Abstract
Myelodysplastic syndromes (MDS) are a heterogeneous group of clonal disorders characterized by ineffective hematopoiesis and frequent progression to acute myeloid leukemia. Within MDS, 5q− syndrome constitutes a distinct clinical entity characterized by an isolated deletion of the long arm of chromosome 5 (5q−), a relatively good prognosis, and infrequent transformation to acute leukemia. The cell of origin in 5q− syndrome as well as in other 5q-deleted MDS patients has not been established, but evidence for involvement of multiple myeloid (but not lymphoid) lineages has suggested that a myeloid-restricted progenitor rather than a pluripotent (lympho-myeloid) stem cell might be the primary target in most patients. Although in 9 patients no evidence of peripheral blood T-cell and only 1 case of B-cell involvement was found, the data herein support that 5q deletions occur in hematopoietic stem cells (HSCs) with a combined lympho-myeloid potential. First, in all investigated patients a minimum of 94% of cells in the minor CD34+CD38− HSC compartment were 5q deleted as determined by fluorescence in situ hybridization. Second, in 3 of 5 patients 5q aberrations were detected in a large fraction (25% to 90%) of purified CD34+CD19+ pro-B cells. Furthermore, extensive functional characterization with regard to responsiveness to early-acting cytokines, long-term culture-initiating cells, and nonobese diabetic/severe combined immunodeficiency repopulating cells supported that MDS HSCs in 5q-deleted patients are CD34+CD38−, but inefficient at reconstituting hematopoiesis.
Introduction
Myelodysplastic syndromes (MDS) represent a heterogeneous group of clonal hematopoietic disorders characterized by hyperproliferative but ineffective hematopoiesis, as reflected in dysplastic changes in myeloid cell lineages and different degrees of anemia, leukopenia, and thrombocytopenia.1-3 Overall, the prognosis of MDS patients is poor, with high mortality resulting from infections and hemorrhage due to refractory cytopenias, and with 25% to 30% of the cases showing progression to acute myeloid leukemia (AML).
Fully 40% to 60% of all MDS patients have identifiable chromosomal aberrations at diagnosis,1-8 providing not only evidence for the clonal nature of MDS, but also a means of staging the cell types involved in the clone through standard karyotyping or interphase fluorescence in situ hybridization (FISH).9,10 FISH has complemented X-linked restriction fragment length polymorphism (RFLP) analysis and detection of oncogene (in particularras) mutations to determine at what stage in the hematopoietic hierarchy the transformation in MDS occurs.11-13
Two alternative and complementary models have been proposed to explain the apparent lineage restriction and heterogeneity of leukemias,14-16 also applicable to MDS. The first interprets the lineage restriction as a consequence of transformation occurring at different levels of commitment in the hematopoietic hierarchy. In contrast, the other model proposes that transformation occurs at the level of a multipotent progenitor/stem cell and that the apparent lineage restriction is a result of the transforming event itself. Clonality studies of myeloid and lymphoid cells have revealed variable results with regard to the potential involvement of multipotent (lympho-myeloid) stem cells in MDS (reviewed in Heaney and Golde,2 Knuutila,13 and Weimar et al17). Although technical issues such as skewed methylation patterns in RFLP studies and potential impurities in lymphocyte preparations might explain some of the discrepancies,2,11,13,17 in most (but not all) cases, the MDS clone appears to involve the myeloid but not lymphoid cell lineages.9,12,18-35 Because MDS, in contrast to the stem cell disease chronic myelogenous leukemia (CML),36 also rarely transforms into acute lymphoblastic leukemia, it has been proposed that the transformation event in MDS occurs most frequently at the level of a committed myeloid progenitor (reviewed in Heaney and Golde2).
Although there is agreement that multiple myeloid cell lineages invariably are involved in the MDS clone, whether lymphocytes are also derived from this clone might depend in part on the specific chromosomal aberration(s) involved. The most powerful tool to enumerate specific chromosomal aberrations in various cell lineages is interphase FISH applied to purified cell populations. In the case of trisomy 8 and monosomy 7, a large number of such FISH studies have suggested that only in a very few cases are the lymphocytes involved in these aberrant clones.9,25,27 29-35
Within MDS, 5q− syndrome constitutes a distinct clinical entity characterized by an isolated deletion of the long arm of chromosome 5 (del(5q) or 5q−), female predominance, macrocytic anemia, modest neutropenia, normal or high platelets, hypolobular megakaryocytes, and a low risk of leukemic transformation.37-41 Clonality studies performed on MDS patients with 5q deletions have revealed no cases of lymphocyte involvement.23,24,28,35,38-40 Whether pluripotent hematopoietic stem cells (HSCs) are involved in the 5q− clone could be important for the use of autologous stem cell transplantation in these patients.42 That lymphocytes appear not to be involved in the 5q-deleted clone does not exclude HSCs as primary targets of 5q deletions. Such a scenario would be feasible if the transformed pluripotent stem cell had lost or reduced its ability to differentiate toward lymphocytes. Thus, addressing the potential involvement of lympho-myeloid HSCs in 5q− syndrome would require a different approach than investigating only the involvement of mature lymphocytes and myeloid cells.
Because multipotent reconstituting HSCs in normal as well as AML bone marrow (BM) have been demonstrated recently to reside almost exclusively in the CD34+CD38− compartment, representing less than 0.1% of normal BM cells,43-46 we purified CD34+CD38− cells from MDS patients with a known 5q deletion. In all patients, FISH analysis demonstrated that virtually all CD34+CD38− cells belonged to the 5q-deleted clone, and functional human stem cell assays supported that 5q-deleted MDS stem cells are CD34+CD38− but are ineffective in reconstituting hematopoiesis. Finally, in some patients a large fraction of pro-B (CD34+CD19+) progenitors also belonged to the 5q− clone. Thus, a lympho-myeloid HSC appears to be the primary target of 5q deletions in MDS.
Patients, materials, and methods
Patients
BM and peripheral blood (PB) samples from 12 MDS patients and 10 normal subjects were collected at the Hematology Sections at Karolinska and Huddinge Hospitals, Sahlgrenska University/Östra Hospital, and University Hospital of Lund after obtaining informed consent and with approval of the ethics committees at the medical faculties of the respective hospitals. Patient characteristics are provided in Table 1. All patients had a confirmed diagnosis of MDS according to the French-American-British classification47 and a confirmed 5q deletion. Patient no. 12 had been treated with chemotherapy (tioguanin + arabinosylcytosine + daunorubicin) plus granulocyte-macrophage colony-stimulating factor (GM-CSF) in 1996 because of progression with greater than 20% blasts in the BM (refractory anemia with excess blasts in transformation; RAEBt). Since then, the clinical picture had been stable without additional treatment.
Purification of BM and PB cell populations
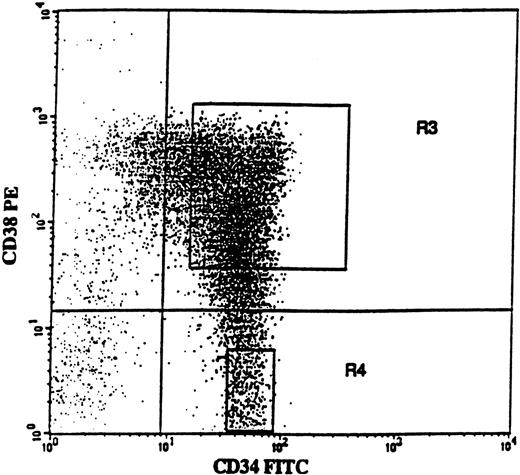
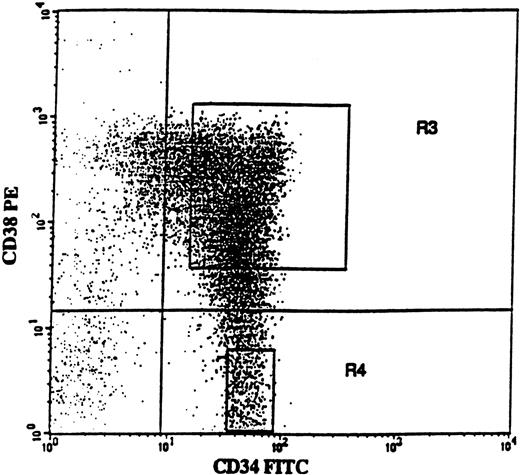
BM cells were obtained from the posterior iliac crest. BM and PB mononuclear cells (MNCs) were isolated by Ficoll-Hypaque (Nycomed, Oslo, Norway) gradient centrifugation. Positive selection of BM CD34+ cells was performed using a MACS (magnetically activated cell sorting) CD34 isolation kit (Miltenyi Biotec, Bergish Gladbach, Germany), as described previously.48Purity of CD34+ cells was 76% to 94% (average 88%). CD34-enriched cells were incubated with CD38-phycoerythrin (PE) and CD34–fluorescein isothiocyanate (FITC) monoclonal antibodies (MoAbs) or with isotype-matched irrelevant control antibodies (all from Becton Dickinson, San Jose, CA). The 75% of CD34+ cells expressing the highest levels of CD38 (CD34+CD38+) and CD34+CD38− cells were sorted on a FACSVantage Cell Sorter (Becton Dickinson). As described previously,48a conservative approach was taken so that only the “lowest” CD34+CD38− cells (mean, 3.7%; range, 2.5% to 6.1%) were sorted (Figure 1; R4 gate), in an effort to obtain a highly enriched population of primitive progenitors. Both CD34+ cell populations reproducibly had a purity of greater than 98% with regard to CD34 expression.
CD34 and CD38 expression profile on CD34-enriched cells from a typical patient with 5q− syndrome.
CD34-enriched cells from patient no. 4 were stained with CD34-FITC and CD38-PE antibody or isotype control antibody. In this patient, CD34+CD38+ and CD34+CD38− cells represented 91.4% and 8.6% of CD34+ cells, respectively. Also shown are the sorting gates for CD34+CD38+ (R3) and CD34+CD38− (R4) cells.
CD34 and CD38 expression profile on CD34-enriched cells from a typical patient with 5q− syndrome.
CD34-enriched cells from patient no. 4 were stained with CD34-FITC and CD38-PE antibody or isotype control antibody. In this patient, CD34+CD38+ and CD34+CD38− cells represented 91.4% and 8.6% of CD34+ cells, respectively. Also shown are the sorting gates for CD34+CD38+ (R3) and CD34+CD38− (R4) cells.
BM CD34+CD19+CD14−CD15−(pro-B) and CD34+CD33+ (myeloid) progenitors were sorted after incubation with allophycocyanin (APC)– or FITC-conjugated CD34 MoAbs, PE-conjugated CD19 or CD33 MoAbs, and FITC-conjugated CD14 and CD15 MoAbs for 15 minutes on ice. PB CD3+, CD15+, and CD19+ cell populations were sorted after incubation of MNCs with CD3-FITC (Becton Dickinson), CD15-FITC (Pharmingen, San Diego, CA), and CD19-PE (Becton Dickinson) MoAbs respectively.
Fluorescence in situ hybridization
Cytospin preparations of various cell populations were pretreated as described previously.49 Briefly, slides were fixed in methanol/acetic acid 3:1 for 15 minutes, dehydrated in a graded (70% to 100%) series of ethanol, and air dried. The slides were then fixed in 1% neutral buffered formalin for 5 minutes, followed by microwave heat treatment in sodium citrate buffer, pH 6.0, for 2 × 5 minutes; 0.01% proteinase K (type VIII; Sigma, St. Louis, MO) digestion for 5 minutes; and postfixation in neutral buffered formalin for 5 minutes before being dehydrated and air dried. The LSI EGRI/D5S721:D5S23 dual-color DNA probe (Vysis Inc, Downers Grove, IL) was used for hybridization according to the manufacturer's instructions. After hybridization, the slides were washed in 1 × SSC for 5 minutes at 72°C, followed by washes in 0.05% TBS-Tween for 3 minutes at room temperature and counterstaining with DAPI (4,6-diamino-2-phenylinol, 1 μg/mL; Sigma). The Vysis dual-color probe hybridizes to band 5q31 (SpectrumOrange LSI EGRI) and to band 5p15.2 (SpectrumGreen LSI D5S721:D5S23) of chromosome 5. In the nuclei of normal cells, the probe appears as 4 distinct signals, 2 orange and 2 green (2O + 2G), whereas patients with del(5q) typically show 1O + 2G. Occasionally the probe may appear as other combinations of orange and green signals, which are excluded when calculating the frequency of 5q-deleted and normal cells in a specific cell population. Unless otherwise stated, 100 to 600 cells were evaluated for each cell population. For all cell populations investigated, cells from normal subjects were treated the same way and used to determine the cutoff value for the FISH method; they always showed less than 5% 1O + 2G cells. When validating the specificity of FISH results on fluorescence-activated cell sorter (FACS)-purified cell populations from MDS patients, one also must consider the likelihood of 1O + 2G cells originating from impurities. On the basis of the purity of sorted populations (greater than 90%) and with the cutoff value of the FISH method, we generally considered findings of less than 10% to 15% 1O + 2G cells (depending on cell populations) to be negative or inconclusive.
Hematopoietic growth factors
Recombinant human (rh) megakaryocyte growth and development factor (MGDF), rh granulocyte colony-stimulating factor (G-CSF), rh stem cell factor (SCF), rh interleukin-3 (IL-3), and rh GM-CSF were generously provided by Amgen Corp. (Thousand Oaks, CA). Rh erythropoietin (Epo) was supplied by Boehringer Mannheim Corp (Mannheim, Germany) and rh fH3 ligand (FL) by Immunex (Seattle, WA).
Expansion cultures
CD34+CD38+ and CD34+CD38− cells from MDS patients and healthy subjects were seeded at 3000 to 10 000 cells/mL in Iscove's modified Dulbecco's medium (IMDM) (BioWhittaker, Walkersville, MD) supplemented with 20% fetal calf serum (FCS; BioWhittaker), 10−4 mol/L 2-mercaptoethanol (Sigma), and cytokines. After 10 to 12 days, the cells were counted and cytospins were examined morphologically after May-Grünwald-Giemsa staining (Sigma) or were subjected to FISH analysis.
Single-cell clonogenic assay
As described previously,50CD34+CD38+ and CD34+CD38− cells were seeded in Terasaki plates (Nunc, Kamstrup, Denmark) at a density of one cell per well in 20 μL serum-depleted medium (X-vivo 15; BioWhittaker) supplemented with 1% bovine serum albumin (Stem Cell Technologies Inc., Vancouver, Canada), 10−4 mol/L 2-mercaptoethanol, and cytokines. Wells were scored for cell growth after 11 to 13 days of incubation.
Long-term culture-initiating assay
Long-term cultures were established with normal BM MNCs, irradiated, and maintained according to previously described methods.48,51 CD34+CD38+(750-7500) and CD34+CD38− (150-750) cells from MDS patients and healthy adults were added to stroma layers (same stroma for MDS and normal controls), and cocultures were maintained by weekly 50% medium changes (Myelocult H5100; Stem Cell Technologies). After 5 weeks, nonadherent and adherent cells were plated in methylcellulose cultures, as described,48 supplemented with MGDF, G-CSF, SCF, FL (all at 25 ng/mL), Epo (5 U/mL), and IL-3 (10 ng/mL). Colony-forming cells (CFC; readout of long-term culture-initiating [LTC-IC] assay) were scored after an additional 12 to 14 days in culture.
Nonobese diabetic/severe combined immunodeficiency (NOD/SCID) repopulating assay
NOD/LtSz-SCID mice (originally from The Jackson Laboratory, Bar Harbor, ME) were bred and housed under sterile conditions in microisolator cages and given autoclaved food and acidified drinking water. Mice were irradiated with 350 to 375 cGy from a137Cs source at 8 to 12 weeks of age and thereafter were given prophylactic ciprofloxacin (100 μg/mL) in the drinking water until analysis (6-8 weeks after transplantation). Tail-vein transplantation/injection of hematopoietic cells suspended in 0.5 to 1.0 mL of medium was performed within 4 hours of irradiation. If hematopoietic cells transplanted were less than 105, 1 × 106 irradiated accessory cells (human MNCs or CD34-depleted cells) were coinjected. Femora and tibiae were collected after asphyxiation with CO2, and engraftment was investigated as described previously.45 52 Briefly, cells were stained with anti-human CD45-FITC and CD71-FITC antibodies as well as antimouse CD45.1(Ly 5.1)-PE. Untransplanted mice (negative controls) and mixtures of 0.1% to 0.5% human cells in murine BM (positive controls) were always included. If engraftment was detected by CD45/71 analysis (detection level less than 0.05%), staining with anti–CD34-FITC, CD19-PE, CD33-PE/CD66b-FITC, and CD15-PE was performed, including antihuman CD45–peridinin chlorophyll protein and antimouse CD45.1 biotin plus streptavidin-APC. BM cells (5 × 104 to 1 × 105) were plated in methylcellulose and human-specific cytokines (GM-CSF, 50 ng/mL; IL-3, 25 ng/mL; and SCF, 25 ng/mL) plus Epo at 5 U/mL, resulting in no colony formation of BM from untransplanted NOD/SCID mice (L.N. and S.E.W.J., unpublished observations). CFU-GM and burst-forming unit-erythroid were scored after 10 to 14 days and transferred to slides for FISH analysis.
Results
Targeting of the 5q deletion to the CD34+CD38− stem cell compartment
In 5 patients with refractory anemia (RA), the 5q deletion was the only diagnosed chromosomal aberration (Table 1), and all of these fulfilled the diagnostic criteria for the 5q− syndrome.39 Of the remaining 7 patients, 1 had an isolated 5q deletion but was of the RAEB subgroup, whereas the others had a 5q deletion combined with other chromosomal aberrations. Morphologically identified blasts in BM aspirates varied from less than 5% (8 of 12 patients) to 17% (patient no. 11).
Although CD34+CD38− cells represent only approximately 1 in 1000 normal BM cells, almost all stem cell activity resides in this minor subfraction, both in normal and AML subjects.43-46 All 4 patients with 5q− syndrome investigated showed a similar distribution between CD34+CD38+ and CD34+CD38− cells as observed in normal BM, with the CD34+CD38− subpopulation representing 10% or less of the CD34+CD38+ cells (Table2, Figure 1). Similarly, in all 5 investigated patients with 5q deletions combined with other chromosomal aberrations, CD34+CD38− cells represented a minority of the total CD34+ cells. Only in patient no. 6 did CD34+CD38− cells represent a major fraction of the CD34+ cells. It is noteworthy that this patient had as many as 24% CD34+ cells in the BM MNCs, whereas CD34+ cells did not exceed 6% in any of the other patients included in this study. Among CD34+CD38+ cells, 88% to 98% had 5q deletions (Table 3). Strikingly, in 8 of the 9 investigated patients, more than 98% of the CD34+CD38− cells carried the 5q deletion, whereas 94% in the last patient (no. 4) were involved (Table 3, Figure2). Thus, in MDS patients with both 5q− syndrome and 5q deletion in combination with other chromosomal aberrations, the vast majority of candidate stem cells appear to be derived from the 5q-deleted clone.
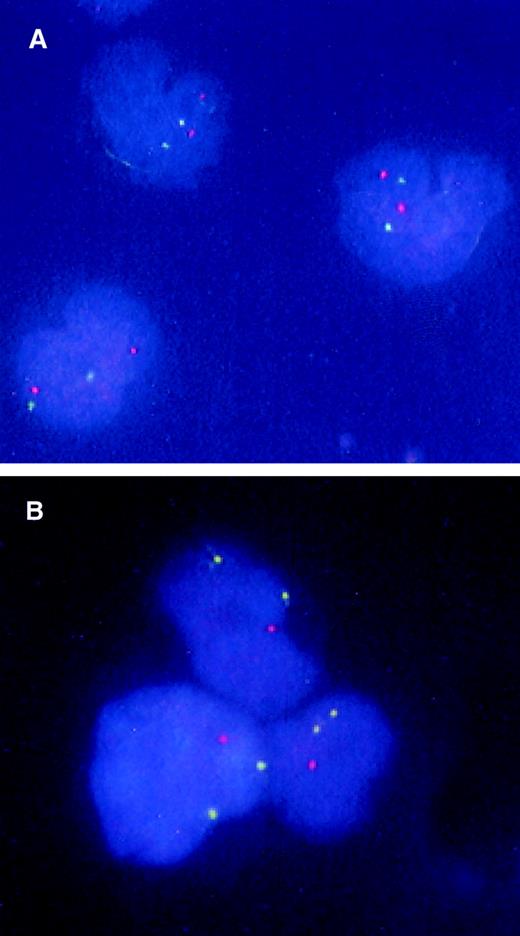
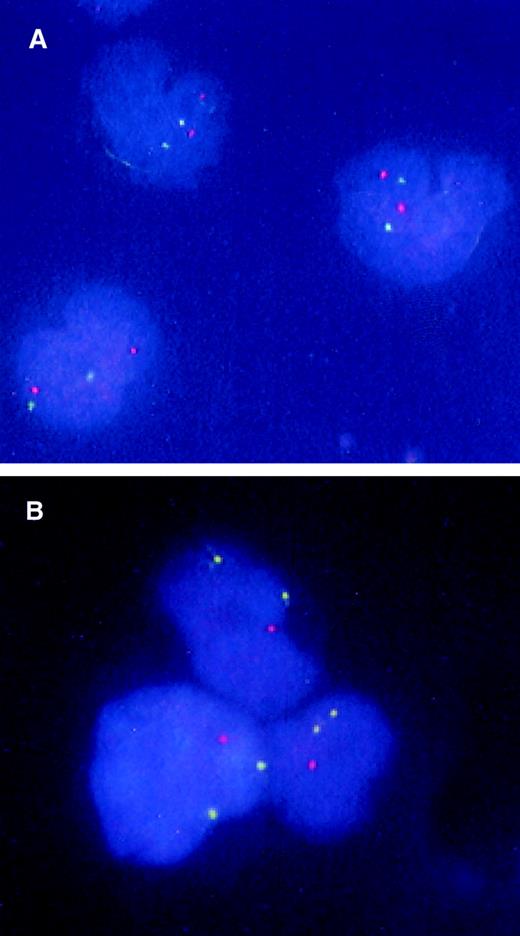
FISH analysis of CD34+CD38− candidate stem cells from a patient with 5q− syndrome.
BM CD34+CD38− cells were isolated from a normal donor (A) and patient no. 1 with 5q− syndrome (B) and subjected to FISH analysis as described in “Materials and methods.” The nuclei of normal cells appear as 2 green (5p) and 2 orange (5q), whereas 5q-deleted cells have 2 green signals and 1 orange signal.
FISH analysis of CD34+CD38− candidate stem cells from a patient with 5q− syndrome.
BM CD34+CD38− cells were isolated from a normal donor (A) and patient no. 1 with 5q− syndrome (B) and subjected to FISH analysis as described in “Materials and methods.” The nuclei of normal cells appear as 2 green (5p) and 2 orange (5q), whereas 5q-deleted cells have 2 green signals and 1 orange signal.
Demonstration of 5q deletions in lymphoid progenitor cells but not mature lymphocytes
Apparently in contrast to the finding that virtually all cells in the phenotypically defined stem cell compartment had the 5q deletion, previous studies had stated that 5q deletions are restricted to myeloid cells and do not involve mature lymphocytes.23,24,28,35,38,39 In the present studies (Table 4), a large fraction of PB CD15+ myeloid cells in all patients were 5q deleted (51% to 99%), whereas the findings on T cells in all patients were within the expected normal range (when corrected for purity of the population and the cutoff value for the FISH method). It is noteworthy that in patient no. 4, a large fraction (30% and 33%; examined on 2 different occasions) of PB CD19+ cells were 5q deleted, whereas B cells in the remaining patients were within the expected normal range. Thus, with the exception of B cells in 1 of 9 patients, our findings of 5q deletions in mature myeloid but not lymphoid cells confirmed those of previous studies.23,24,28 35 Thus, we hypothesized 2 potential explanations for the apparent involvement of HSCs, but not mature lymphocytes, in the 5q− clone: (1) The 5q deletion in multipotent HSCs is incompatible with lymphoid commitment; or (2) the 5q deletion is incompatible with or disadvantageous for lymphocyte maturation.
To distinguish between these possibilities, we sought to purify the earliest identifiable lymphoid progenitors from 5q-deleted MDS BM. Probably because of the myeloid hyperplasia in these patients, the frequencies of pro-T (CD34+CD7+) and pro-B (CD34+CD19+) cell progenitors were very low when compared with those of normal subjects (L.N. and S.E.W.J., unpublished observations). Thus, to obtain enough purified cells to perform meaningful FISH analysis, we could investigate only pro-B cells (Table 5). These pro-B cells were sorted based on coexpression of CD34 and CD19 and also lack of myeloid cell surface antigens (Figure 3). Five of the 9 patients investigated revealed distinct CD34+CD19+ pro-B cells sufficient to allow meaningful sorting. In 3 patients (2 with 5q− syndrome), a sizeable fraction of CD34+CD19+ cells with 5q deletion (67%, 90%, and 25% for patients 2, 4, and 9, respectively) was identified. The majority of CD34+CD33+ myeloid progenitors in all investigated patients had the 5q deletion (Table 5). Finally, in one patient (no. 1), we sorted a distinct CD34+CD7+ pro–T-cell population representing 7% of the total CD34+ cell population, of which 98% had the 5q deletion (L.N. and S.E.W.J., unpublished observations). Thus, the finding of the 5q deletion in lymphoid-committed as well as myeloid-committed progenitor cells lends further support to the theory that the 5q deletion is of multipotent (lympho-myeloid) stem cell origin.
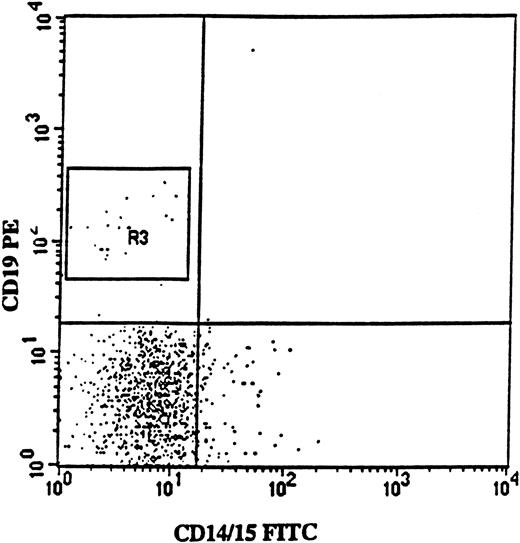
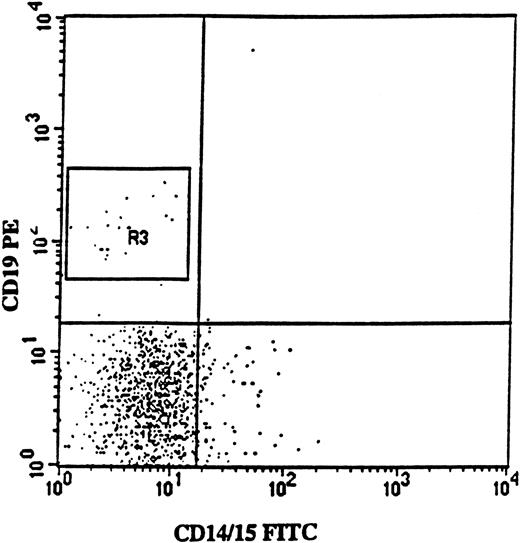
Isolation of CD34+CD19+ pro-B cells from a patient with 5q− syndrome.
FACS profile shows CD19 (PE) versus myeloid (CD14/15-FITC) antigen expression on BM cells from patient no. 2, gated on CD34 (APC)-positive BM cells. Note the lack of coexpression of CD19 and myeloid antigens. In this patient, CD34+CD19+ cells represented 2% of the CD34+ cells, and 67% of these cells were 5q− according to FISH analysis.
Isolation of CD34+CD19+ pro-B cells from a patient with 5q− syndrome.
FACS profile shows CD19 (PE) versus myeloid (CD14/15-FITC) antigen expression on BM cells from patient no. 2, gated on CD34 (APC)-positive BM cells. Note the lack of coexpression of CD19 and myeloid antigens. In this patient, CD34+CD19+ cells represented 2% of the CD34+ cells, and 67% of these cells were 5q− according to FISH analysis.
Functional characterization of the CD34+CD38− candidate stem cell compartment in MDS patients with 5q deletions
The development of better functional stem cell assays has facilitated the identification and characterization of human stem cells in recent years. Among these are in vitro assays of purified candidate stem cells revealing characteristic patterns of responsiveness to early-acting cytokines53 and reconstitution of hematopoiesis in long-term in vitro stroma-supported cultures or in in vivo xenograft models.54-57
CD34+CD38+ and CD34+CD38− cells were purified from 5 patients and investigated for their responsiveness to a predetermined optimal combination of cytokines (Table 6). In 4 of the patients (nos. 4, 7, 8, and 10), CD34+CD38− cells cultured for 10 to 14 days maintained a much higher frequency of immature/blast cells than CD34+CD38+ cells, comparable to normal donors, supporting the more primitive nature of CD34+CD38− cells. However, the in vitro expansion potential of both the CD34+CD38+ and CD34+CD38− cells in all of these patients was markedly lower than that of the parallel normal controls, and most of the cells derived from both populations had 5q deletions (Table 6). However, it is interesting that in most patients (with the exception of patient no. 12; discussed later), a much higher frequency of cells with normal 5q status was produced in culture than observed in the starting CD34+CD38+ populations, suggesting a proliferative advantage of cells with a normal 5q status. In the CD34+CD38− populations, a similar tendency was observed for patient no. 8. In the last patient (no. 12), whose BM in all assays showed a unique and hyperresponsive pattern, the CD34+CD38+ cells also maintained a high level of blast cells following culture, and the expansion potential of the CD34+CD38− cells was somewhat higher than that of the normal control. Almost all cells in both populations from this patient contained the 5q deletion.
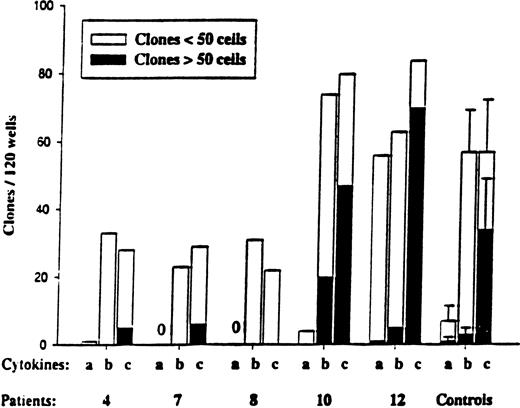
We next investigated the responsiveness of CD34+CD38+ and CD34+CD38− progenitors to the early-acting cytokines SCF, FL, and MGDF. Normal human CD34+CD38− stem cells show a unique response pattern to these cytokines, in that a combination of all 3 optimally recruits CD34+CD38− cells into proliferation, whereas SCF+FL in the absence of MGDF has little or no such ability.58 CD34+CD38− cells isolated from patients 4, 7, 8, and 10 all showed such a normal response pattern (Figure 4), whereas CD34+CD38+ cells (such as their normal counterparts) showed a higher response to SCF+FL in the absence of MGDF (L.N. and S.E.W.J., unpublished observations). Again, the CD34+CD38− cells isolated from patient 12 displayed enhanced responsiveness, in particular to SCF+FL, when compared with the other patients as well as normal controls.
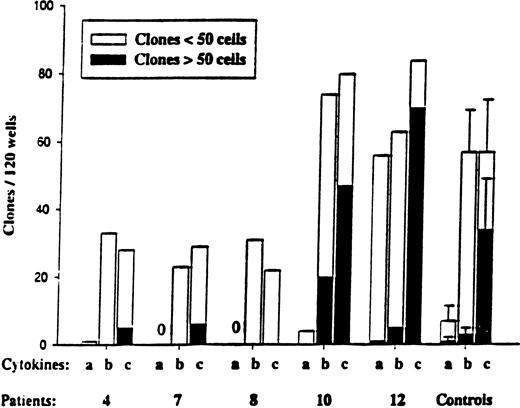
Responsiveness of CD34+CD38−MDS cells to early-acting cytokines.
CD34+CD38− cells were plated at the single-cell level as described in “Materials and methods” and cultured in 3 different cytokine combinations: a = SCF + FL; b = SCF + FL + MGDF; and c = cocktail (SCF + FL + MGDF + IL-3 + G-CSF + GM-CSF + Epo). Five normal subjects were used as controls. Wells were scored for total clones per 120 wells and for size (3-50 cells and more than 50 cells) after 11 to 13 days of incubation. No clones were observed for MDS patients 7 and 8 in response to cytokine combination “a.” In general, the clones formed from CD34+CD38−cells were too small to allow FISH analysis.
Responsiveness of CD34+CD38−MDS cells to early-acting cytokines.
CD34+CD38− cells were plated at the single-cell level as described in “Materials and methods” and cultured in 3 different cytokine combinations: a = SCF + FL; b = SCF + FL + MGDF; and c = cocktail (SCF + FL + MGDF + IL-3 + G-CSF + GM-CSF + Epo). Five normal subjects were used as controls. Wells were scored for total clones per 120 wells and for size (3-50 cells and more than 50 cells) after 11 to 13 days of incubation. No clones were observed for MDS patients 7 and 8 in response to cytokine combination “a.” In general, the clones formed from CD34+CD38−cells were too small to allow FISH analysis.
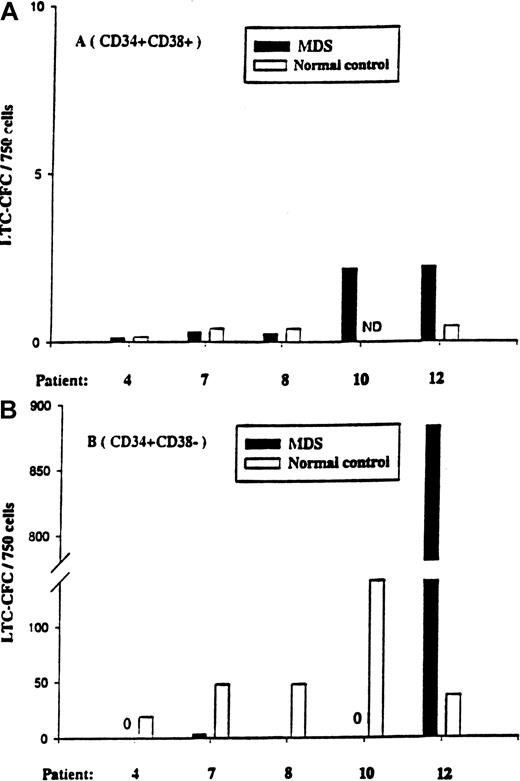
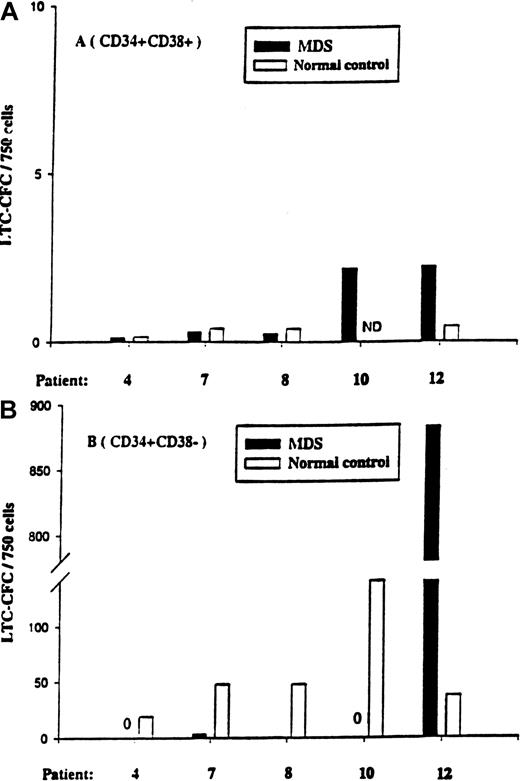
The CD34+CD38+ cell population from all 5 patients (and the normal controls) contained little or no LTC-IC activity (Figure 5). At variance with the normal donors, CD34+CD38− cells from patients 4, 7, 8, and 10 contained little or no LTC-IC activity, even when plated at 5-fold higher cell numbers than the normal control. In striking contrast, CD34+CD38− cells from patient no. 12 had greater than 20-fold more LTC-IC activity than the normal control population, and all (12 of 12) investigated colonies were 5q deleted, again supporting the hyperresponsiveness of the 5q-deleted CD34+CD38− cells in this patient.
LTC-IC activity of CD34+CD38+and CD34+CD38− cells in 5q− MDS patients.
Five-week LTC-IC activity of MDS BM CD34+CD38+cells (A) and CD34+CD38− cells (B) was evaluated as described in “Materials and methods.” CD34+CD38+ and CD34+CD38− cells isolated from normal subjects were included as controls. After 5 weeks of culture, the number of CFCs produced was evaluated in methylcellulose, as described in “Materials and methods.” Results are the means of 3 to 6 replicate wells from each cell population and patient.
LTC-IC activity of CD34+CD38+and CD34+CD38− cells in 5q− MDS patients.
Five-week LTC-IC activity of MDS BM CD34+CD38+cells (A) and CD34+CD38− cells (B) was evaluated as described in “Materials and methods.” CD34+CD38+ and CD34+CD38− cells isolated from normal subjects were included as controls. After 5 weeks of culture, the number of CFCs produced was evaluated in methylcellulose, as described in “Materials and methods.” Results are the means of 3 to 6 replicate wells from each cell population and patient.
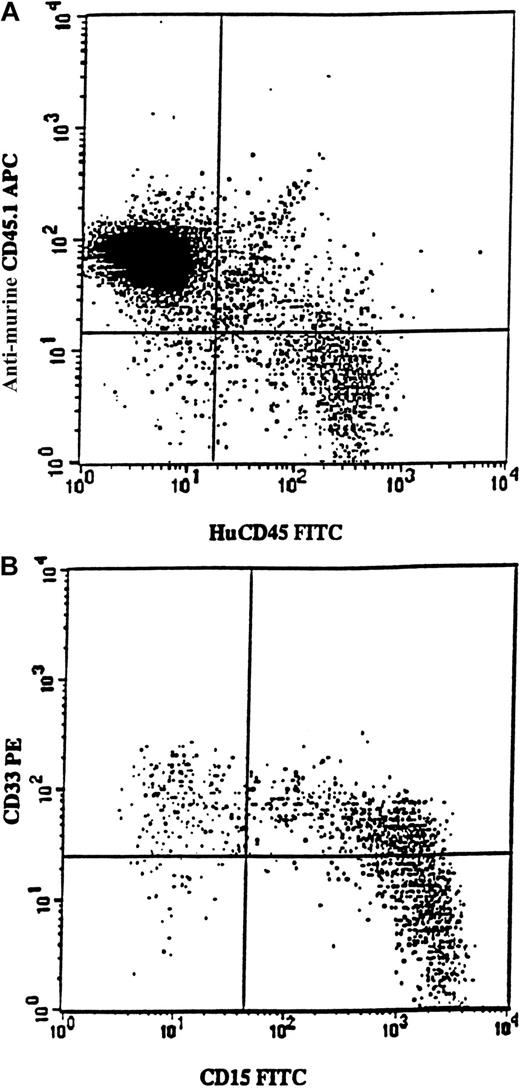
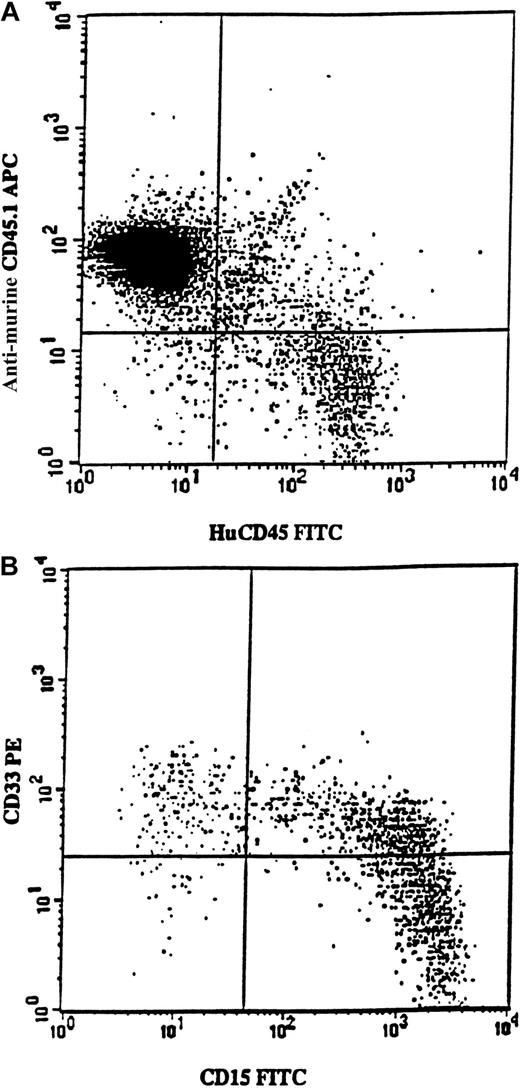
Samples from 7 5q-deleted patients (nos. 3, 4, 6, 7, 8, 11, and 12) were transplanted into sublethally irradiated mice, either as CD34+ cells (250 000-700 000 per mouse) or as purified CD34+CD38− cells (6000-17 000 per mouse). In agreement with the cells' inability to achieve long-term reconstitution in vitro, no mice transplanted with cells from patients 3, 4, 6, 7, 8, or 11 showed any signs of human reconstitution (as determined by FACS and human colony formation; L.N. and S.E.W.J., unpublished observations). However, mice receiving transplants of 700 000 CD34+ cells from patient no. 12 showed as much as 12% human (CD45) engraftment (Figure6A). Most reconstituted cells expressed the myeloid markers CD15 and/or CD33 (Figure 6B). Human CD45+CD15+ cells were purified from the murine BM and, when investigated by FISH, all proved to be 5q deleted (256 of 256 evaluated cells).
Exclusive myeloid repopulation by 5q-deleted NOD/SCID reconstituting stem cells.
FACS profile shows BM from 4 pooled NOD/SCID mice each having transplantation with 700 000 CD34+ cells isolated from MDS patient no. 12. (A) BM cells were stained with antimouse CD45.1-APC (Ly 5.1) and antihuman CD45-FITC. Human CD45+ cells constituted 12% of the whole mouse BM. (B) Coexpression of CD15 and CD33 on human CD45+ cells. BM was gated on huCD45+ cells and investigated for the expression of huCD15 and huCD33. Note that virtually all cells expressed myeloid markers, as confirmed by the absence of CD19 expression (data not shown).
Exclusive myeloid repopulation by 5q-deleted NOD/SCID reconstituting stem cells.
FACS profile shows BM from 4 pooled NOD/SCID mice each having transplantation with 700 000 CD34+ cells isolated from MDS patient no. 12. (A) BM cells were stained with antimouse CD45.1-APC (Ly 5.1) and antihuman CD45-FITC. Human CD45+ cells constituted 12% of the whole mouse BM. (B) Coexpression of CD15 and CD33 on human CD45+ cells. BM was gated on huCD45+ cells and investigated for the expression of huCD15 and huCD33. Note that virtually all cells expressed myeloid markers, as confirmed by the absence of CD19 expression (data not shown).
Discussion
Identification of the cell of origin in MDS not only should facilitate a better understanding of MDS pathogenesis, but also could have important therapeutic implications. Currently, allogeneic stem cell transplantation represents the only curative treatment for MDS.59 However, because of the advanced age of most MDS patients and limited donor availability, only a small fraction of MDS patients are eligible for this option. As a consequence, autologous stem cell transplantation has become a new therapeutic alternative pursued in MDS,42 in which the status and involvement of the reconstituting stem cell pool could be of crucial importance to the clinical outcome.
Most studies exploring the potential involvement of HSCs in MDS have done so by investigating to what degree the lymphoid as well as myeloid cell lineages are involved in the malignant clone. As outlined in the “Introduction,” such studies have predominantly concluded that in most MDS patients, lymphocytes do not appear to be derived from the malignant clone.9,23,25-31,33,35 This appears particularly clear when chromosomal aberrations are used as markers for the clonal disease.9,25,27,28,30-32,34,35 An alternative and less explored avenue of establishing whether HSCs are transformed in MDS is to investigate whether highly purified HSC populations belong to the transformed clone or not. Whereas FISH analyses of purified HSC populations from AML patients have revealed cytogenetically aberrant cells in the stem cell compartment,60,61 a recent study of MDS patients with trisomy 8 demonstrated that the stem cell compartment was not part of the +8 clone.62 In striking contrast, we here provide data to support that in MDS patients with a 5q deletion, virtually all cells in the HSC compartment appear to be involved in the 5q-deleted clone, although evidence for mature T cells with 5q deletions could not be obtained in any of the 10 investigated patients, and in the case of B cells, in only 1 patient (no. 9). Despite this, multiple lines of evidence supported that primitive lympho-myeloid HSCs represent the cell of origin for 5q deletions: (1) There is massive involvement (in most patients 99% or more) of the minor CD34+CD38− HSC compartment. (2) The presence of a large fraction of 5q-deleted CD34+CD19+pro-B cells was found in 3 of 5 investigated patients and of pro-T cells (CD34+CD7+) in 1 investigated patient. In 1 patient (no. 4), both CD34+CD19+ pro-B cells and CD19+ B cells were found to be involved on 2 separate occasions. (3) Extensive functional characterization suggested that CD34+CD38− cells represent the HSC compartment in 5q-deleted MDS, as demonstrated previously in normal and AML subjects.43-46 58
As expected, given that the nature of the disease is one of ineffective hematopoiesis, the 5q-deleted HSCs (with one notable exception, patient no. 12) were inefficient at reconstituting hematopoiesis (in vitro as well as in vivo). Although the control subjects used in these studies were considerably younger than the investigated MDS patients, the high number of cells used in the LTC assay suggests a dramatic reduction in stem cell activity in these patients. However, in future studies it will be important to use age-matched controls and to further increase the number of input cells in these assays. It will also be important to assay CD34−cells for potential stem cell activity, although in patient no. 12 the stem cell activity resided in the CD34+CD38−population. In agreement with the finding that most CD34+CD38− cells are 5q deleted, the frequency of normal CD34+CD38− HSCs in 5q-deleted patients appears to be very low. Thus, phenotypic, cytogenetic, and functional studies strongly implicate involvement of the CD34+CD38− HSC compartment in MDS with 5q deletions. This is in striking contrast to the lack of +8 HSCs in MDS patients with trisomy 8.62 The only notable methodologic difference between the studies was that Saitoh et al sorted HSCs based on coexpression of CD34 and Thy1,62 63 whereas we studied CD34+CD38− cells. However, because these populations in BM have been demonstrated to largely overlap functionally and phenotypically, this is unlikely to explain the contrasting conclusions reached.
Because MDS are heterogeneous, the different findings in patients with trisomy 8 and 5q deletions could be explained in that the clonal disease originates in committed myeloid progenitors in patients with trisomy 8 and in pluripotent HSCs in 5q-deleted patients. Based on previous studies, it has been proposed that the neoplastic event in most MDS patients occurs at a committed myeloid progenitor level.2 However, the basis for proposing primary transformation events in MDS occurring in myeloid-restricted progenitors has been the lack of evidence for lymphocyte involvement in most cases.2,9,12,18-23;25-31,33,35 Because our results as well as previous studies suggest that mature lymphocytes are not derived from the 5q-deleted clone,23,24,28 35 del(5q) could also have been predicted to occur predominantly in myeloid-restricted progenitors. Thus, on the basis of our findings, we would rather propose that HSCs could be the cell of origin in many cases of MDS and that direct studies of highly purified HSCs will be an important avenue to establish this.
There are multiple possible explanations for why B and T cells in MDS patients with 5q deletions appear not to be derived from the 5q-deleted HSCs. Because reconstituting common lymphoid progenitors have been identified64,65 and lymphoid progenitors can be long-lived,66 it is possible that the B and T cells are derived from lymphoid-restricted progenitors generated before the 5q deletion. Alternatively, B and T cells might be produced from a low fraction of normal stem cells. Regardless, the lack of lymphopenia in 5q− patients suggests an efficient compensatory mechanism for lymphocyte production by “normal” hematopoietic cells in these patients. It is important to emphasize that because of the limitations intrinsic to the methods used in this study (FACS and FISH) as well as in other studies, it is impossible to conclude or exclude that a minor fraction of B and T cells are derived from the 5q-deleted clone. In fact, several studies of MDS patients have suggested that a low fraction of T and, in particular, B cells might be involved in the malignant clone (reviewed in Knuutila13). We propose that 5q-deleted stem cells are either deficient in their ability to differentiate toward mature lymphocytes or that these are efficiently deleted. It is noteworthy that similar findings, compatible with deficient lymphocyte (in particular T-cell) differentiation, have been reported recently for CML.67
Before these studies, we expected that the del(5q) FISH analysis on cells in the CD34+CD38− HSC compartment might prove more heterogeneous in patients with multiple aberrations than in the more distinct group of 5q− syndrome. However, also in all 5 patients with a complex karyotype, a minimum of 99% of the CD34+CD38− cells proved to have the 5q deletion. This suggests that 5q deletions frequently represent an early event in MDS transformation.
Autologous transplantation has been launched as a new and potentially curative treatment for MDS.42 In that regard, it has been argued that a key challenge is to obtain normal stem cell grafts uncontaminated with transformed stem cells.2 Although the presence of lymphoid as well as, in some cases, myeloid cells with a normal 5q karyotype argues for the likely coexistence of normal HSCs, the finding that a majority of HSCs in 5q-deleted MDS might carry the 5q deletion and have the same phenotype as normal HSCs suggests that purification and selection for normal HSCs will prove difficult.
Acknowledgments
We thank Drs Janet Nichol and Graham Molineux (both of Amgen) and Stewart Lyman (of Immunex) for generous contributions of cytokines for these studies. The expert advice and assistance of David Bryder and Anna Fossum in the NOD/SCID analysis; Gunilla Gärdebring, Carl-Magnus Högerkorp, and Sverker Segrén in cell sorting; and Lilian Wittman, Eva Gynnstam, Irene Persson, and Kristina Sundgren in mouse work are highly appreciated. We also thank Lisa Palm for help with cryopreservation. We are particularly grateful to Bertil Johansson (Department of Clinical Genetics) for helpful advice and educating discussions and to Drs John E. Dick and Connie J. Eaves for crucial advice during the establishment of the NOD/SCID assay. We also thank all patients and bone marrow volunteers for their contributions; the staff at the Department of Hematology for assistance with the aspirations; Inge Olsson, P. G. Nilsson, Jan Westin, Bengt Sallerfors, Ingemar Winqvist, Rolf Billström, Tor Olofsson, Ingunn Dybedal, Ewa Sitnicka, Yutaka Sasaki, and Corinne Rusterholz for discussions or critical review of the manuscript; and Stig Rödjer and Nils Mauritzon for providing crucial bone marrow samples. Prof Stefan Karlsson's contributions through helpful discussions and critical review of the manuscript are highly appreciated.
Supported by grants from the Swedish Cancer Society (4148-B98-01XAB and 3794-B98-03XAB), the Cancer Society in Stockholm (98:111 and 99:151), The Craaford Foundation, Georg Danielsson Foundation, Harald Jeansson Foundation, Nilsson's Cancer Foundation, Åke Wiberg Foundation, Anna-Lisa and Sven-Eric Lundgren Foundation, John and Augusta Persson Foundation, John Persson Foundation, Tobias Foundation, Government Public Health (ALF) Grants, Skåne Landsting, and the Medical Faculty, University of Lund.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Sten E. W. Jacobsen, Stem Cell Laboratory, Institute of Laboratory Medicine, University Hospital of Lund, 22185, Lund, Sweden; e-mail: sten.jacobsen@molmed.lu.se.