in the D3 domain of the VWF protein. These 2 sequence alterations were identical to the amino acid sequence of the deposited VWF sequence from the C57BL/6 murine strain (GenBank accession number AAP41950) and the A/J strain (GenBank accession number ABC86573). Because no bleeding phenotype was associated with these murine strains, these substitutions appeared to be normal polymorphisms in the murine gene without functional consequence. The CASA/RKJ strain (GenBank accession number ABC86574) also has these 2 substitutions. The CASA/RKJ strain has higher VWF levels than the C57BL/6 and A/J strains. The CASA/RKJ strain does not have an abnormal bleeding phenotype, but when crossed with an ADAMTS13 knockout mouse, it does develop thrombocytopenic purpura when stressed with Shiga toxin.43
Prepublished online as Blood First Edition Paper, April 25, 2006; DOI 10.1182/blood-2005-06-2330.
Supported in part by The Malcolm Hewitt Wiener Foundation, Greenwich, CT; and the Will Rogers Memorial Fund, Los Angeles, CA.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Drs N. Hackett, B. Coller, and M. Jirouskova for helpful advice; P.J. Lenting (University Medical Center, Utrecht, The Netherlands) and C.V. Denis (INSERM U143, Le Kremlin-Bicêtre, France) for murine VWF cDNA; D.D. Wagner for VWF knockout mice (CBR Institute for Biomedical Research, Department of Pathology, Harvard Medical School, Boston, MA); E. Westein for technical assistance; and N. Mohamed for help in preparing the manuscript.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal