Abstract
Adoptive transfer of T-cell receptor (TCR) genes has been proposed as an attractive approach for immunotherapy in cases where the endogenous T-cell repertoire is insufficient. While there are promising data demonstrating the capacity of TCR-modified T cells to react to foreign antigen encounter, the feasibility of targeting tumor-associated self-antigens has not been addressed. Here we demonstrate that T-cell receptor gene transfer allows the induction of defined self-antigen–specific T-cell responses, even when the endogenous T-cell repertoire is nonreactive. Furthermore, we show that adoptive transfer of T-cell receptor genes can be used to induce strong antigen-specific T-cell responsiveness in partially MHC-mismatched hosts without detectable graft versus host disease. These results demonstrate the feasibility of using a collection of “off the shelf” T-cell receptor genes to target defined tumor-associated self-antigens and thereby form a clear incentive to test this immunotherapeutic approach in a clinical setting.
Introduction
Major histocompatibility complex (MHC) molecules present peptides on the cell surface irrespective of whether they are derived from foreign proteins or from self-proteins. Different tissue types within the body each express a unique set of proteins, and peptide epitopes derived from such tissue-specific proteins can in principle be used as tumor-rejection antigens.1 However, because most of these tumor-associated antigens (TAAs) are nonmutated self-antigens, the T-cell repertoire specific for such antigens is generally small in size and low in avidity. Indeed, both preclinical studies and clinical trials have provided evidence that a lack of T cells with the required reactivity is a major factor limiting T-cell–based immunotherapy. For instance, murine studies have demonstrated that tumor-specific T-cell responses against foreign tumor-associated antigens can readily be induced by vaccination. However, when the same tumor-associated antigen is considered “self” by the available T-cell repertoire, reactivity to these antigens is highly reduced.2,3 In line with this, replacement of the endogenous T-cell compartment through a combination of allogeneic stem cell transplantation (allo-SCT) and donor lymphocyte infusion (DLI) forms an effective treatment strategy for patients with hematologic malignancies such as chronic myeloid leukemia (CML).4 Importantly, the antileukemic effect of allo-SCT/DLI is dependent on the recognition of minor histocompatibility antigens (MiHAgs) of the recipient as “nonself” by the infused donor lymphocytes, and the development of T-cell responses against such antigens is predictive of remission.5 The effects of DLI following allo-SCT provide an excellent example of how an endogenous antigen can become foreign by introduction of a novel T-cell compartment and how recognition of endogenous antigens by this exogenous T-cell compartment is associated with remission. The major drawback of this treatment protocol is that the introduced T-cell reactivity against self-antigens is not specifically directed toward defined tissues but is strictly determined by the available MiHAg differences between donor and recipient. As a consequence, allo-SCT/DLI may fail to result in tumor regression in cases where the immunodominant MiHAgs are not expressed on tumor cells. Furthermore, expression of the immunodominant MiHAgs on nonmalignant cell types results in graft versus host disease (GvHD), a common complication of allo-SCT/DLI with severe morbidity and mortality.
To introduce a new T-cell repertoire that is more specifically directed toward tumor cells, it has been suggested to transfer genes encoding TAA-specific T-cell receptors (TCRs) into autologous T cells. As an example, HLA-A2.1–restricted T-cell receptors specific for melanocyte antigens or for MiHAgs may be isolated from patients experiencing tumor remission following adoptive T-cell therapy for melanoma6,7 or allo-SCT for CML,5,8 and such TCRs may then be used for the treatment of HLA-A2.1–positive patients that share this disease (hereafter referred as allogeneic TCR gene transfer).
The potential of TCR gene transfer has been demonstrated in a recent series of studies that demonstrated both in vitro9-11 and in vivo12,13 that TCR gene transfer suffices to redirect T cells to antigens of choice. However, 2 issues that are essential for the clinical implementation of TCR gene transfer have not been addressed; first: whether TCR gene transfer can be used to break tolerance toward a defined self-antigen; and second: whether TCR gene transfer is feasible in settings where the TCR recipient and TCR donor are partially MHC mismatched, an essential requirement for the development of collections of tumor-specific TCR genes that can be used for the treatment of larger patient groups.
Here we demonstrate that TCR gene transfer meets these 2 essential requirements for clinical use. First, we show that autologous T cells that are transduced with an allogeneic high-affinity TCR specific for a defined self-antigen can specifically target a tissue or tumor expressing this self-antigen. Second, we demonstrate that TCR-modified T cells do function in a variety of partially MHC-mismatched recipients without detectable off-target reactivity. Based on these data we feel that there is sufficient incentive to analyze the feasibility of inducing defined tumor-specific T-cell responses by TCR gene transfer in clinical trials.
Materials and methods
Mice
C57BL/6 (H-2b) (B6), Balb.B (H-2b), and F1 offspring of C57BL/6 and Balb/C (H-2d), SJL (H-2s), B10.BR (H-2k), or FvB (H-2q) mice were obtained from the Experimental Animal Department of The Netherlands Cancer Institute. RIP-OVAhi mice14 were kindly provided by Dr C. Kurts (Friedrich-Wilhelms-Universitat, Bonn, Germany). All animal experiments were performed in accordance with institutional and national guidelines and were approved by the Experimental Animal Committee of The Netherlands Cancer Institute (DEC).
Retroviral constructs and retroviral transduction of T cells
The TCRα and TCRβ fragments of both the F5 and OT-I TCR, separated by an internal ribosomal entry sequence (IRES), were cloned into the pMX retroviral vector15 to obtain pMX-F5α-IRES-F5β 16 and pMX-OT-Iα-IRES-OT-Iβ constructs. Mouse splenocytes were modified by retroviral transduction as described previously.12
Generation of tumor lines expressing ovalbumin and tumor treatment
The C-terminal part of ovalbumin (amino acids [aa] 161 to 385) and the murine CD4 molecule were cloned into the pMX retroviral vector separated by IRES to obtain pMX-OVA-IRES-CD4. B16 cells were transduced with this construct, and the B16-OVA-IRES-CD4 (B16-OVA) cell line was obtained as a single cell clone selected for high CD4 expression. Melanoma cells were washed with HBSS (Gibco) to remove serum components, and 1 × 105 cells were injected in 200 μL HBSS subcutaneously. Tumors were measured with calipers, and mice were killed after tumors reached an average diameter of 12.5 mm.
Flow cytometry
Surface TCR expression was measured 24 hours after transduction by flow cytometry. Cells were stained with FITC- or PE-conjugated anti-TCR Vα2 and anti-TCR Vβ5 monoclonal antibodies (mAbs) (OT-I TCR), anti-TCR Vβ11 mAb and anti-TCR Vβ2, 3, 4, 5.1, 8, 9, and 10b mAb (anti-Vβ-pool) (F5 TCR), or MHC tetramers, in combination with PE- or APC-conjugated anti-CD8α mAb (all mAbs from BD Pharmingen [San Diego, CA] except PE-conjugated anti-CD8α mAb from Caltag [Burlingame, CA]).17 Propidium iodide (Sigma, St Louis, MO) was used to select for live cells. For the measurement of T-cell responses, peripheral-blood samples were taken at the indicated days after transfer. Following removal of erythrocytes by NH4Cl treatment, the cells were washed twice with PBS with 0.5% BSA and 0.02% NaN3 (PBS/BSA). Cells were stained with the relevant antibodies and analyzed by flow cytometry. Intracellular IFN-γ stainings were performed as previously described.12 Data acquisition and analysis was done on a FACSCalibur (Becton Dickinson, MountainView, CA) with CellQuest software.
Viral infection
For live influenza A infections, anesthetized mice were infected by intranasal administration of 50 μL HBSS (Life Technologies, Grand Island, NY) containing 200 plaque forming units (PFU) of influenza A/WSN/33 (WSN)–OVA(I)18 virus (hereafter referred to as inflova) or 25 hemagglutinating units (HAU) of influenza A/NT/60/68 virus. For vaccinia infections, 2 × 107 PFU was intraperitoneally injected. Vaccinia recombinant for GFP-OVA257-264 was kindly provided by Dr J. Yewdell (National Institutes of Health, Bethesda, MD).19
Allogeneic bone marrow transplantation
Transplantation of female B6 bone marrow and splenocytes into lethally irradiated male Balb.B recipients was performed as described previously.20
Histopathology
Extensive necropsy was done on F1 mice that received TCR-transduced cells and subsequent viral infection and on control mice that only received a viral infection. Tissues were sampled in buffered formalin (skin, liver, salivary glands, gastrointestinal tract, spleen, pancreas, heart and lungs, urogenital system, secondary sex glands, head, extremities, and spinal cord). Sections were stained with hematoxylin and eosin and examined blindly for indications of autoimmune pathology, with a special emphasis on liver, skin, and intestine. The sections were reviewed with a Zeiss Axioskop2 Plus microscope (Carl Zeiss Microscopy, Jena, Germany) equipped with Plan-Apochroma (×5/0.16, ×10/0.45, ×20/0.60, and ×40/0.95) and Plan-Neofluar (×2.5/0.075) objectives. In addition to the objectives, there was an extra enlargement device included in the body of the microscope. Images were captured with a Zeiss AxioCam HRc digital camera and processed with AxioVision 4 software (both from Carl Zeiss Vision, München, Germany).
Measurement of blood-glucose levels and treatment of diabetes
Blood-glucose levels in RIP-OVAhi mice were monitored by Accu-Check Compact (Roche Diagnostics, Mannheim, Germany) measurement. Mice were considered diabetic when blood-glucose levels were 20 mM or above. For long-time follow-up, diabetic mice were treated with subcutaneous insulin implants according to the manufacturer's protocol (LinShin Canada, Scarborough, ON).
Immunohistochemistry
Immunohistochemistry was carried out on frozen tissue sections. Sections were preincubated with PBS/4% BSA/5% normal goat serum. As primary antibodies, rabbit anti-OVA (Sigma) and rat anti–mouse CD8α (BD PharMingen) were used. Anti-OVA staining was visualized using the Rabbit Envision kit (DAKO, Glostrup, Denmark); anti-CD8 staining was visualized by a 2-step immunoenzymatic procedure. First, biotin-labeled goat-anti–rat immunoglobulins (Santa Cruz Biotechnology, Santa Cruz, CA) were applied, followed by HRP-labeled avidin-biotin complex (ABC) (DAKO). AEC (Sigma) was used as a substrate chromagen, and slides were counterstained with hematoxylin. Images were processed with the same microscopy device and program as described under “Histopathology.”
Results
In vivo function of TCR-transduced T cells in a self-tolerant setting
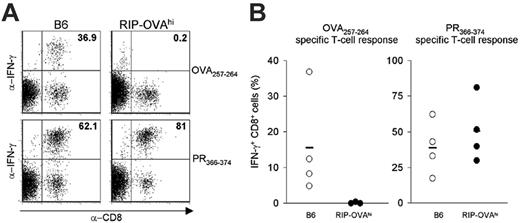
We first addressed whether TCR gene transfer can be used to break tolerance to a defined tissue-specific self-antigen. To this end, we examined the feasibility of inducing a β-cell–specific T-cell attack in RIP-OVAhi mice that express the ovalbumin protein in the insulin-producing β cells of the pancreas. To test whether these mice are tolerant toward ovalbumin, RIP-OVAhi mice were infected intranasally with inflova, an influenza A strain recombinant for the MHC class I–restricted epitope of ovalbumin (OVA257-264), and peripheral-blood samples were analyzed by MHC-tetramer staining. As an internal control, mice were also analyzed for T-cell responses toward PR366-374, the MHC class I–restricted epitope present within the influenza A nucleoprotein of inflova. In none of the RIP-OVAhi mice could OVA257-264-specific T cells be detected over background, whereas in control B6 mice the average response was 2.5% of the CD8+ population. This lack of T-cell responsiveness is selective for the self-protein ovalbumin, as the PR366-374-specific T-cell responses were of a similar magnitude in both strains (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). To test the absence of OVA-specific CD8+ cells in RIP-OVAhi mice more stringently, splenocytes were isolated 6 weeks after infection for an in vitro restimulation assay with either the OVA257-264 or the PR366-374 epitope and subsequent intracellular IFNγ staining. In cultures obtained from RIP-OVAhi mice, no CD8+ cells specific for the OVA257-264 epitope could be detected, whereas CD8+ cells specific for the PR366-374 epitope were present in large numbers (average, 51% of CD8+ cells) (Figure 1). These data show that the OVA257-264 epitope is considered “self” in RIP-OVAhi mice and that any residual T-cell reactivity toward this antigen is below background levels.
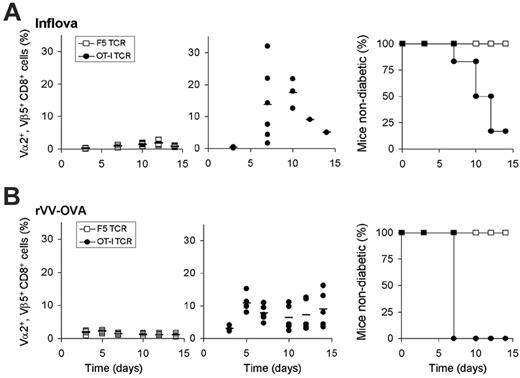
Having established that the endogenous T-cell repertoire is nonreactive toward the OVA257-264 self-epitope, we have used this model to determine whether such self-antigens can be targeted by autologous T cells that are redirected by introduction of a high-affinity TCR that recognizes this antigen. To generate T cells redirected toward ovalbumin, RIP-OVAhi–derived splenocytes were transduced with the OT-I TCR (Vα2+, Vβ5.1+) (Figure S2). After adoptive transfer of 1 × 105 OT-I TCR+ CD8+ cells and subsequent vaccination with inflova, a marked expansion of the Vα2+, Vβ5.1+ population was observed (average, 14%; maximum, 31% of total CD8+ cells). As a control, in RIP-OVAhi mice that received T cells transduced with the F5 TCR that recognizes the MHC class I–restricted epitope of the nucleoprotein of influenza A/NT/60/68 (NP366-374), no expansion of the Vα2+, Vβ5.1+ T-cell population could be detected (Figure 2A). Notably, the kinetics and magnitude of the OT-I TCR-modified T-cell response in RIP-OVAhi mice were comparable to T-cell responses observed in B6 mice, indicating that self-tolerance does not affect the capacity of redirected T cells to react to antigen encounter. Furthermore, when RIP-OVAhi mice are rechallenged with antigen 6 weeks later, expansion of the redirected T cells is again comparable with that observed in B6 mice, indicating that redirected T cells maintain the capacity to react to antigen encounter, even in a tolerant host (M.C., unpublished observations, October 2005). If redirected T cells would also be capable of performing effector function in vivo upon encounter of a self-antigen, OT-I TCR-transduced T cells should be able to target the β cells of the pancreas and thereby induce type I diabetes. To examine this, blood-glucose levels were analyzed at multiple time points following infusion of either OT-I or F5 TCR-transduced T cells. After adoptive transfer of OT-I TCR-transduced T cells, more than 80% of the mice developed diabetes within 14 days (blood-glucose levels of 20 mM or above), whereas the control group stayed normoglycemic (Figure 2A). Also, when in vivo activation of OT-I TCR-modified cells was achieved by a different vaccination strategy using a vaccinia strain recombinant for ovalbumin (rVV-OVA), OT-I TCR-transduced T cells proliferated extensively, and β-cell destruction resulting in diabetes was observed in 100% of the mice (Figure 2B). Adoptive transfer of OT-I transduced T cells without vaccination did not result in detectable T-cell responses or the development of diabetes, indicating that endogenous antigen does not induce substantial activation of the infused T cells (data not shown).
Tolerance in RIP-OVAhi mice. (A) Flow cytometric analysis of in vitro–restimulated splenocytes of inflova-infected B6 (left) or RIP-OVAhi mice (right). An IFNγ assay was performed 14 days after restimulation with 5 × 10–4 μg/mL OVA257-264 peptide (top) or PR366-374 peptide (bottom). (B) IFNγ production 14 days after restimulation with the OVA257-264 peptide (left) or PR366-374 peptide (right) of splenocytes from inflova-infected B6 (○) or RIP-OVAhi (•) mice. Each circle represents 1 mouse; bars, averages.
Tolerance in RIP-OVAhi mice. (A) Flow cytometric analysis of in vitro–restimulated splenocytes of inflova-infected B6 (left) or RIP-OVAhi mice (right). An IFNγ assay was performed 14 days after restimulation with 5 × 10–4 μg/mL OVA257-264 peptide (top) or PR366-374 peptide (bottom). (B) IFNγ production 14 days after restimulation with the OVA257-264 peptide (left) or PR366-374 peptide (right) of splenocytes from inflova-infected B6 (○) or RIP-OVAhi (•) mice. Each circle represents 1 mouse; bars, averages.
In vivo function of TCR-modified T cells in a self-tolerant setting. (A) Analysis of blood cells and blood-glucose levels of RIP-OVAhi mice that received 1 × 105 F5 (□) or OT-I (•) transduced T cells followed by inflova infection. (B) Analysis of blood cells and blood-glucose levels of RIP-OVAhi mice that received 1 × 106 F5 (□) or OT-I (•) transduced T cells followed by rVV-OVA infection; blood was sampled 3 to 15 days after infection. (Left and middle) Closed circles and open squares represent TCR-transduced T-cell responses in individual mice; bars, averages. (Right) Blood-glucose levels were measured to monitor development of type I diabetes. RIP-OVAhi mice that were infected with inflova were killed upon development of diabetes; RIP-OVAhi mice that were infected with rVV-OVA were treated with insulin implants upon development of diabetes.
In vivo function of TCR-modified T cells in a self-tolerant setting. (A) Analysis of blood cells and blood-glucose levels of RIP-OVAhi mice that received 1 × 105 F5 (□) or OT-I (•) transduced T cells followed by inflova infection. (B) Analysis of blood cells and blood-glucose levels of RIP-OVAhi mice that received 1 × 106 F5 (□) or OT-I (•) transduced T cells followed by rVV-OVA infection; blood was sampled 3 to 15 days after infection. (Left and middle) Closed circles and open squares represent TCR-transduced T-cell responses in individual mice; bars, averages. (Right) Blood-glucose levels were measured to monitor development of type I diabetes. RIP-OVAhi mice that were infected with inflova were killed upon development of diabetes; RIP-OVAhi mice that were infected with rVV-OVA were treated with insulin implants upon development of diabetes.
To visualize infiltration of OT-I TCR-transduced T cells in the islets of Langerhans, pancreata were harvested 7 days after adoptive transfer of either OT-I or F5 TCR-transduced cells and subsequent inflova infection. CD8+ cells infiltrating the islets of Langerhans were observed only when mice received T cells directed against ovalbumin (Figure 4C-D). Furthermore, the boundary of the β-cell islets of mice that had received OT-I TCR-transduced T cells displayed a jagged appearance, indicating that some β cells had already been killed prior to the clinical onset of diabetes (Figure 4A-B).
If tissue destruction by redirected T cells is restricted to the cell type that expresses the targeted self-antigen, the only clinical effect of adoptive transfer of OT-I TCR-transduced T cells in RIP-OVAhi mice should be the development of type I diabetes. To address this experimentally, severe diabetes was induced by adoptive transfer of 1 × 106 OT-I TCR-transduced cells and subsequent vaccination with rVV-OVA. This strategy results in a diabetic coma if mice remain untreated. Directly after the onset of diabetes, mice were treated with insulin implants to allow for long-term monitoring. Insulin administration was sufficient to reverse the clinical signs of diabetes such as polydipsia and polyuria. Importantly, following treatment of diabetes, the animals displayed no detectable morbidity during a 3-month follow-up, suggesting that pancreatic β cells are the major or sole target of the infused TCR-modified T cells. Subsequently, pancreata were isolated and analyzed for ovalbumin expression. In mice that received a single adoptive transfer of OT-I cells, the number of islets was decreased approximately 10-fold as compared with control mice (Figure 5). These data demonstrate that a one-time infusion of OT-I TCR-transduced T cells can be used to target a defined tissue-specific self-antigen and that such targeting appears highly selective.
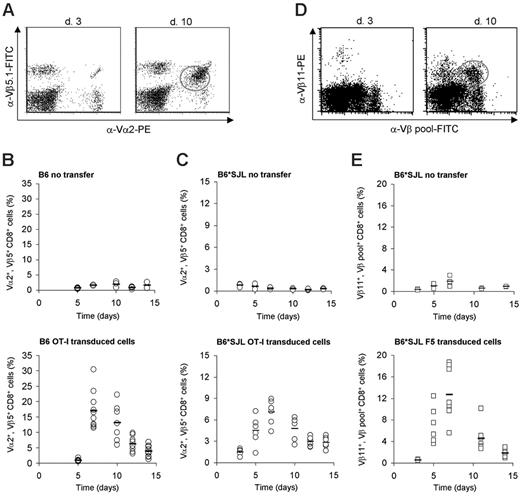
In vivo expansion of TCR-transduced T cells in partially MHC-mismatched recipients. (A) Flow cytometric analysis of blood samples at day 3 (left) or day 10 (right) after infection from B6 mice that received 1 × 105 OT-I TCR-transduced T cells followed by inflova infection. Activated TCR-transduced T cells can be distinguished from the endogenous Vα2/Vβ5+ CD8+ fraction by a lower expression of the Vα2/Vβ5 TCR chains. (B-C) Analysis of blood samples from B6 mice (B) and B6*SJL mice (C) that received no modified T cells (top) or 1 × 105 OT-I TCR-transduced T cells (transduction efficiency in B6*SJL mice, 23% of CD8+ cells) (bottom) followed by inflova infection. Blood was sampled 5 to 14 days after infection and stained as in panel A. Open circles indicate T-cell responses in individual mice; bars, averages. (D) Flow cytometric analysis of blood samples at day 3 (left) or day 10 (right) after infection from B6*SJL mice that received 1 × 105 F5 TCR-transduced T cells (transduction efficiency, 3.5% of CD8+ cells) followed by A/NT/60/68 infection. The percentage of F5 TCR-transduced cells was calculated from the fraction of Vβ11+ cells within the population of Vβ-pool+ cells. Note that a Vβ11dull population that is Vβ-pool negative is present in mice that received F5 TCR-modified T cells. This represents TCR-transduced T cells that express an endogenous Vβ chain for which no antibody was available. (E) Analysis of blood samples from B6*SJL mice that received no modified T cells (top) or 1 × 105 F5 TCR-transduced T cells (bottom) followed by A/NT/60/68 infection. Blood was sampled 5 to 14 days after infection and stained as in panel D. Open squares indicate T-cell responses in individual mice; bars, averages.
In vivo expansion of TCR-transduced T cells in partially MHC-mismatched recipients. (A) Flow cytometric analysis of blood samples at day 3 (left) or day 10 (right) after infection from B6 mice that received 1 × 105 OT-I TCR-transduced T cells followed by inflova infection. Activated TCR-transduced T cells can be distinguished from the endogenous Vα2/Vβ5+ CD8+ fraction by a lower expression of the Vα2/Vβ5 TCR chains. (B-C) Analysis of blood samples from B6 mice (B) and B6*SJL mice (C) that received no modified T cells (top) or 1 × 105 OT-I TCR-transduced T cells (transduction efficiency in B6*SJL mice, 23% of CD8+ cells) (bottom) followed by inflova infection. Blood was sampled 5 to 14 days after infection and stained as in panel A. Open circles indicate T-cell responses in individual mice; bars, averages. (D) Flow cytometric analysis of blood samples at day 3 (left) or day 10 (right) after infection from B6*SJL mice that received 1 × 105 F5 TCR-transduced T cells (transduction efficiency, 3.5% of CD8+ cells) followed by A/NT/60/68 infection. The percentage of F5 TCR-transduced cells was calculated from the fraction of Vβ11+ cells within the population of Vβ-pool+ cells. Note that a Vβ11dull population that is Vβ-pool negative is present in mice that received F5 TCR-modified T cells. This represents TCR-transduced T cells that express an endogenous Vβ chain for which no antibody was available. (E) Analysis of blood samples from B6*SJL mice that received no modified T cells (top) or 1 × 105 F5 TCR-transduced T cells (bottom) followed by A/NT/60/68 infection. Blood was sampled 5 to 14 days after infection and stained as in panel D. Open squares indicate T-cell responses in individual mice; bars, averages.
Homing of TCR-transduced T cells to self-antigen–expressing peripheral tissue. RIP-OVAhi mice received 1 × 105 F5 (left) or OT-I (right) transduced T cells followed by inflova infection. Pancreatic islet infiltration by CD8+ cells and islet integrity were determined on day 7. Immunohistochemical analysis for ovalbumin (top) or CD8 (bottom) is shown. Original magnification, ×20.
Homing of TCR-transduced T cells to self-antigen–expressing peripheral tissue. RIP-OVAhi mice received 1 × 105 F5 (left) or OT-I (right) transduced T cells followed by inflova infection. Pancreatic islet infiltration by CD8+ cells and islet integrity were determined on day 7. Immunohistochemical analysis for ovalbumin (top) or CD8 (bottom) is shown. Original magnification, ×20.
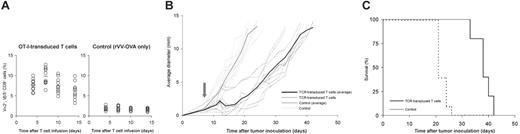
To address whether TCR-transduced T cells can also target a tumor expressing this antigen in a self-tolerant setting, we generated a variant of the poorly immunogenic B16 melanoma that expresses the C-terminal part of ovalbumin (aa 161 to 407). Importantly, the cells were generated using the murine CD4 molecule as a selection marker to ensure that the marker gene product would not constitute a neoantigen. In line with many prior vaccination studies, when the resulting B16-OVA cells are injected in wild-type B6 mice, for which OVA is a foreign antigen, vaccination suffices to suppress tumor growth. However, the same vaccination is ineffective when RIP-OVAhi are challenged with B16-OVA cells, even when vaccination is given at the day of tumor inoculation (Figure S3). This indicates that the presence or absence of an OVA-specific T-cell repertoire is a critical determinant in this model. To test whether OT-I TCR-transduced T cells could substitute for the absence of a tumor-reactive T-cell repertoire in RIP-OVAhi mice, the mice were challenged with 1 × 105 B16-OVA cells followed by an adoptive transfer of 1 × 106 OT-I transduced cells plus vaccination with rVV-OVA on day 7 after tumor inoculation. Upon inoculation in tumor-bearing mice, OT-I transduced T cells expanded (Figure 6A) and induced severe autoimmune diabetes on days 5 and 6 after infusion (data not shown). Also the tumor was targeted by OT-I TCR-transduced T cells, as shown by a transient regression of the tumor in all mice. This initial regression was followed by a short plateau phase, after which the tumor grew out (Figure 6B). It is noted that in this tumor model, the one-time infusion of OT-I transduced T cells that was used results in a transient response of redirected T cells in peripheral blood that is apparently not maintained by the tumor. Nevertheless, this T-cell response does prolong survival from an average of 21 days in the nontreated group to an average of 38 days in the treated group (Figure 6C).
Destruction of ovalbumin-expressing β cells by TCR-transduced T cells. RIP-OVAhi mice received 1 × 106 F5 (left) or OT-I (right) transduced T cells followed by rVV-OVA infection. Mice were treated with insulin implants upon onset of diabetes, and pancreata were harvested 3 months after transfer. Three nonserial sections per mouse were assessed for ovalbumin-positive islands by immunohistochemistry. Per section, the number of islets per view was counted using ×2.5 magnification. Shown are (A) 2 representative sections and (B) the average numbers of islets per view for mice that received F5 or OT-I TCR-transduced T cells. Original magnification, ×5.
Destruction of ovalbumin-expressing β cells by TCR-transduced T cells. RIP-OVAhi mice received 1 × 106 F5 (left) or OT-I (right) transduced T cells followed by rVV-OVA infection. Mice were treated with insulin implants upon onset of diabetes, and pancreata were harvested 3 months after transfer. Three nonserial sections per mouse were assessed for ovalbumin-positive islands by immunohistochemistry. Per section, the number of islets per view was counted using ×2.5 magnification. Shown are (A) 2 representative sections and (B) the average numbers of islets per view for mice that received F5 or OT-I TCR-transduced T cells. Original magnification, ×5.
Safety and feasibility of allogeneic TCR gene transfer in a partially MHC-mismatched setting
In the second part of the study we addressed whether TCR gene transfer is feasible in partially MHC-disparate recipients, in which the introduced TCR will encounter MHC–self-peptide complexes that were not present during thymic selection. In this setting, 2 scenarios could potentially unfold that may impair the feasibility of TCR gene transfer. If T cells transduced with an allogeneic TCR would engage with an allogeneic MHC allele product complexed with a self-antigen with a broad tissue distribution, inactivation of the infused T cells could occur.21 Alternatively, if TCR-transduced T cells recognize allogeneic MHC molecules complexed with organ-specific self-peptides, the resulting off-target recognition could lead to autoimmune pathology. In addition to these potential problems that are specific for TCR gene transfer in a partially MHC-disparate setting, TCR gene transfer could conceivably lead to off-target autoimmune pathology via 2 other mechanisms (see “Discussion”), and the occurrence of such autoimmune reactivity has only been tested in a small cohort of mice.12
Antitumor effect of TCR-transduced T cells in a self-tolerant setting. RIP-OVAhi mice were challenged with 1 × 105 B16-OVA cells subcutaneously. On day 7, mice received either 1 × 106 OT-I TCR-transduced T cells followed by vaccination with rVV-OVA (left panel in A, black line in B, solid line in C) or vaccination alone (right panel in A, gray line in B, dashed line in C). (A) Blood was sampled and analyzed for the presence of OT-I transduced T cells on day 4 to 14 after adoptive transfer. Each circle represents one mouse; bars, average immune responses. (B) Tumor growth was measured 3 times a week starting at day 7 after inoculation. Dashed lines represent growth curves in individual mice; solid lines, average growth curves; arrow, the start of treatment. (C) Survival curve: Mice were killed when the average tumor diameter exceeded 12.5 mm.
Antitumor effect of TCR-transduced T cells in a self-tolerant setting. RIP-OVAhi mice were challenged with 1 × 105 B16-OVA cells subcutaneously. On day 7, mice received either 1 × 106 OT-I TCR-transduced T cells followed by vaccination with rVV-OVA (left panel in A, black line in B, solid line in C) or vaccination alone (right panel in A, gray line in B, dashed line in C). (A) Blood was sampled and analyzed for the presence of OT-I transduced T cells on day 4 to 14 after adoptive transfer. Each circle represents one mouse; bars, average immune responses. (B) Tumor growth was measured 3 times a week starting at day 7 after inoculation. Dashed lines represent growth curves in individual mice; solid lines, average growth curves; arrow, the start of treatment. (C) Survival curve: Mice were killed when the average tumor diameter exceeded 12.5 mm.
To test the feasibility of TCR gene transfer in a large cohort of partially MHC-disparate recipients, we generated offspring of H2b mice with a number of different non-H2b strains. Subsequently, peripheral T cells of these mice were used as recipient cells for the F5 TCR and the OT-I TCR. Because both the F5 and OT-I TCR were originally isolated from H-2b mice, these TCRs are nonreactive with endogenous antigens complexed with the H-2b class I and class II alleles (H-2Kb, Db, I-Ab). In line with this, when T cells modified with the F5 TCR12 or with the OT-I TCR (Figure 3) are adoptively transferred into B6 mice, these cells show a classic proliferative response upon antigen encounter (ie, infection with influenza A strains expressing either the NP366-374 or OVA257-264 epitope).
To determine whether infusion of TCR-modified T cells can also be used to generate antigen-specific T-cell responses in a partially MHC-mismatched setting, splenocytes of F1 offspring of C57BL/6 and Balb/C (H-2d), SJL (H-2s), B10.BR (H-2k), or FvB (H-2q) mice were transduced with the OT-I TCR, and 1 × 105 OT-I TCR-transduced CD8+ cells were adoptively transferred into syngeneic F1 recipients. Subsequently, mice were intranasally infected with inflova, and T-cell responses were measured in peripheral-blood samples by Vα2, Vβ5.1 staining (Figure 3A). As a control, T-cell responses were followed in F1 mice that had only received inflova virus. In all 4 groups of partially MHC-disparate recipients the OT-I TCR-transduced T cells showed a very marked expansion upon inflova infection with a peak response around day 7 (Figures 3C and S4).
To expand these data to a second T-cell receptor, 1 × 105 F5 TCR-transduced CD8+ cells were adoptively transferred into the various partially MHC-mismatched recipients, followed by an intranasal influenza A/NT/60/68 infection. Because no Vα-specific antibody is available for the F5 TCR and because MHC tetramer staining does not allow one to distinguish between endogenous and exogenous NP366-374-specific T cells, we developed an alternative procedure for tracing TCR-modified T cells ex vivo. Rearrangement of TCRβ genes is subject to allelic exclusion and, as a consequence, conventional αβ T cells only express a single TCRβ subunit.22,23 In contrast, TCR gene–modified T cells express both the endogenous TCR and the newly introduced TCR and may therefore be detected by screening for such dual Vβ expression (Figure 3D). As was the case for the OT-I TCR-modified T cells, F5 TCR-transduced T cells showed a very marked expansion upon viral infection in all groups of partially MHC-mismatched recipients tested. Consistent with the notion that these Vβ11dullVβ-pool+ cells represent F5 TCR-modified T cells, no population of Vβ11dullVβ-pool+ cells was detectable in control groups (Figures 3E and S5).
To examine the occurrence of possible TCR gene transfer–associated autoimmunity in this large group of animals, all partially MHC-mismatched mice that received TCR-modified T cells were clinically observed for at least 6 weeks after transfer. Mortality induced by transfer of TCR-modified cells in MHC-disparate recipients was 0% (0 of 66 mice), and none of the mice developed clinically manifest autoimmune disease. To screen for autoimmune pathology in more detail, all mice were killed to perform an extensive pathologic examination. Special attention was given to gut, liver, and skin, the classic target organs of GvHD (Tables S1-S2).24 In all groups, no signs of pathology were observed in intestine and skin. In liver tissue, a mild inflammatory process could be observed in most animals. These lesions displayed a random distribution rather than the periportal localization that is a hallmark of liver GvHD. More importantly, the observed liver pathology was found not only in mice that received TCR-transduced T cells (OT-I, 90%; F5, 76%) but also in control groups (“only inflova,” 71%; “only influenza A/NT/60/68,” 83%), indicating that this pathology is unlikely to be a consequence of the adoptive transfer of TCR-transduced T cells (Figure 7A-B). In addition, in many of the mice that were infected with inflova, pneumonia-related lesions were detected. Again, these lesions were found irrespective of transfer of OT-I TCR-transduced T cells, strongly suggesting that this pathology is virus induced (Table S1). Apart from these 2 histopathologic observations detected irrespective of TCR gene transfer, no consistent pattern of autoimmune pathology was observed (Tables S1-S2). To be able to compare these data to a situation in which GvHD is known to occur, we transplanted MHC-matched bone marrow from B6 into Balb.B mice, where minor histocompatibility differences between the 2 strains are known to result in acute liver and gastrointestinal (GI) tract GvHD.25 Balb.B recipients of B6 bone marrow developed clinical GvHD on days 18 to 24, upon which they were killed. Histopathologic analysis of these mice revealed severe colitis (100% of mice) and liver pathology with periportal lymphoid infiltrates (89% of mice) characteristic of GvHD (Figure 7C; Table S3).
To test for possible chronic GvHD in mice that received TCR-modified T cells, we looked for signs of autoimmunity in F1 offspring of B6 and Balb/C mice 8 months after transfer of OT-I TCR-transduced T cells. Also during this long-term follow-up, the mice displayed no clinical autoimmunity, and pathologic examination again did not reveal any sign of autoimmunity above that observed in control mice. Collectively, these data demonstrate that for 2 different TCRs in 4 different groups of partially MHC-mismatched recipients tested, adoptive transfer of TCR-transduced T cells does not lead to detectable off-target autoimmunity.
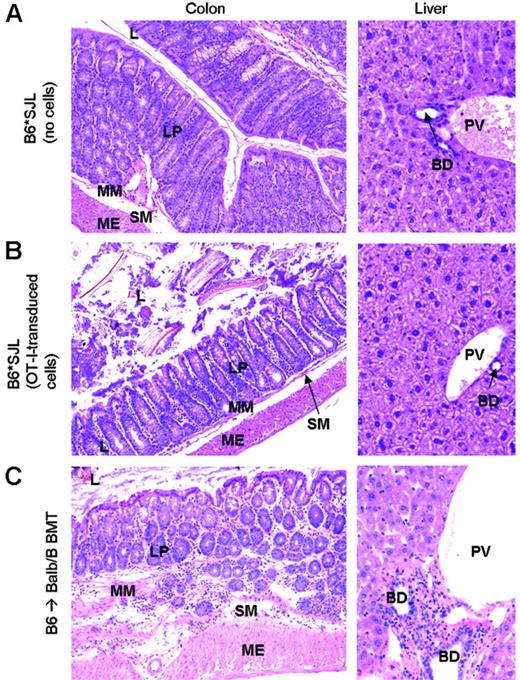
TCR-transduced T cells do not induce graft versus host pathology in partially MHC-mismatched recipients. Colon and liver sections were obtained either 6 weeks after viral infection (A-B) or at the onset of clinical GvHD (C). L indicates lumen; LP, lamina propria; MM, muscularis mucosae; SM, submucosa; ME, muscularis externa; BD, bile duct; PV, portal vein. Lymphocytic infiltrates are present throughout the intestinal wall and in the periportal area of Balb.B recipients (C), whereas intestine and periportal area of partially MHC-mismatched recipients are unaffected (A-B). Original magnification, ×16 (A-B, left panels); ×32 (A-B, right panels); ×125 (C, left panel); and ×20 (C, right panel).
TCR-transduced T cells do not induce graft versus host pathology in partially MHC-mismatched recipients. Colon and liver sections were obtained either 6 weeks after viral infection (A-B) or at the onset of clinical GvHD (C). L indicates lumen; LP, lamina propria; MM, muscularis mucosae; SM, submucosa; ME, muscularis externa; BD, bile duct; PV, portal vein. Lymphocytic infiltrates are present throughout the intestinal wall and in the periportal area of Balb.B recipients (C), whereas intestine and periportal area of partially MHC-mismatched recipients are unaffected (A-B). Original magnification, ×16 (A-B, left panels); ×32 (A-B, right panels); ×125 (C, left panel); and ×20 (C, right panel).
Discussion
Most human tumors do not have a viral etiology, and the shared antigens that are available for T-cell attack are therefore largely restricted to nonmutated self-antigens. As a consequence, the success of T-cell–based immunotherapy relies in large part on the ability to generate tumor-specific T cells that can efficiently target defined self-antigens.
In a murine model expressing a β-cell–specific self-antigen, we have demonstrated that T cells transduced with a TCR recognizing this self-antigen expand upon vaccination and target both pancreatic cells and tumor cells expressing this self-antigen. These data provide the first in vivo evidence that TCR gene transfer can be used to generate a T-cell compartment specific for self-antigens and thereby overcome self-tolerance. It is noted that the effect of single-dose infusion of TCR-transduced T cells left approximately 10% of β-cell islets intact and also is insufficient to result in complete tumor regression. The fact that the response of the TCR-transduced T cells is transient and apparently not maintained by either β-cell or tumor-cell–derived antigen is likely to be important in this respect, and an obvious next step will be the analysis of the effect of repetitive infusion/vaccination.26,27 In addition, it seems useful to test the value of additional treatments that are currently used in clinical trials such as lymphodepletion and anti-CTLA4 treatment for a possible potentiating effect. Such protocols may first be evaluated in the type of self-tolerant models used in the present study but should ultimately be validated in murine models for sporadic tumor development, where tumor-induced immune suppression may form an obstacle.28
In this study we circumvented self-tolerance by introduction of an ovalbumin-specific TCR that was originally obtained from a mouse for which OVA was not a self-antigen and that was therefore of high affinity. Although we did not compare these data with the adoptive transfer of a low-affinity OVA-specific TCR, prior data on the analysis of the antitumor effects of low-avidity self-specific T cells make it likely that the use of a high-affinity T-cell receptor is essential (reviewed by de Visser et al29 ). Analogous to the development of platforms for the isolation of high-affinity antibodies against human antigens, several strategies to obtain high-affinity TCRs specific for human tumor-associated self-antigens have been described in recent years (reviewed by Coccoris et al30 ), and a comparison of the specificity and activity of TCRs obtained by the methods will be an important goal for the coming period.
In the second part of this study, we have demonstrated—for 2 TCRs—that TCR transfer into T cells of a large cohort of partially MHC-mismatched recipients results in redirected T cells that function in vivo and that this experimental therapy is not associated with detectable autoimmunity. While TCR-gene modified T cells have displayed the intended MHC and antigen specificity in in vitro assays,31 infusion of TCR-transduced T cells could—at least in theory—induce “off-target” autoimmunity via 3 different mechanisms.32 Specifically, introduction of an exogenous TCR will not only result in expression of the introduced TCR, but the introduced T-cell receptor chains can also form mixed heterodimers with endogenous T-cell receptor α and β chains, and such mixed TCRs may potentially be reactive toward self-peptides. Secondly, the TCR may be introduced into ignorant self-reactive T cells.33 If such T cells become activated via the introduced TCR, this will result in an expanded population of activated autoreactive T cells with an increased ability to infiltrate peripheral tissues. If either of these 2 mechanisms would result in autoimmune pathology with appreciable frequency, such pathology would be expected to occur irrespective of possible MHC disparities between TCR donor and recipient. The third mechanism via which TCR-transduced T cells could induce off-target autoimmunity is when the introduced TCR recognizes MHC molecules in the recipient that were absent in the original TCR donor. In this study we have shown the safety of TCR gene transfer in a group of more than 60 partially MHC-mismatched recipient mice, and these results reveal 2 things. First, these data suggest that activation of ignorant self-specific T cells or the formation of mixed heterodimers does not pose a serious risk factor for the development of autoimmune disease upon TCR gene transfer. Second, these data demonstrate that MHC mismatches between TCR donor and recipient can be compatible with effective and safe allogeneic TCR gene transfer. In line with this, an absence of detectable side effects has also been observed in clinical trials in which EBV- and CMV-specific T-cell lines were used for the treatment or prophylaxis of posttransplantation viral infections, although in this case in general under conditions of immunosuppression34 While encouraging, it is important to note that both sets of data should not be taken as evidence that TCR transfer will never be complicated by MHC mismatches between TCR donor and recipient, because the propensity for reactivity with allogeneic MHC varies between different TCRs. Rather, the data should be taken to indicate that it is feasible to identify TCRs that are safe and effective in a large number of partially MHC-mismatched recipients. As a consequence, it should be possible to generate collections of TCR genes that can be used to induce desired TAA-specific T-cell responses in clinical trials. Should clinical trials nevertheless reveal undesired alloreactivity, it may be possible to limit such toxicity by inclusion of a suicide switch (reviewed by Straathof et al35 ).
Prior data have shown the capacity of TCR gene-modified T cells to react to foreign antigen encounter in immunocompetent mice. The current data expand these studies by demonstrating the feasibility of targeting a defined self-antigen by TCR gene transfer and by demonstrating the lack of toxicity of TCR gene transfer in the clinically relevant setting of a partial MHC disparity. Collectively these studies provide sufficient support to test the safety and efficacy of TCR gene therapy in clinical trials. Such trials will be essential to define under which conditions adoptive immunotherapy with T-cell receptors can be of clinical value and should provide further leads for optimization in preclinical models.
Prepublished online as Blood First Edition Paper, April 6, 2006; DOI 10.1182/blood-2005-08-009357.
Supported by grants from the Netherlands Organization for Scientific Research (NWO Pioneer grant 00-03), the Dutch Cancer Society (NKI 2003-2860), and the EU 6th Framework program (ATTACK).
M.A.d.W. and M.C. designed and performed research, analyzed data, and wrote the paper; M.C.W. designed and performed research; M.D.v.d.B. and E.M.M. performed research; J.-Y.S. analyzed data, M.v.d.V. performed research and analyzed data; J.B.A.G.H. designed research; and T.N.M.S. designed research, analyzed data, and wrote the paper.
M.A.d.W. and M.C. contributed equally to this work.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank M. Toebes for technical support, C. Kurts for the RIP-OVAhi mice, D. Topham for the influenza A/WSN/33 (WSN)–OVA(I) virus, and J. Yewdell for vaccinia virus recombinant for GFP-OVA257-264.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal