Abstract
Fms-like tyrosine kinase 3 (FLT3) receptor mutations as internal tandem duplication (ITD) or within the kinase domain are detected in up to 35% of patients with acute myeloid leukemia (AML). N-benzoyl staurosporine (PKC412), a highly effective inhibitor of mutated FLT3 receptors, has significant antileukemic efficacy in patients with FLT3-mutated AML. Mutation screening of FLT3 exon 20 in AML patients (n = 110) revealed 2 patients with a novel mutation (Y842C) within the highly conserved activation loop of FLT3. FLT3-Y842C-transfected 32D cells showed constitutive FLT3 tyrosine phosphorylation and interleukin 3 (IL-3)-independent growth. Treatment with PKC412 led to inhibition of proliferation and apoptotic cell death. Primary AML blasts bearing FLT3-Y842C mutations showed constitutive FLT3 and signal transducer and activator of transcription 5 (STAT-5) tyrosine phosphorylation. Ex vivo PKC412 treatment of primary blasts resulted in suppression of constitutive FLT3 and STAT-5 activation and apoptotic cell death. Inspection of the FLT3 structure revealed that Y842 is the key residue in regulating the switch from the closed to the open (= active) conformation of the FLT3 activation loop. Overall, our data suggest that mutations at Y842 represent a significant new activating mutation in AML blasts. Since FLT3 tyrosine kinase inhibitors (TKIs) such as PKC412 are currently being investigated in clinical trials in AML, extended sequence analysis of FLT3 may be helpful in defining the spectrum of TKI-sensitive FLT3 mutations in AML. (Blood. 2005;105:335-340)
Introduction
Fms-like tyrosine kinase 3 (FLT3) is a member of the class III receptor tyrosine kinase (RTK) family including FMS, platelet-derived growth factor receptor (PDGFR), and c-KIT.1 FLT3 is expressed by cells of the hematopoietic stem cell compartment and early myeloid progenitor cells.2,3 Stimulation of FLT3 by its ligand (FLT3 ligand [FL]) synergizes with other hematopoietic growth factors and induces cellular proliferation of progenitor cells.4,5 Recent studies have demonstrated that mutations in the FLT3 gene occur in about one third of adult patients with AML. In 20% to 27% of patients with AML, an internal tandem duplication (ITD) in the juxtamembrane region of FLT3 can be detected.6-8 Another 7% of patients have mutations within the activation loop of the second kinase domain, predominantly substitutions of aspartate at residue 835 (D835),9,10 while additional mutations at residues 836 and 840 have also been described.11,12 FLT3 mutations are detected at initial diagnosis of AML, but several patients have been reported to have gained new, secondary FLT3 mutations at relapse.13 Altogether, FLT3 is the single most commonly activated gene in AML. Mutations in FLT3 are associated with higher leukocyte and blast counts, an increased relapse rate, and decreased overall survival.8,14,15 Expression of mutated FLT3 receptors results in constitutive tyrosine phosphorylation of FLT3; subsequent activation of downstream signaling molecules such as signal transducer and activator of transcription 5 (STAT-5), Akt, and mitogen-activated protein kinase (MAPK); and factor-independent growth of murine hematopoietic cell lines, resembling myeloproliferative disease in transplantation models.9,16-20 N-benzoyl staurosporine (PKC412), a derivative of the naturally occurring alkaloid staurosporine,21 is an inhibitor of several kinases, including PKC-α, -β, and -γ; vascular endothelial growth factor receptor 2 (VEGF-R2 KDR); c-KIT; and PDGFR-α and -β. Further, screening analysis identified PKC412 to be a highly active inhibitor of mutant FLT3 receptors with a 50% inhibitory concentration (IC50) of less than 10 nM, as revealed by in vitro and in vivo models.22 Recently, PKC412 treatment of patients with AML having either mutant FLT3-ITD or FLT3-D835 receptors resulted in an at least 50% reduction of peripheral blood blasts and an at least 50% reduction of bone marrow blasts in 14 (70%) of 20 and 5 (25%) of 20 patients, respectively.23 Here, we describe the identification and characterization of a novel activating mutation (Y842C) within the kinase domain of FLT3. Treatment of primary AML leukemic blasts harboring FLT3-Y842C mutation with PKC412 resulted in induction of apoptosis.
Patients, materials, and methods
Mutation analysis
DNA samples were obtained from AML patients enrolled in phase 2 studies investigating PKC41223 or imatinib mesylate24 or from patients with AML treated with standard chemotherapy protocols at the University of Mainz. In addition, samples of AML patients treated at the University of Muenster were also analyzed. Informed consent was obtained from all patients. The clinical studies were conducted in accordance with the Declaration of Helsinki and upon institutional review board processing. Genomic DNA from bone marrow (BM) was extracted using the QIAmp DNA Blood Mini-Kit (Qiagen, Hilden, Germany). FLT3 exon 20 was amplified by standard polymerase chain reaction (PCR) and mutation analysis was performed as described previously.9,13 In addition, the following primers specific for flanking genomic sequences of exon 20 were used according to standard protocols and resulting PCR products were sequenced: FLT3genom-f (catcaccggtacctcctact); and FLT3genom-r (ccacagtgagtgcagttgtt).
RNA from bone marrow (BM) was extracted using the QIAgen RNeasy Mini-Kit (Qiagen) and cDNA was generated with SuperScript First-Strand Synthesis System for reverse transcriptase-PCR (RT-PCR) according to the manufacturer's protocol (Invitrogen, Groningen, The Netherlands).
The coding sequence of FLT3 was amplified by PCR and sequenced using the following primers: FLT3mRNA-f (tgccgctgctcgttgtttt); FLT3mRNA-r (agaaggccttggatgcaga); FLT3mf2 (caaactcctcagaccacattg); FLT3mr2 (catgatatctcgagccaatcc); FLT3mf3 (gaggagttgtttccatggtc); FLT3mr3 (gtgtaattggtagcttcactctg); FLT3mf4 (acggatacagcatatccaag); and FLT3mf5 (gcactcatgtcagaactcaag).
DNA constructs and vectors
Human FLT3-wild-type (FLT3-WT) and human FLT3-ITD constructs, both subcloned into the pAL expression vector under control of the 5′ long terminal repeat (LTR) of the Moloney murine sarcoma virus (MoMSV) and the plasmid pMAM/BSD, were used.17 The FLT3-Y842C point mutation was introduced into full-length human FLT3-WT cDNA by Medigenomix (Martinsried, Germany). The correct sequence of the construct was confirmed by nucleotide sequencing.
Transfection of 32D cells
Murine 32D cells were kindly provided by T. Skorski (Philadelphia, PA) and were maintained in RPMI 1640 with 10% fetal calf serum (FCS) and 10% Wehi-conditioned medium as a source of interleukin 3 (IL-3). Ten micrograms of plasmid DNA and 1 μg pMAM/BSD were cotransfected into 32D cells by electroporation. Cells were selected with 15 μg/mL blasticidin (Invitrogen) in IL-3-supplemented culture. Polyclonal cell lines were used for further experiments after 24 or 3 hours' Wehi starvation as indicated.
Detection of CD135 expression by flow cytometry
Cells were incubated for 2 hours at 4°C with a mouse phycoerythrin (PE)-labeled isotype-matched control antibody or anti-CD135-PE antibody detecting human FLT3 (Becton Dickinson, Krefeld, Germany). Viable cells were analyzed using a flow cytometer (Beckman Coulter, Heidelberg, Germany).
MTT assay
32D cells (12 × 103) expressing FLT3-Y842C were seeded in triplicate in 96-well plates and grown in RPMI 1640 medium containing PKC412 (100 nM), FL (100 ng/mL), 10% Wehi as a source of IL-3, or combinations as indicated. Wells were assayed for uptake of MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl tetrazolium bromide) at several time points as indicated using standard methods.
Cell cycle analysis
Cell cycle analysis was performed as previously described.25
Colony assay
Stably transfected 32D cells were pelleted, extensively washed in phosphate-buffered saline (PBS), and seeded in 6-well culture plates at a fixed density (3000 cells/mL) in complete RPMI 1640/methylcellulose medium without IL-3 containing 10% FCS, according to the manufacturer's instructions (Sigma, Munich, Germany). Cultures were maintained for 14 days in a 37°C, 5% CO2 incubator, after which colonies were scored.
Isolation of primary AML blasts and cell culture
Heparin-treated BM samples and 20 mL of heparin-treated peripheral blood (PB) samples were obtained from patient PN 1 (patient number 1) before starting with PKC412 therapy. In addition, 3 PB samples from AML patients (PN 2-PN 4) also enrolled in the above-mentioned study were obtained after informed consent. Clinical samples were processed without time delay and mononuclear cells (MNCs) were isolated by means of Ficoll-Hypaque (Seromed, Berlin, Germany) density-gradient centrifugation. For Western blotting, freshly isolated PB MNCs were directly used after Ficoll isolation and were incubated in RPMI 1640 medium supplemented with 20 mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid), pH 7.3; 50 μM β-mercaptoethanol; 2 mM L-glutamine; and penicillin G (100 U/mL)/streptomycin (100 μg/mL). For cell cycle analysis, BM MNCs were maintained in RPMI 1640 medium supplemented as described above plus 10% FCS.
Protein extract preparation, immunoprecipitation, and Western blotting
Cells (1 × 107/mL) were incubated in the presence of 100 ng/mL FL (R&D Systems, Minneapolis, MN), 100 ng/mL stem cell factor (SCF; CellConcepts, Umkirch, Germany), 50 or 100 nM PKC412 (kindly provided by T. Meyer, Novartis, Basel, Switzerland), 1 μM imatinib mesylate (kindly provided by Dr Buchdunger, Novartis), and in combinations for 15 minutes at 37°C as indicated. Preparation of cellular lysates was performed as described.24 Antibody coupled to agarose beads and immunoprecipitation buffer (HEPES 50 mM, 150 mM NaCl, 10% [vol/vol] glycerol, 1% Triton X-100, 5 mM EGTA [ethylene glycoltetraacetic acid], 1.5 mM MgCl2, 1 mM phenylmethylsulfonyl fluoride, 10 μg/mL aprotinin, 10 μg/mL leupeptin, 0.2 mM sodium orthovanadate, 50 mM NaF, 1 μM okadaic acid) were added to 500 μg protein lysates to a total of 200 μL and incubated for 4 hours on a rotating platform at 4°C. Immunoprecipitates were washed 3 times with 1 mL immunoprecipitation buffer and separated on 10% sodium dodecyl sulfate (SDS) polyacrylamide gels. Protein lysates were subjected to SDS-polyacrylamide gel electrophoresis (PAGE) and blotted onto nitrocellulose membrane (Amersham, Freiburg, Germany) as previously described.24 The following antibodies were used: anti-phospho-Flt3, anti-phospho-STAT-5, anti-phospho-Akt, anti-phospho-extracellular signal-related kinase 1/2 (Erk1/2), Akt, and Erk1/2 (all Cell Signaling Technology, Frankfurt, Germany); Flt3 and STAT-5 (N20; Santa Cruz, Heidelberg, Germany); and antiphosphotyrosine (Biomol, Hamburg, Germany; and Santa Cruz).
Analysis of the FLT3 structure
Results
Mutation analysis
Genomic DNA of patients (n = 30) enrolled into the PKC412 phase 2 trial was analyzed for known activating mutations of FLT3, such as internal tandem duplication (ITD) of the juxtamembrane (JM) coding region and point mutations of codon 835, using standard methods.9 Upon start with PKC412 treatment, in several patients considered to be wild-type, hematologic responses were observed. Therefore, to screen for further activating mutations within the second kinase domain of FLT3, genomic PCR using primers flanking exon 20 was performed. Sequence analysis revealed a novel point mutation in exon 20 of FLT3, resulting in substitution of a tyrosine residue for cysteine (Y842C). No further mutations in the entire FLT3 cDNA sequence from AML blasts of this patient (PN 1) were detected. The same mutation was detected in a second patient (PN 5) enrolled into the PKC412 phase 2 study. Both patients bearing the novel Y842C mutation in leukemic blasts had relapsed after initial standard chemotherapy. Patient PN 1 with an AML French-American-British (FAB) subtype M2 was refractory after second relapse; patient PN 5 (AML FAB subtype M5) experienced his first relapse soon after induction chemotherapy. In patient PN 1, cytogenetic analysis demonstrated trisomy 8 in 86% of metaphases; cytogenetics of patient PN 5 showed one translocation, t(11;15). Mutation screening was then extended and 80 additional patients were analyzed. Altogether, in 110 AML patients, including patients at first diagnosis or at relapse and including patients with normal or abnormal cytogenetics, 2 patients harboring the novel point mutation (Y842C) in exon 20 of FLT3 were identified. Results of sequence analysis of all patients are depicted in Table 1.
Results of sequence analysis of all patients
. | FLT3-WT, n = 79 . | FLT3-ITD, n = 22 . | FLT3-D835, n = 7 . | FLT3-Y842C, n = 2 . |
|---|---|---|---|---|
| Disease status | ||||
| First diagnosis | 47 | 15 | 4 | 0 |
| Relapsed | 25 | 4 | 3 | 2 |
| Unknown | 7 | 3 | 0 | 0 |
| Cytogenetics | ||||
| Normal | 21 | 9 | 2 | 0 |
| Abnormal | 30 | 5 | 3 | 2* |
| t(8;21) or t(15;17) | 1 | 3 | 0 | 0 |
| Unknown | 27 | 5 | 2 | 0 |
. | FLT3-WT, n = 79 . | FLT3-ITD, n = 22 . | FLT3-D835, n = 7 . | FLT3-Y842C, n = 2 . |
|---|---|---|---|---|
| Disease status | ||||
| First diagnosis | 47 | 15 | 4 | 0 |
| Relapsed | 25 | 4 | 3 | 2 |
| Unknown | 7 | 3 | 0 | 0 |
| Cytogenetics | ||||
| Normal | 21 | 9 | 2 | 0 |
| Abnormal | 30 | 5 | 3 | 2* |
| t(8;21) or t(15;17) | 1 | 3 | 0 | 0 |
| Unknown | 27 | 5 | 2 | 0 |
Karyotypes: PN 1: 47,XY, +8; PN5: 46,XY, t(11;15).
Overexpression of FLT3-Y842C results in constitutive tyrosine phosphorylation of FLT3
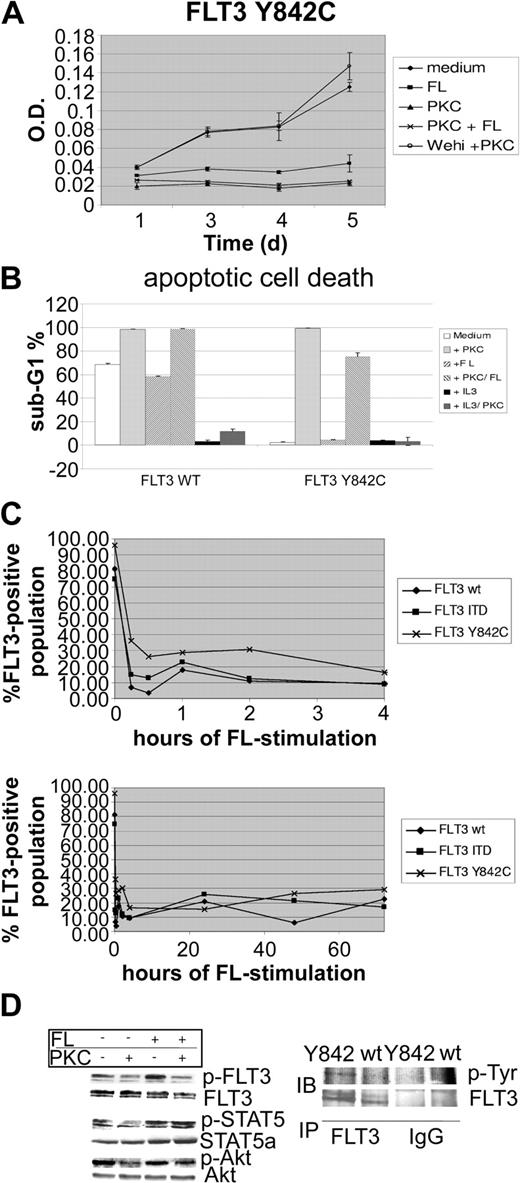
To further characterize the functional significance of this novel mutation, we generated FLT3-wt-, FLT3-ITD-, and FLT3-Y842C-expressing 32D cells. Surface expression of FLT3 was confirmed by fluorescence-activated cell sorter (FACS) analysis (data not shown). Constitutive tyrosine phosphorylation of FLT3-Y842C (Figure 1D left) was detected as revealed by Western blot analysis using a specific antiphosphotyrosine FLT3 antibody (Tyr591). As a confirmatory experiment, FLT3 immunoprecipitation followed by Western blot using an antiphosphotyrosine antibody was performed (Figure 1D right). Stimulation with FL caused a modest increase of FLT3 activation, indicating subtotal tyrosine phosphorylation in cells expressing FLT3-Y842C. However, compared with FLT3-ITD-bearing cells, the intensity of FLT3 tyrosine phosphorylation was significantly less in FLT3-Y842C mutants (data not shown). In addition, whereas FLT3-ITD causes tyrosine phosphorylation of the glycosylated (isoform with higher molecular weight) and hypoglycosylated isoform (lower molecular weight band) of FLT3, Y842C mutants appeared to preferentially activate the glycosylated isoform (Figure 1D). In cell culture experiments, 32D cells expressing the mutant FLT3-Y842C receptor showed IL-3-independent proliferation (Figure 1A). Treatment with PKC412 (100 nM) resulted in significant inhibition of factor-independent growth. Cell cycle analysis, performed upon 48 hours of PKC412 treatment, revealed extensive apoptotic cell death (Figure 1B). PKC412-induced inhibition of cell growth and apoptotic cell death was rescued by addition of IL-3, indicating that the growth inhibitory effect of PKC412 was due to direct inhibition of the mutant FLT3-Y842C receptor. Stimulation with FL was not able to revert the growth inhibitory effects of PKC412 (Figure 1A). Interestingly, incubation with FL alone led to a significant decrease in IL-3-independent proliferation (Figure 1A). As revealed by flow cytometry analysis, stimulation with FL was followed by rapid FLT3 receptor down-regulation within 15 minutes and was maintained during the incubation period lasting up to 72 hours (Figure 1C). Since FLT3-Y842C-expressing cells in IL-3-depleted medium are completely dependent on a constitutively active FLT3 receptor, this FL-induced down-regulation of the FLT3 receptor most likely accounts for the inhibitory effects of FL stimulation. However, despite reduced cell growth, stimulation with FL resulted in a partial rescue of PKC412-induced apoptotic cell death (Figure 1B). At a protein level, treatment of 32D-FLT3-Y842C cells with PKC412 led to strong suppression of FLT3 tyrosine phosphorylation (Figure 1D). FL stimulation further enhanced FLT3 activation and partially inhibited PKC412 effects on phosphorylation of FLT3 (Figure 1D). Investigation of downstream pathways showed constitutive activation of STAT-5 (Figure 1D). Compared with FLT3-ITD-bearing cells, STAT-5 tyrosine phosphorylation was significantly weaker (data not shown). PKC412 treatment resulted in strong inhibition of STAT-5 tyrosine phosphorylation. Again, the phospho-STAT-5 signal was enhanced upon stimulation with FL, and FL stimulation was able to revert PKC412-induced suppression of tyrosine phosphorylation. Investigation of Akt showed constitutive phosphorylation, and PKC412 treatment resulted in inhibition of Akt phosphorylation. Stimulation with FL partially inhibited PKC412-induced down-regulation of the p-Akt signal. To investigate the capability of clonal growth, colony assays using 32D-FLT3-Y842C, FLT3-wt, and FLT3-ITD cells were performed. Upon growth factor withdrawal, 32D-FLT3-Y842C cells were not able to form colonies, whereas strong colony formation was observed in 32D-FLT-ITD cells (data not shown).
The FLT3-Y842C mutant is constitutively tyrosine phosphorylated and inhibits apoptotic cell death upon growth factor withdrawal. 32D cells were transfected with either pAL-FLT3-WT, pAL-FLT3-ITD, or pAL-FLT3-Y842C. (A) 32D-FLT3-Y842C cells were grown in the absence of IL-3 and incubated with PKC412 (100 nM), FL (100 ng/mL), 10% Wehi-conditioned medium, or in combinations as indicated. MTT assays were performed at several time points. Shown is 1 representative of 2 experiments. (B) 32D-FLT3-Y842C and FLT3-WT cells were treated for 48 hours as indicated and cell cycle analysis was performed. Shown is 1 representative of 2 experiments. (C) Analysis of FLT3 membrane expression by flow cytometry. 32D cells transfected with FLT3-WT, FLT3-ITD, and FLT3-Y842C were stimulated with FL (100 ng/mL) for the time points indicated. FLT3 receptor expression was determined by flow cytometry using a specific PE-conjugated anti-FLT3 antibody. (D) FLT3-Y842C mutants were treated with FL (100 ng/mL), PKC 412 (100 nM), and in combination for 15 minutes as indicated. Whole-cell lysates were prepared and analyzed by Western blot (IB). To control equal loading, the blot was stripped and reprobed with antibodies as indicated. In addition, immunoprecipitation (IP) using a specific anti-Flt3 antibody followed by Western blot analysis with an antiphosphotyrosine (p-Tyr) antibody was performed. IgG indicates immunoglobulin G. *Error bar indicates standard deviation.
The FLT3-Y842C mutant is constitutively tyrosine phosphorylated and inhibits apoptotic cell death upon growth factor withdrawal. 32D cells were transfected with either pAL-FLT3-WT, pAL-FLT3-ITD, or pAL-FLT3-Y842C. (A) 32D-FLT3-Y842C cells were grown in the absence of IL-3 and incubated with PKC412 (100 nM), FL (100 ng/mL), 10% Wehi-conditioned medium, or in combinations as indicated. MTT assays were performed at several time points. Shown is 1 representative of 2 experiments. (B) 32D-FLT3-Y842C and FLT3-WT cells were treated for 48 hours as indicated and cell cycle analysis was performed. Shown is 1 representative of 2 experiments. (C) Analysis of FLT3 membrane expression by flow cytometry. 32D cells transfected with FLT3-WT, FLT3-ITD, and FLT3-Y842C were stimulated with FL (100 ng/mL) for the time points indicated. FLT3 receptor expression was determined by flow cytometry using a specific PE-conjugated anti-FLT3 antibody. (D) FLT3-Y842C mutants were treated with FL (100 ng/mL), PKC 412 (100 nM), and in combination for 15 minutes as indicated. Whole-cell lysates were prepared and analyzed by Western blot (IB). To control equal loading, the blot was stripped and reprobed with antibodies as indicated. In addition, immunoprecipitation (IP) using a specific anti-Flt3 antibody followed by Western blot analysis with an antiphosphotyrosine (p-Tyr) antibody was performed. IgG indicates immunoglobulin G. *Error bar indicates standard deviation.
Expression of Y842C in primary AML blasts results in constitutive activation of FLT3 and STAT-5
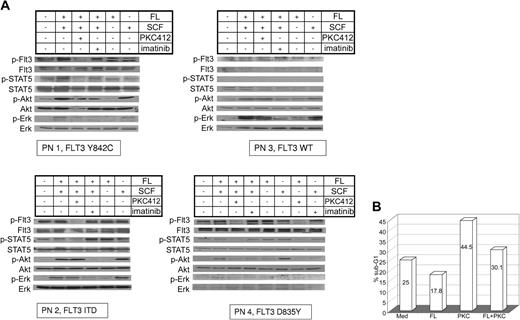
In addition to 32D cells overexpressing FLT3-Y842C or FLT3-ITD, we investigated activation of FLT3, STAT-5, Akt, and Erk in primary AML blasts. Primary AML MNCs (PN1-PN4) were incubated with FL, SCF, and PKC412 and compared with AML blasts of patients with Flt3-ITD, Flt3-D835, and wt-Flt3. In all 3 receptor mutants, constitutive tyrosine phosphorylation of FLT3 and activation of STAT-5 could be demonstrated, whereas in wild-type FLT3 cells neither activation of FLT3 nor STAT-5 was detected (Figure 2A lane 1). In contrast to FLT3-ITD, stimulation with FL further enhanced FLT3 tyrosine phosphorylation in D835Y- and Y842C-FLT3 mutants (Figure 2A lanes 2, 5). Constitutive phosphorylation of FLT3 and STAT-5 in AML blasts with mutated FLT3 receptors was strongly suppressed upon treatment with the FLT3 kinase inhibitor PKC412 (50 or 100 nM; Figure 2A lane 3). In order to analyze the specificity of PKC412, primary AML blasts were incubated with SCF, FL, and imatinib mesylate (Figure 2A lane 4). No effects of imatinib mesylate on FLT3 or STAT-5 activation were seen. For wt-FLT3 as well as mutated FLT3, minimal constitutive phosphorylation of Akt was detected (Figure 2A lane 1). Further induction of Akt phosphorylation was dependent on SCF stimulation, whereas FL had no effect (Figure 2A lanes 5-6). Similar results were obtained for the activation of Erk1/2 (Figure 2A). SCF-induced activation of Akt and Erk was suppressed by imatinib mesylate (Figure 2A lane 4). We further investigated the effect of PKC412 on the survival of primary AML blasts harboring the FLT3-Y842C mutation. In long-term culture upon growth factor withdrawal, no proliferation was seen using MTT assays (data not shown), therefore, cell cycle analysis was performed. Incubation with PKC412 caused significant cell death, whereas simultaneous stimulation with FL resulted in a partial rescue (Figure 2B).
Signal transduction patterns of mutated and wt-Flt3 in primary AML cells and cell cycle analysis. (A) PB was taken before start with PKC412. MNCs were separated and treated with FL (100 ng/mL), SCF (100 ng/mL), 100 nM PKC 412, imatinib mesylate (1 μM) and in combination for 15 minutes as indicated. Whole-cell lysates were analyzed by Western blot. Constitutive tyrosine phosphorylation of FLT3 and STAT-5 in primary AML blasts harboring mutated FLT3 using specific antiphosphotyrosine antibodies for FLT3 and STAT-5 was detected. Akt and Erk1/2 activation were investigated using specific antiphosphoserine-Akt and antiphosphotyrosine-Erk antibodies, respectively. Further, FL or SCF effects on the investigated signaling pathways are demonstrated. To control equal loading, the blot was stripped and reprobed. (B) Primary MNCs obtained from AML patient PN 1 were maintained in RPMI 1640 without any growth factor supplementation and incubated with or without PKC412 (50 nM), FL (100 ng/mL), or in combination for 90 hours as indicated, and cell cycle analysis was performed.
Signal transduction patterns of mutated and wt-Flt3 in primary AML cells and cell cycle analysis. (A) PB was taken before start with PKC412. MNCs were separated and treated with FL (100 ng/mL), SCF (100 ng/mL), 100 nM PKC 412, imatinib mesylate (1 μM) and in combination for 15 minutes as indicated. Whole-cell lysates were analyzed by Western blot. Constitutive tyrosine phosphorylation of FLT3 and STAT-5 in primary AML blasts harboring mutated FLT3 using specific antiphosphotyrosine antibodies for FLT3 and STAT-5 was detected. Akt and Erk1/2 activation were investigated using specific antiphosphoserine-Akt and antiphosphotyrosine-Erk antibodies, respectively. Further, FL or SCF effects on the investigated signaling pathways are demonstrated. To control equal loading, the blot was stripped and reprobed. (B) Primary MNCs obtained from AML patient PN 1 were maintained in RPMI 1640 without any growth factor supplementation and incubated with or without PKC412 (50 nM), FL (100 ng/mL), or in combination for 90 hours as indicated, and cell cycle analysis was performed.
Discussion
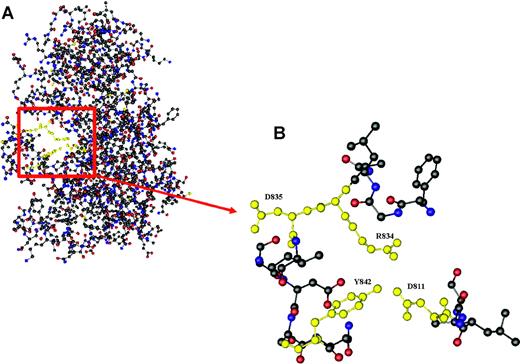
Here, we describe a new, yet unrecognized mutation (Y842C) within the kinase domain of FLT3, which is sensitive to the FLT3 kinase inhibitor PKC412 at the cellular level in vitro. Overexpression studies showed constitutive FLT3 tyrosine phosphorylation, activation of downstream pathways like STAT-5 and Akt, and Il-3-independent proliferation. Further, molecular characterization of primary AML blasts from a patient bearing the novel mutation demonstrated constitutive activation of FLT3 and of the downstream molecule STAT-5. Thus, the newly identified Y842C mutation demonstrated similar signaling patterns as previously described for FLT3-D835. No constitutive activation of Akt or Erk could be detected in our sample of primary AML cells. This is in contrast to 32D cells overexpressing FLT3-Y842C. However, investigations of cells overexpressing FLT3-ITD or FLT3-tyrosine kinase domain (TKD) mutations in an experimental context may not reflect situations as observed in in vivo studies. In our experiments, activation of Erk and Akt seems to be regulated by c-kit and SCF. Further, ex vivo treatment with PKC412 resulted in significant inhibition of constitutive FLT3 and STAT-5 tyrosine phosphorylation and apoptotic cell death. In contrast, no effect was seen using imatinib mesylate (Figure 2A). Upon PKC412 treatment, apoptotic cell death was observed in primary AML blasts. Recently, a FLT3-Y842H mutation, acquired in Ba/F3 FLT3-ITD cells during treatment with the tyrosine kinase inhibitor (TKI) SU5614 and resulting in resistance to the compound, was identified.27 However, this mutation was detected in the context of a resistance model, whereas the mutation described here was identified in primary AML blasts from patients previously untreated with tyrosine kinase inhibitors. It is interesting to note that the recently described crystal structure of FLT3 reveals a critical role for Y842 in regulating the switch from the closed to the open (active) conformation of the FLT3 activation loop (Figure 3).28 Since our data are consistent with the concept that the Y842C mutation results in constitutive activation of FLT3, it is tempting to speculate that the exchange of tyrosine for cysteine at position 842 disrupts the autoinhibited state of the FLT3 activation loop.
Crystal structure of the kinase and JM domain of human Flt3. (A) The section highlighted in red shows Tyr842 pointing into the active site of the activation loop, thereby stabilizing the inhibited conformation of Flt3 by forming a hydrogen bond with Asp811, which in turn is linked to Arg834 through an ion pair. (B) Relative conformation of Tyr842 (Y842), Asp811 (D811), Arg834 (R834), and Asp835 (D835) in autoinhibited Flt3.
Crystal structure of the kinase and JM domain of human Flt3. (A) The section highlighted in red shows Tyr842 pointing into the active site of the activation loop, thereby stabilizing the inhibited conformation of Flt3 by forming a hydrogen bond with Asp811, which in turn is linked to Arg834 through an ion pair. (B) Relative conformation of Tyr842 (Y842), Asp811 (D811), Arg834 (R834), and Asp835 (D835) in autoinhibited Flt3.
Mutations affecting codon 835 within the kinase domain of FLT3 have been detected in about 7% of AML patients.9,10 Several other mutations within the activation loop, as FLT3-Ile836del, FLT3-840GS, or FLT3-N841, or within exon 14 of the FLT3 gene, have been described recently.11,12,29,30 In addition, similar activating mutations of c-KIT (D816V, E839K, V825A) and PDGFR (D842V, DelDIM842-845, DelHDSN845-848P), both members of the RTK III family involved in malignant transformation, have been reported.31,32 All these mutations are located within a highly conserved region of the activation loop. The novel FLT3-Y842C mutation was detected in 2 of 110 patients. However, upon selection for patients lacking FLT3-ITD and FLT3-D835, 2 of 81 patients harbor the novel mutation. Further, the analysis depicted in Table 1 points out that the FLT3-Y842C mutation appears to occur preferentially in patients with relapsed AML (n = 2/25 patients) and/or with cytogenetic abnormalities (n = 2/30 patients). Given that the novel mutation described here and other mutations described recently11,12,29,30 could only be identified by direct sequencing, it is likely that the number of mutations in this region of FLT3 is currently underestimated. Thus, extended sequence analysis of this mutational hot spot or use of high-throughput sequencing techniques33 may be helpful in further defining the spectrum of TKI-sensitive FLT3 mutations in AML.
Prepublished online as Blood First Edition Paper, September 2, 2004; DOI 10.1182/blood-2004-02-0660.
Two of the authors (G.H., C.P.S.) are employed by a company (Novartis Pharmaceuticals) whose product was studied in the present work.
T.K. and F.B. contributed equally to this manuscript.
Presented in part at the 45th Annual Meeting of the American Society of Hematology, San Diego, CA, December 6-9, 2003.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We are grateful to B. Carius and K. Kunz for expert technical assistance.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal